American Journal of Analytical Chemistry
Vol.3 No.12A(2012), Article ID:26198,11 pages DOI:10.4236/ajac.2012.312A128
Quality of Canola Oil Obtained by Conventional and Supercritical Fluid Extraction
1Food Science Department, Faculty of Agriculture (Saba Basha), Alexandria University, Alexandria, Egypt
2Richardson Centre for Functional Foods & Nutraceuticals, Winnipeg, Canada
3Human Nutritional Science Department, University of Manitoba, Winnipeg, Canada
Email: *rykhattab@ud.edu.sa, kh_rkh@yahoo.com
Received November 15, 2012; revised December 15, 2012; accepted December 25, 2012
Keywords: Canola Oil; Quality; Supercritical Fluid Extraction
ABSTRACT
Quality of canola oil obtained by the supercritical fluid extraction (SFE), using CO2 with ethanol as a co-solvent, was evaluated and compared to that of the conventionally-obtained oils using either n-hexane or chloroform methanol mixture. Physical characteristics, chemical properties, fatty acid composition and phenolic profile of oils were investigated. The SFE oil showed significantly lower melting point, peroxide value (PV) and higher free fatty acids (FFAs) and iodine value (IV) than the n-hexane-extracted one. There were no significant differences in the fatty acid composition of different oils. The SFE oil showed significantly higher phenolic content (35.91, 10.15, 3.16, 0.32 and 47.48 mg/g of sinapic acid, sinapine, sinapoyl glucose, canolol and total phenolics) as compared to 0.08, 0.70, 0.88, 0.45 and 0.71 mg/g, respectively in the n-hexane-extracted oil. These results indicate the superiority of SFE and advocate its use for the extraction of highly stable and functional canola oil for further health and nutraceutical uses. The present results have an industrial and technological relevance as SFE could be competitive with the traditional extraction techniques providing an environmental approach and enhancing the obtained oil quality and stability. After recovery of the initial installation costs, SFE could be more economic than conventional extraction. However, further economical studies are needed to validate this last conclusion.
1. Introduction
Canola (Brassica napus) is one of the top five oilseed crops cultivated worldwide and the major oilseed crop in Canada with an annual seeded area and production in excess of 7.5 million hectares and 14.2 million metric tonnes, respectively during 2011/2012 season [1]. It is mainly utilized for its oil which is one of the most common edible and healthy cooking oils due to its low content of saturated fatty acids (~7.0%), high content of the mono-unsaturated fatty acids (C18:1n9, oleic acid; ~60.0%), and adequate content of n3 fatty acids (C18:3n3, a-linolenic acid; 8.0% - 12.0%). It contains almost 2:1 ratio of n6 to n3 fatty acids which has been reported to be preferred from the health point of view [2,3] as well as high content of vitamin E [4]. Canola seed is much richer in phenolic compounds than any other oilseed [5]. Its phenolics are mainly sinapic acid derivatives; most commonly sinapine, the choline ester of sinapic acid. Sinapic acid may also exist in its free form or as a glucosidic ester, glucopyranosyl sinapate [6]. Only a small proportion of the phenolic compounds is transferred to the pressed oil while the rest is retained in the defatted residue. The content of these compounds in the crude oil further decreases when the oil is processed [7]. The most prolific phenolic compound responsible for the antioxidant activity in the crude canola oil was identified as vinylsyringol (canolol), which is formed via sinapic acid decarboxylation during pressing of the oil at high temperature and pressure [8,9]. Owing to their high phenolic content canola extracts were reported to have powerful antioxidative properties stronger than many synthetic antioxidants [10,11]. Oilseeds are usually extracted with organic solvents intended to remove the neutral lipids. In routine laboratory analysis the commonly used official extraction methods include those of the German Fat Science Society [12], the American Oil Chemists’ Society [13], the International Organization for Standardization [14], and the Federation of Oils, Seeds and Fats Associations [15]. Most of these methods require long time to complete and consume significant amounts of solvents. n-Hexane has been the most widely used solvent in the oil industry. However, for technological, economical and environmental safety issues, the research is currently geared towards the development of alternative extraction procedures using environment-friendly technologies as well as other organic solvents [16,17]. Common gases, such as carbon dioxide (CO2), in their supercritical state have properties and extraction capacities very similar to liquids [18]. Over the past two decades, supercritical fluid extraction (SFE) using CO2 has been investigated as a possible replacement for n-hexane-based oil extraction [19-22] owing to its numerous advantages [23-25]. By using CO2, SFE could be utilized selectively to extract neutral lipids leaving the polar compounds like phenolics and phospholipids in the defatted meal. To retrieve those components, a polar co-solvent like ethanol can be added to the supercritical carbon dioxide [26]. Effect of SFE on oil yield [24,27], tocopherol content [28] and oxidative stability [29] was investigated. However, studies to compare the effect of different extraction techniques on canola oil composition and quality are rare.
Investigating the physical and chemical properties, fatty acid composition and phenolic profile as quality parameters to compare SFE oil with that obtained by other conventional techniques is needed for the appropriate assessment of the prospective of SFE of canola oil. The only relevant study was conducted by Jenab, Rezaei and Emam-Djomeh [29] who compared canola oils extracted by SC-CO2 (340.0 bar and 40.0˚C) and a commercial organic solvent (70% n-hexane) and reported that the SFE showed lower extraction yield and slightly higher poly-unsaturated fatty acids (PUFAs). Reports on the phenolic content of canola oil from the SFE are lacking.
Adding the pre-extracted phenolics back into the refined oil has been reported to enhance its oxidative stability [30,31] and is a known option for utilizing the natural antioxidants present in canola seed. The other worthy approach is to modify the extraction or refining processes to maximize the extraction and retention of those phenolics in the obtained oil. SFE could be a good practice to produce canola oil with enhanced composition and better quality. Based on the role of canola phenolics as antioxidants, increasing their content in the oil would produce value-added oil with an enhanced stability and longer shelf life. The aim of this work was to evaluate the quality of canola oil obtained by SFE (with ethanol as a co-solvent) as compared to the conventionally solvent-extracted one.
2. Materials and Methods
2.1. Materials
Canola seeds were obtained from the Canadian Grain Commission (Winnipeg, MB, Canada) for the laboratory oil extraction. Two commercial canola oils were obtained from Viterra Associated Proteins (Ste. Agathe, MB, Canada) (expeller-pressed oil) and Bunge Canada (Oakville, ON, Canada) (solvent-extracted oil) for comparison.
Phenolic standards; sinapic, ferulic, caffeic, t-cinnamic, syringic, r-coumaric, salicylic, vanillic, gallic acid, kaempferol and syringaldehyde were purchased from Sigma Aldrich (St. Louis, MO, USA). Sinapinaldehyde was purchased from ChromaDex, Inc. (Irvine, CA, USA). Sinapine thiocyanate was purchased from EPL Bio Analytical Services (Niantic, IL, USA). Sinapoyl glucose was kindly donated by Dr. A. Baumert, IPB-Halle, Germany.
2.2. Experimental Procedures
2.2.1. Sample Preparation
Canola seeds were cleaned manually to remove husks and foreign matter, homogenously ground using a coffee grinder, sieved through 60 mesh (250 µm) screen, and stored at 4˚C in polyethylene bags until used for the extraction.
2.2.2. Oil Extraction
2.2.2.1. Soxtec Extraction Oil was extracted from the ground canola seeds using the Soxtec 2050 (Foss-Tecator, Foss North America, MN, USA) according to AOCS Am 2.93 [15] following the manufacturer’s application guidelines. Samples (12 g each) were put in the thimbles which were then loaded in the Soxtec (unit temperature at 135˚C). The pre-dried aluminum cups were then inserted into the extraction unit and 80 mL of either n-hexane or chloroform/methanol (2:1; v/v) was added to each sample. The system was programmed to start as following: boiling (15 min), rinsing (60 min) and recovery (20 min). Samples were extracted in two cycles.
2.2.2.2. Folch Extraction Oil was extracted from the ground seeds using the Folch method [32]. The ground seeds were homogenized with chloroform/methanol (2:1; v/v) to a final volume of 20 times the weight of the sample (1 g in 20 ml of solvent mixture). After dispersion, the whole mixture was agitated on the magnetic stirrer overnight at room temperature. The homogenate was filtrated using a folded filter paper to recover the liquid phase. The defatted residues were re-extracted another two times (2 hr each) and the extracts from the three extractions were combined.
2.2.2.3. Supercritical Fluid Extraction (SFE)
SFE was carried out in a lab scale unit (SFE 2000, Thar Technologies, Inc., Pittsburgh, PA) equipped with 1200 ml extraction vessel and a high pressure pump according to the procedure of Li, Wu, Rembel and Thiyam [33]. The system was operated by passing supercritical CO2 through a fixed bed of sample particles, precipitating liquid extract in cyclone separators and finally releasing CO2 to the ambient surroundings. The pump was precooled to 4.0˚C in order to deliver liquid CO2 from a storage cylinder (Medigas Manitoba Ltd., Winnipeg, MB, Canada) to the extraction vessel efficiently. A total of 150 g of the ground canola seed was loaded into the extraction vessel in each run. Extraction temperature, pressure and CO2 flow rate were set at 50˚C, 40,000 kPa (400 bar) and 70 g/min, respectively. The co-solvent (food grade ethanol 95%; Commercial Alcohols, Brenntag Canada) was added by pouring a certain volume (500 ml representing 4.70% of the total weight of CO2 used during the extraction) directly into the sample extraction vessel before each extraction process, and the extraction continued for 2 hr.
The oil/solvent mixture after each extraction process was evaporated under vacuum at ambient temperature (20˚C ± 2˚C) in a rotary evaporator (BÜCHI Rotavapor R-205, BÜCHI Labortechnik AG, Postfach, Switzerland) to remove the solvent, and the resultant oil was weighed to calculate the oil content and then kept frozen until further analyses.
2.2.3. Physicochemical Properties of the Oils
Physicochemical properties of the obtained oils were investigated in terms of specific gravity (Method Cc 10a- 25), melting point (Method Cc 1-25), free fatty acids (FFA%) (Method Ca 5a-40), peroxide value (PV) (Method Cd 8-53), r-anisidine value (Method Cd 18-90), and iodine value (IV) (Methods Cd 1d-92 and Cd 1c-85) according to the AOCS official methods [13]. The color of the oils was determined by either the Lovibond PFX 995 Tintometer (The Tintometer Limited, Amesbury, Salisbury, UK) with the 10.0 mm sample cell using the Lovibond RYBN color scale according to the AOCS (Method Cc 13b-45) [13] or by the spectrophotometric method [34] through measuring the absorption of the oil samples at 420 nm against water as blank using the DU 800 UV/ Visible Spectrophotometer (Beckman Coulter Inc., Mississauga, ON, Canada) and reporting the results as the color index (CI).
2.2.4. Fatty Acid Composition
Oil samples were saponified in 0.5 mol/L methanolic KOH. The fatty acids methyl esters (FAMEs) were prepared with 14% boron trifluoride in methanol [35]. FAMEs were analyzed by a GC-6890 Agilent gas chromatography system (Agilent Technologies Canada Inc., Mississauga, ON, Canada) equipped with a DB225-M/S capillary column (30.0 m × 0.25 mm i.d.; J&W Scientific, Folsom, CA) and a flame ionization detector (FID). The initial temperature was set at 70˚C and increased to 195˚C at the rate of 20˚C/min for 5.0 min followed by an increase to 220˚C at 2˚C/min for 15.0 min, and then 240˚C at 20˚C/min for 5.0 min. Hydrogen was used as carrier gas. FAMEs were identified by comparison of retention times with fatty acid methyl ester standard (NuChek Prep 461; Elysian, MN, USA) and results were expressed as area percentages.
2.2.5. Phenolic Profile of Canola Oil
2.2.5.1. Extraction of Phenolic Compounds Extraction of phenolic compounds from the oil samples was carried out following the procedure of Koski et al. [7] with some modifications. Oil samples (2.5 g each) were mixed with 5 ml of n-hexane and 5 ml of 17.28 mol/L aqueous methanol followed by vortex for 2 min and centrifugation at 2236 xg for 10 min at 4˚C using the Sorvall RC6 200505A351 (Mandel, Ashville, NC, USA). The bottom layer (the methanol layer) was pipetted out using a glass pipette to a large test tube. The n-hexane residues were re-extracted the same way and the bottom layer was combined with the previous one in the same test tube and totally dried under nitrogen. The residues were redissolved in 2.5 ml of 17.28 mol/L aqueous methanol, shaken in ultrasound water bath for 1 min and then filtered through a 0.45 µm syringe filter.
2.2.5.2. Characterization of Phenolic Compounds Using HPLC-DAD Analysis of phenolic compounds for the resultant filtrates was done by reversed-phase DAD-HPLC (Ultimate 3000; Dionex, Sunnyvale, CA, USA) equipped with on-line degasser, binary pump, auto sampler, column heater and diode array detector according to our procedure [36]. A gradient elution was performed using water/methanol (90:10; v/v) with 1.25% o-phosphoric acid as solvent A, and methanol (100%) with 0.1% o-phosphoric acid as solvent B using C18 column; Synergi 4 m Fusion-RP 80 Å; 150 × 4.0 mm 4 micron (Phenomenex, Canada) at 0, 7, 20, 25, 28, 31 and 40 min with 10, 20, 45, 70, 100, 100 and 10% of solvent B. Chromatograms were acquired at 275 and 330 nm and data were analyzed using the Chromeleon software (Version 6.8). Peaks were identified by comparing their relative retention times with those of the authentic standards. The major identified phenolics; sinapine thiocyanate, sinapoyl glucose, and sinapic acid were used in the preparation of the calibration curves by plotting the concentration of each compound against the area. Total phenolic content was estimated as sinapic acid equivalents (SAE) from the total area of all peaks including the unidentified ones based on sinapic acid calibration curve.
2.3. Statistical Analysis
The data presented are means of three replicates ± standard deviation. Data were analyzed using one factor analysis of variance (ANOVA) and Tukey mean separation for multiple comparisons with the statistical analysis system (SAS) Program (SAS Institute, Carey, NC). Significance was accepted at p ≤ 0.05.
3. Results and Discussion
3.1. Oil Yield from Different Extraction Techniques
Many factors are known to affect the oil yield obtained from the SFE including pressure, temperature, moisture content of the raw material, particle size, flow rate, processing time, and use of co-solvent [37]. These parameters may work individually or cooperatively on the extraction efficiency. Furthermore, some of these factors may increase the oil yield but may not be optimum for the extraction of phenolics and other bioactive components. By controlling these factors, the extraction yield of canola oil ranged from 59.06% [29] to be very close to 100% [22] of that obtained by the solvent extraction. The oil content of canola seeds using different extraction methods is shown in Table 1. Extraction by Soxtec using a combination of chloroform and methanol revealed significantly higher oil yield. It is due to the use of a polar solvent which is able to extract the polar materials (such as phospholipids) is addition to the neutral triacylglycerols.
Despite using a polar co-solvent (ethanol), the SFE showed the lowest oil yield (81.46% of the n-hexane yield) leaving behind the highest oil content in the defatted substrate. The oil contents of the defatted residues were 6.95 ± 0.17, 2.25 ± 0.12, 5.44 ± 0.42 and 14.90 ± 0.23 g/100g after Soxtec (n-hexane), Soxtec (Chl:MeOH), Folch and supercritical fluid extraction, respectively. During this work, we were more interested in the oil composition and quality than in the oil yield which could be significantly improved through applying certain combinations of the operating conditions mentioned above. Moreover, SFE can offer further advantages over the solvent extraction as the extracted oil may carry higher premiums with avoiding the refining losses and environmental and health concerns. In that view, it is sensible to investigate the effect of this environment-friendly technique on the quality of the produced canola oil.
3.2. Physicochemical Properties of Oils
3.2.1. Physical Characteristics
The investigated physical characteristics of the obtained canola oils from different extraction methods as well as the commercial ones included specific gravity, melting point and color (Table 2). Specific gravity of the obtained oils ranged from 0.935 to 0.998 with no significant differences among different extraction techniques although the SFE oil showed slightly higher value. Generally, specific gravity of oils decreases with molecular weight, yet increases with unsaturation levels. Timms [38] reported that between 20˚C and 60˚C, density of vegetable oils changes by 0.30 mg/cm3 for each unit increase in saponification value, 0.14 mg/cm3 for each unit increase in iodine value, −0.68 mg/cm3 for each unit increase in temperature (˚C), −0.20 mg/cm3 for each 0.10% increase
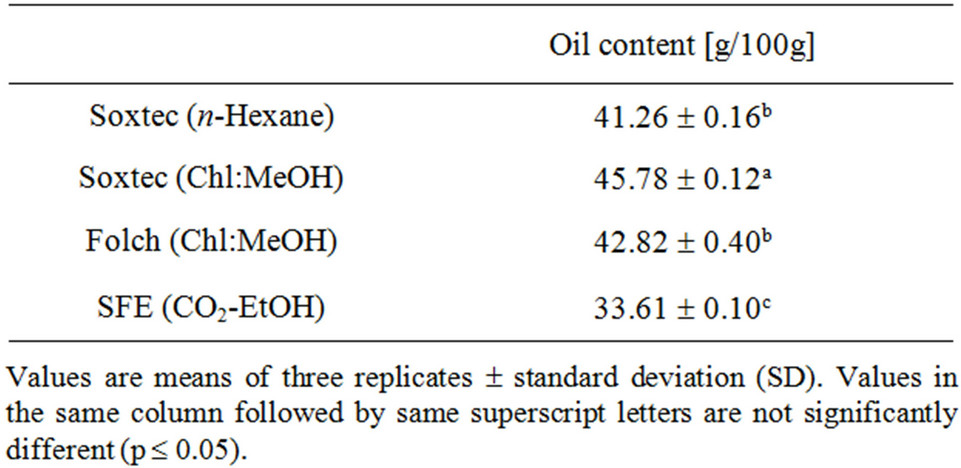
Table 1. Oil content of canola seeds using different extraction techniques.
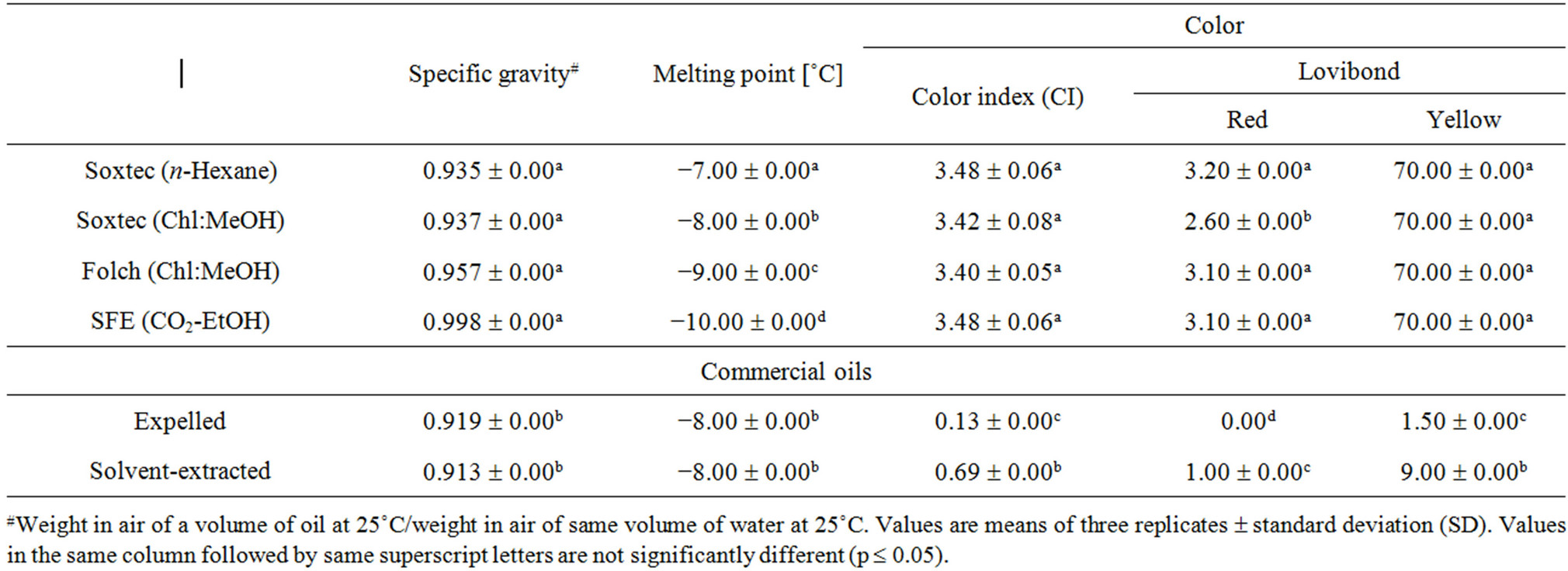
Table 2. Physical characteristics of canola oils from different extraction techniques.
in the free fatty acids, and 0.80 mg/cm3 for each 1.0% increase in moisture. Ackman and Eaton [39] indicated that a different proportion of C18 polyunsaturated fatty acids could be a major factor for the increase in the specific gravity of canola oil, and it is temperature-dependent and decreases when the temperature increases. The commercial oils showed significantly lower specific gravity values. This could be attributed to the effect of refining processes to which these oils were subjected compared to the laboratory-extracted crude oils. Specific gravity of refined canola oil ranges from 0.914 to 0.917 [40]. Melting behavior of vegetable oils influences their functionality in the food matrices. Unlike pure compounds, vegetable oils do not have sharp melting points because they are made up of complex mixtures of triglycerides that pass through a gradual softening before becoming fully liquid.
The melting points of the major canola oil fatty acids were reported to be −11.58, −7.15 and 12.82˚C for linolenic (C18:3n-3), linoleic (C18:2n-6) and oleic (C18:1n-9), respectively [41]. Fasina, Craig-Schmidt, Colley and Hallman [42] indicated that the amount of monoor polyunsaturated fatty acids was highly correlated (R2 = 0.91) with the melting temperature of 12 vegetable oils including canola. They stated that canola oil could start melting at −28.44˚C reaching its melting peak at −13.56˚C and will be completely melted at −4.38˚C. Melting points of the obtained oils ranged from −7.00˚C to −10.00˚C. The SFE oil showed significantly lower melting point followed by the Folch-extracted one. It could be attributed to the delicate effect of these methods (room temperature at Folch; low temperature and oxygen absence in the SFE) which could keep the long chain fatty acids with the lower melting points. Results from the fatty acid composition (Table 4) revealed that the SFE oil had slightly higher n-3 and total polyunsaturated fatty acids than the others. These melting point values are attuned with the literature and come in the normal range of canola oil. It was also reported that canola oil will not crystallize at temperature above −10˚C [43].
Crude canola oil contains various compounds which must be removed to ensure a product with good stability and shelf-life. These impurities include phospholipids, mucilaginous gums, free fatty acids and color pigments. The refined, bleached and deodorized (RBD) canola oil should be essentially bland in taste and light yellow in color. The color scores of the crude oils obtained by different extraction methods (determined by either spectrophotometic or Lovibond methods) showed no significant differences (Table 2) except for the Chl/MeOH extracted oil (using Soxtec) which showed lower value on the red scale. The spectrophotometic color index (CI) values ranged from 3.40 to 3.48. The Lovibond scores were 2.60 - 3.20 red and 70.00 yellow. The commercial oils showed significantly lower color scores compared to the laboratory-extracted oils. It is mainly because their color pigments have been removed during bleaching. The CI of the RBD canola oil was reported to be 0.10 and reached 1.09 after being used for frying (180˚C) up to 16 hr [44]. According to the Canadian general standards board requirements, RBD canola oil should have a Lovibond color scores (133.4 mm cell) of 1.5 red and 15.0 yellow [45].
3.2.2. Chemical Properties
Chemical properties of canola oils are shown in Table 3. The content of free fatty acids (FFAs%) differed significantly according to the extraction procedure. The Soxtec-extracted oil (either by using n-hexane or chloroform/methanol) showed the lowest FFAs content. The highest value was noticed in the Folch-extracted oil (3.65%) followed by that of the SFE (1.49%). This could be attributed to the use of polar solvent (methanol in Folch or ethanol in SFE) which could extract these FFAs. Molecules of fatty acids are composed of a non-polar carbon chain with a polar carboxyl group (COOH). Free fatty acids are mainly those of short hydrocarbon chains which make them more polar than triglycerides and thus readily extracted with the polar solvents. Although polar
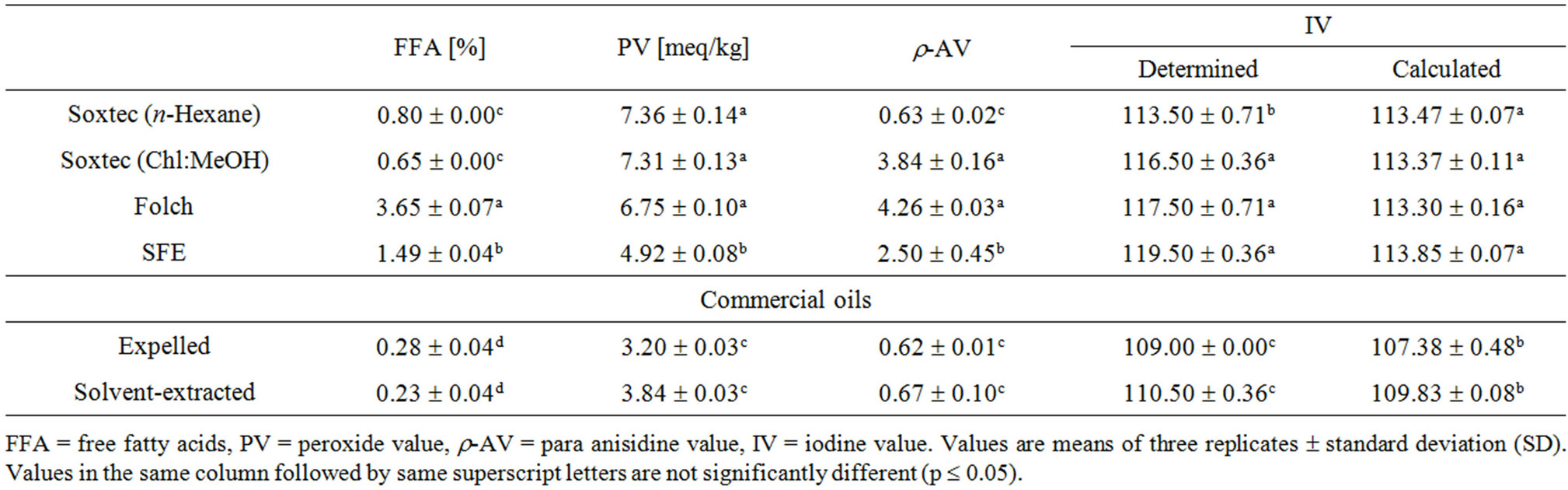
Table 3. Chemical properties of canola oils from different extraction techniques.
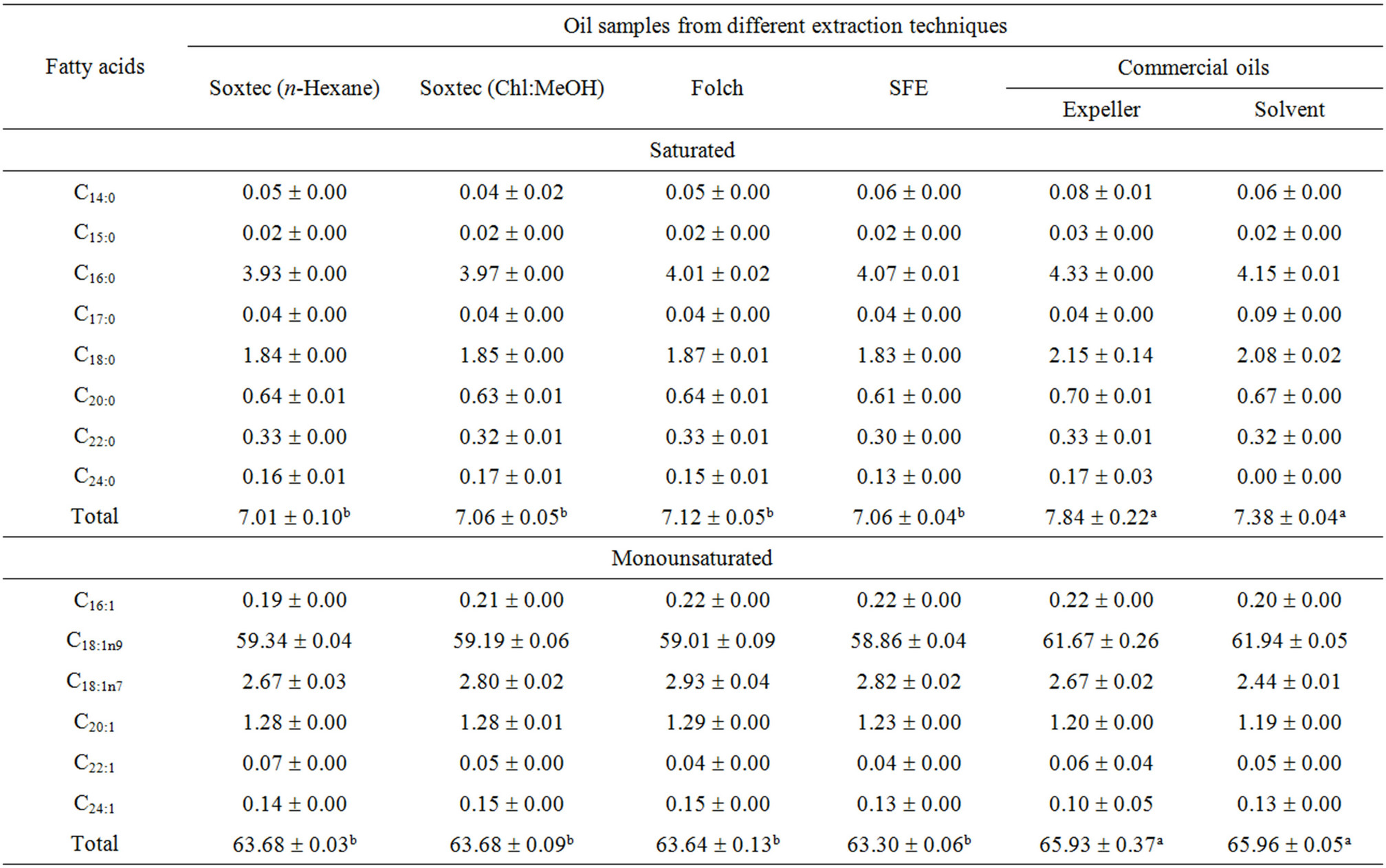
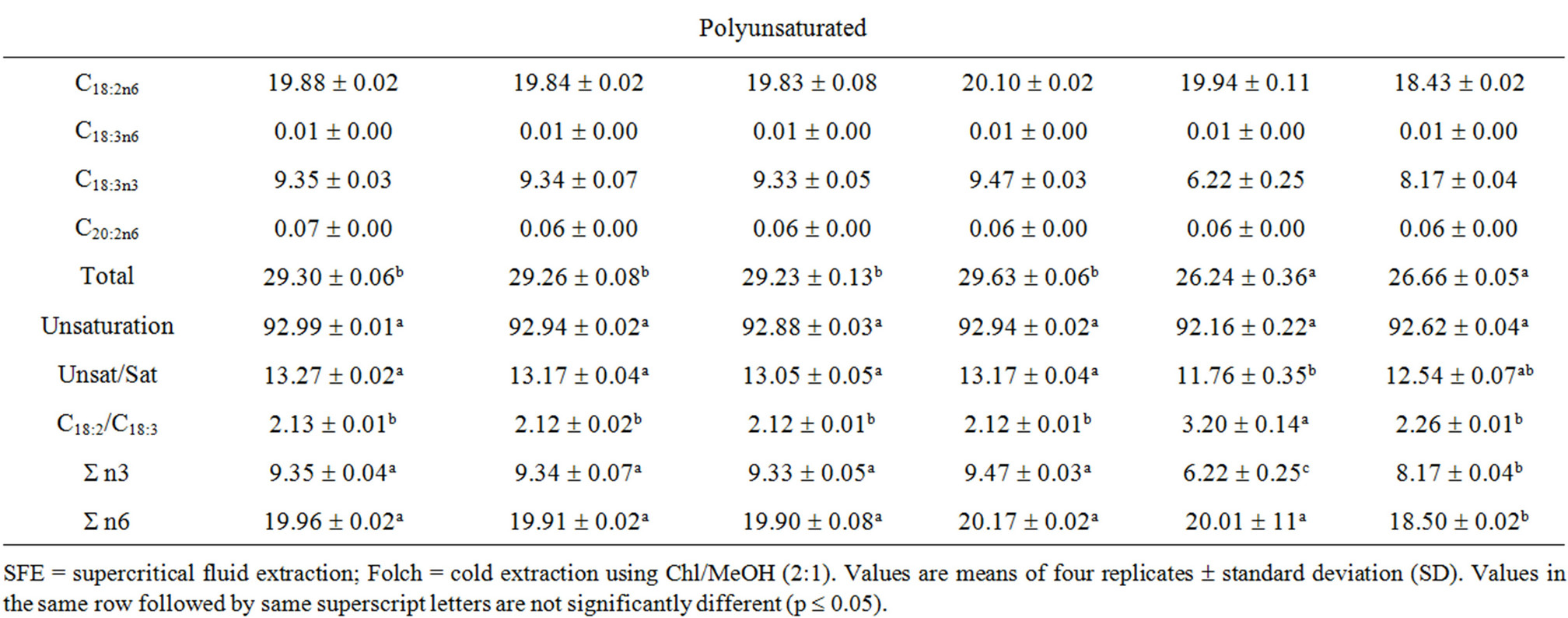
Table 4. Fatty acid composition [%, w/w] of canola oils extracted by different techniques.
solvents were used in Soxtec (Chl/MeOH), Folch and SFE, the FFAs’ contents are significantly different. This could be due to the effect of temperature during the extraction as well as the extraction selectivity of those polar components according to the solvent polarity. The formed FFAs have undergone a breakdown process at higher rates in Soxtec extraction (70˚C - 80˚C) compared to 50˚C in the SFE and 25˚C (room temperature) in Folch extraction. These results agree with other published work. Kim and Yoon [46] reported that the acid value of Folchextracted crude soybean oil was almost 3 times higher than the n-hexane-extracted one. Despite its higher FFAs, they found that Folch-extracted oil revealed much better oxidative stability as the polar solvent was able to extract the phospholipids that acted as a synergist for the primary antioxidant present in crude oil (tocopherols).
It has been also reported the SFE oils showed significantly higher FFAs content than their solvent-extracted counterparts; however the high amounts of FFAs were not considered an indication of increased hydrolysis during extraction procedure, but are due to poor recovery [21,47,48].
The significantly lower peroxide value (4.92 meq/kg) of the SFE oil could be attributed to the minimization of the oxidative reactions in this closed media (while working under CO2) in the absence of oxygen as well as to the lower temperature as compared to the Soxtec extraction. The other factor could be the higher content of sinapic acid and its derivatives in the SFE oil. These phenolic compounds have been reported to pose a stronger antioxidative potential than many synthetic antioxidants [11]. The r-anisidine value (r-AV) of the SFE oil was significantly higher than that extracted by n-hexane, but lower than that of the Soxtec (Chl:MeOH) and Folchextracted oils. This higher r-AV (despite the lower PV compared to the n-hexane-extracted oil) is a reflection of the higher FFAs content. The free fatty acids are more prone to the oxidative deterioration and breakdown to further components including aldehydes and ketones [49,50] which is detected through the r-AV test. Iodine value was experimentally determined using cyclohexaneacetic acid (Method Cd 1d-92) as well as calculated from the fatty acid composition (Method Cd 1c-85). The calculated IV ranged from 113.30 to 113.85 with insignificant differences between oils from different extraction techniques. As IV is a measure of unsaturation (number of double bonds), the higher the content of PUFAs the higher the expected IV. Results of the fatty acid composition (Table 4) support this fact. The SFE oil, which revealed slightly higher content of the C18:3 alinolenic acid, showed slightly higher IV (Table 3).
The calculated IV of the oil based on the fatty acid composition provides a value for only the fatty acids found as a component of this oil. However, other nontriglyceride materials such as partial esters, phosphorlipids, sterols, chlorophylls, fat-soluble vitamins and pigments may be extracted along with the triglycerides and affect the IV based on the number of unsaturated bonds in these molecules. To obtain the actual IV of oils, the chlorinated Wijs method was used [13]. The actual IV obtained for different oils ranged from 113.50 to 119.50 with SFE oil showing the highest value. Incorporating a polar solvent in the extraction increased the IV of the obtained oil compared to the n-hexane extracted one. The higher IV of the SFE oil indicates the ability of this extraction technique to extract more non-triglyceride lipids as well as non-lipid fractions such as vitamins and polyphenols with potent antioxidant properties. The SFE oil, therefore, is expected to pose better stability and nutraceutical performance. Results of PV and IV also conform to those reported by Eggers and Sievers [51] and Rui, Zhang, Li and Pan [48]. The commercially-extracted oils showed significantly lower FFAs, PV, r-AV and IV which come in the normal range of canola oil [45]. This is mainly due to the effect of refining processes.
3.3. Fatty Acid Composition
The effect of extraction method on the fatty acid composition of canola oil was not really significant. The contents of the major fatty acids in the obtained crude oils were 58.86% - 59.34%, 19.83% - 20.10% and 9.33% - 9.47% for oleic, linoleic and linolenic, respectively.
The SFE oil showed slightly higher n3, n6 and total PUFAs than both Soxtecand Folch-extracted oils. This slight increment in the PUFAs is a result of the benign treatment implied in the cold extraction at lower temperature and minimal oxygen availability. The C18:2/C18:3 ratio was almost the same (2.12 - 2.13) apart from the extraction method used. This ratio is recommended from the health point of view [3]. A diet rich in n6 and lacking in n3 fatty acids tends to increase thrombi, blood aggregation and cardiovascular disease as well as allergies, inflammation and diabetes [2].
Canola oil is characterized by its low level of saturated and high level of monounsaturated fatty acids, with an ideal balance between the n6 and n3 fatty acids. Fatty acid profile of canola oil investigated in this study is shown in Figure 1 and Table 4. The major fatty acids were oleic (C18:1n9), linoleic (C18:2n6), linolenic (C18:3n3) and palmitic (C16:0). Other identified fatty acids were mostly myristic (C14:0), palmitoleic (C16:1), eicosenoic (C20:1), behenic (C22:0) and nervonic (C24:1) acids. The obtained fatty acid profile conforms to the normal fatty acid pattern of canola oil [4]. The results of the present study agree with Jenab et al. [29] who found no significant differences in the fatty acid profiles of canola oils extracted by either SFE or n-hexane; however, the linoleic acid concentration of the oil extracted with SFE was somewhat higher. Moreover, the SFE flaxseed oil showed significantly higher n3 and total PUFAs than the Soxhlet-extracted oil using petroleum ether [52].
It was also reported that the fatty acid compositions of white pitaya oil obtained by traditional Soxhlet extraction, microwave-assistant extraction (MAE), SFE and aqueous enzymatic extraction (AEE) were different and depended on the extraction conditions. The highest content of linoleic acid was obtained by SFE [48].
3.4. Phenolic Profile of Canola Oils
The major identified phenolic compounds (Figure 2) were sinapine, sinapoyl glucose, sinapic acid and canolol (2,6-dimethoxy-4-vinyl-phenol). The latter was identified based on its absorbance properties according to the literature. Wijesundera, Ceccato, Fagan and Shen [53] found that canolol had a UV spectrum with maxima at 220 and 270 nm which agrees with our finding (Figure 3). Similarly, Koski, Pekkarinen, Hopia, Wahala, and Heinonen [8] found that canolol has an absorption maximum occurring at 275 nm. The contents of the major phenollics in different oils are shown in Figure 4. The SFE
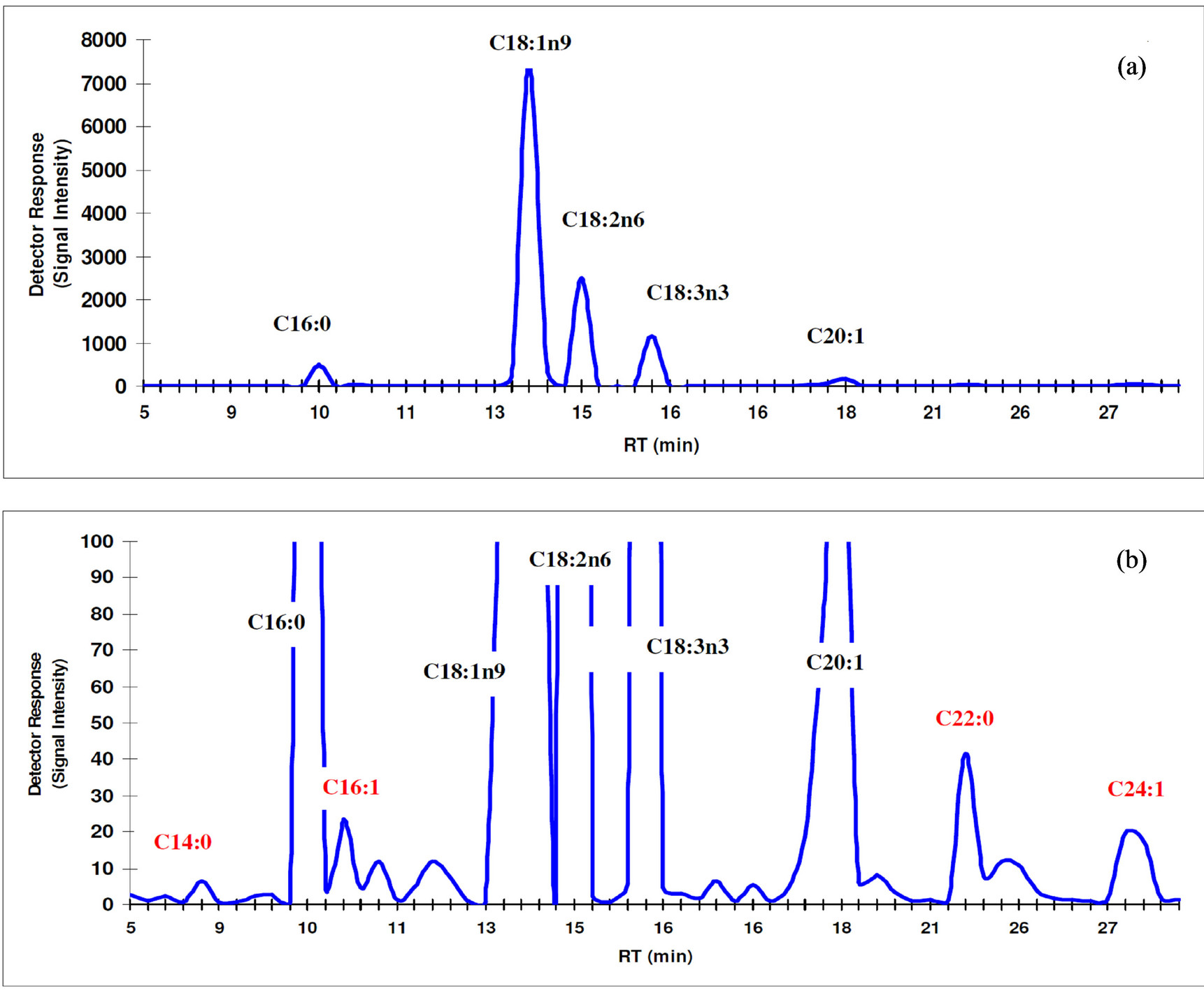
Figure 1. Fatty acid profile of the investigated canola oils. (a) An original view showing only the major fatty acids present; (b) A magnified detailed view showing most fatty acid profile.
oil showed significantly the highest total phenolic content (47.48 mg/g) followed by that extracted by Folch and Soxtec (chloroform/methanol) (34.49 and 25.31 mg/g, respectively).
The n-hexane-extracted oil, on the other hand, showed the lowest value (0.71 mg/g). This could be attributed to the presence of a polar solvent (ethanol in SF and methanol in both Folch and Soxtec extractions) which could extract the polar phenolics with the oil. Furthermore, the SFE oil showed much higher sinapic acid (35.91 mg/g), sinapine (10.15 mg/g) and significantly higher sinapoyl glucose (3.16 mg/g) contents as compared to n-hexane extraction. The significantly higher phenolic content of the SFE oil could be attributed to the presence of polar co-solvent (ethanol) as well as to mild conditions under which the extraction has been done (low temperature and limited oxygen availability). Canolol was found in the oils obtained by n-hexane (Soxtec), chloroform/methanol (Soxtec) and SFE (0.45, 0.76 and 0.32 mg/g, respectively). There was no significant difference in the content of this compound between n-hexane-extracted and SFE oils.
The commercially extracted oils either from the expeller pressing or the solvent extraction did not show any phenolic content. This is due to the retaining of those polar components in the defatted residues as well as the effect of refining processes which would impart a complete degradation of any traces of those compounds. These results agree with other researchers who reported that when pressing the oil, most of the phenolics remain in the meal and the only little amount going to the crude oil will further degrade when the oil is further processed and may not be detectable in the fully refined oil [7,8].
4. Conclusion
The SFE oil showed significantly lower melting point and peroxide value but higher FFAs and iodine value. It contained significantly higher phenolic content and is expected to be more stable than the traditionally extracted one. SFE oil with its high phenolic content provides an excellent choice from the nutrition and health point of view as well as from the technological and economical considerations as it will reveal better stability and extended shelf life. SFE could be competitive with the conventional techniques as it provides environmental
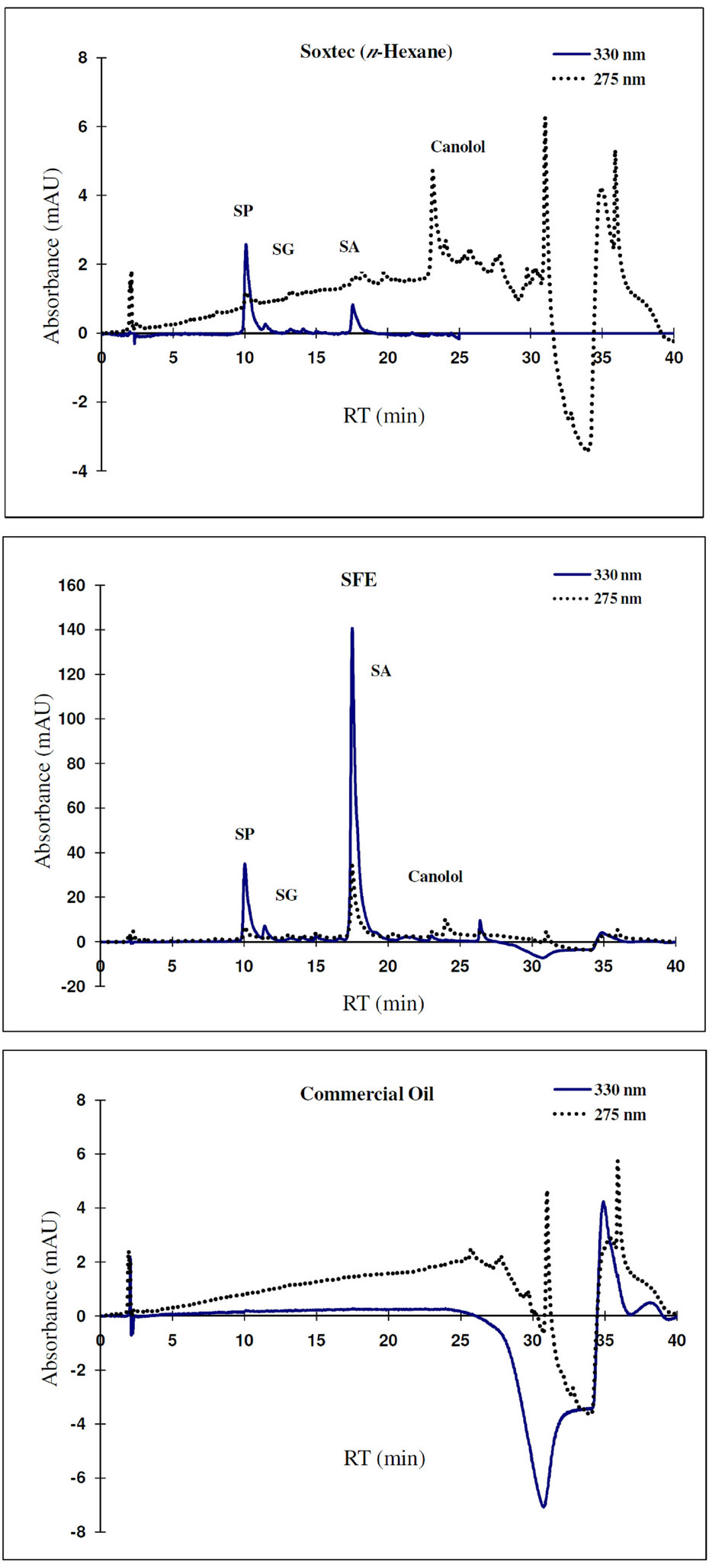
Figure 2. HPLC-DAD Chromatograms of the phenolic profile of the SFE canola oil as compared to both commercially and laboratory-n-hexane extracted ones.
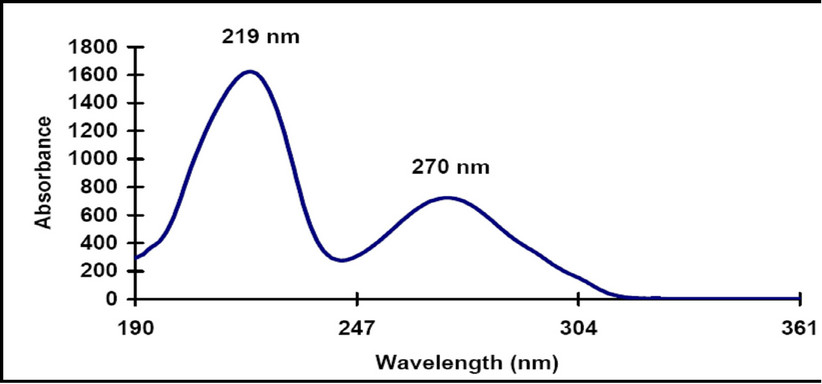
Figure 3. UV spectrum of the identified canolol.
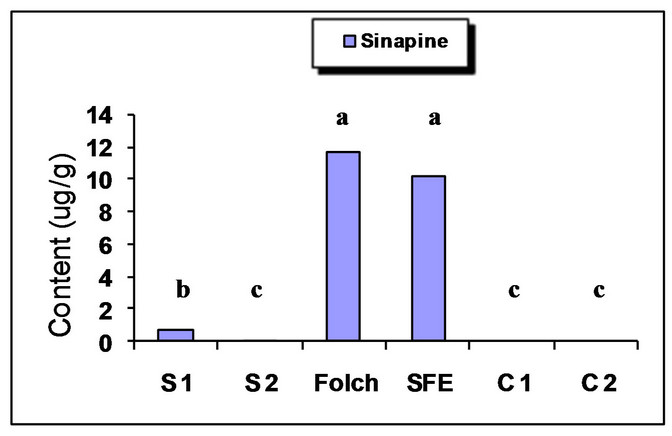
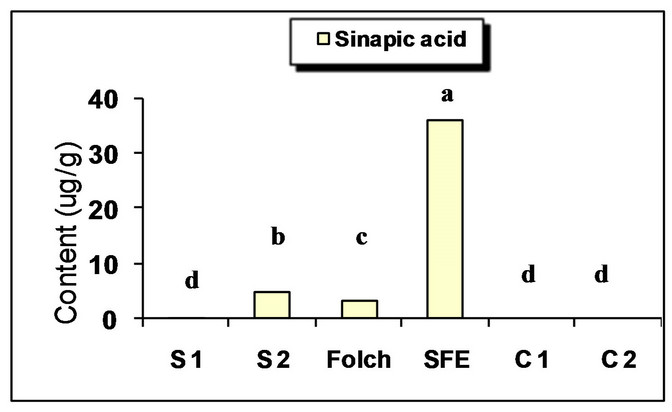
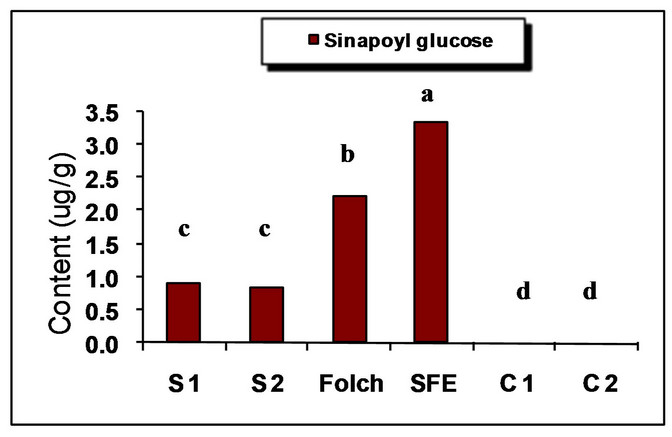
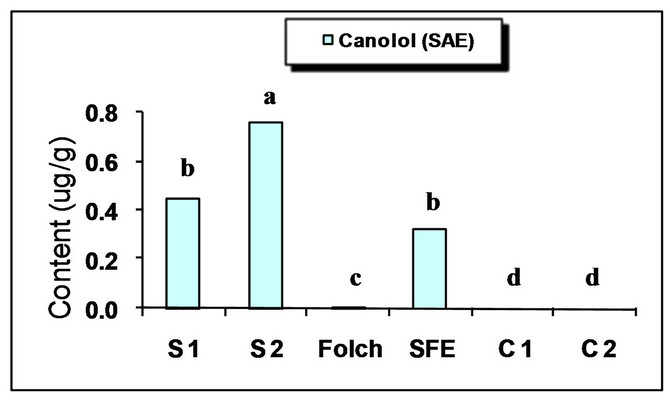
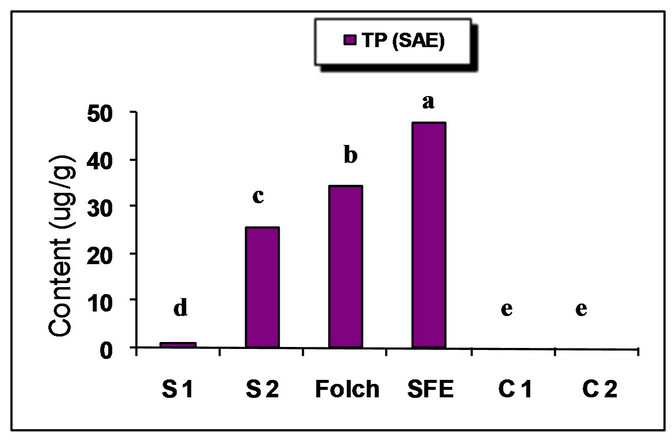
Figure 4. Phenolic content of canola oil from different extraction techniques. S1 = Soxtec (n-hexane); S2 = Soxtec (Chl/MeOH); C1 = commercial oil (expelled); C2 = commercial oil (solvent-extracted); TP = total phenolics as sinapic acid equivalents. Columns topped with same letters are not significantly different (p ≤ 0.05).
approach and enhances the oil quality. Oxidative stability and shelf life studies of the SFE as compared to the conventionally-obtained oil are currently under investigation. Further studies to assess the nutritional and nutraceutical properties of the obtained oil are needed.
5. Acknowledgements
This work has been conducted at the University of Manitoba funded by NSERC, Canola Council of Canada, and Syngenta Crop Protection Inc, Canada. The authors like to acknowledge Jun Wu, Haiyan Li and Dennis Labossiere for their technical help during supercritical fluid extraction and fatty acid analysis.
REFERENCES
- Statistics Canada, “Cereals and Oilseeds Review,” Minister of Industry, Vol. 35, No. 6, 2012, p. 31.
- R. Rice, “Mediterranean Diet,” Lancet, Vol. 344, No. 8926, 1994, pp. 893-894. HUdoi:10.1016/S0140-6736(94)92869-XU
- World Health Organization, “Life in 21st Century. A Vision for All, World Health Report,” WHO, Geneve, 1994.
- R. G. Ackman, “Canola Fatty Acids—An Ideal Mixture for Health, Nutrition and Food Use,” In: F. Shahidi, Ed., Canola and Rapeseed: Production, Chemistry, Nutrition and Processing Technology, Van Nostrand Reinhold, New York, 1994, pp. 81-89.
- M. Naczk, R. Amarowicz, A. Sullivan and F. Shahidi, “Current Research Developments on Polyphenolics of Rapeseed/Canola: A Review,” Food Chemistry, Vol. 62, No. 4, 1998, pp. 489-502. HUdoi:10.1016/S0308-8146(97)00198-2U
- H. Kozlowska, M. Naczk, F. Shahidi and R. Zadernowski, “Phenolic Acids and Tannin in Rapeseed and Canola,” In: F. Shahidi, Ed., Canola and Rapeseed: Production, Chemistry, Nutrition and Processing Technolog, Van Nostrand Reinhold, New York, 1994, pp. 193-210.
- A. Koski, E. Psomiadou, M. Tsimidou, A. Hopia, P. Kefalas, K. Wahala and M. Heinonen, “Oxidative Stability and Minor Constituents of Virgin Olive Oil and ColdPressed Rapeseed Oil,” European Food Research and Technology, Vol. 214, No. 4, 2002, pp. 294-298. HUdoi:10.1007/s00217-001-0479-5U
- A. Koski, S. Pekkarinen, A. Hopia, K. Wahala and M. Heinonen, “Processing of Rapeseed Oil: Effects on Sinapic Acid Derivative Content and Oxidative Stability,” European Food Research and Technology, Vol. 217, No. 2, 2003, pp. 110-114. HUdoi:10.1007/s00217-003-0721-4U
- H. Kuwahara, A. Kanazawa, D. Wakamatu, S. Morimura, K. Kida, T. Akaike and H. Maeda, “Antioxidant and Antimutagenic Activities of 4-Vinyl-2, 6-Dimethoxyphenol (Canolol) Isolated from Canola Oil,” Journal of Agricultural and Food Chemistry, Vol. 52, No. 14, 2004, pp. 4380-4387. HUdoi:10.1021/jf040045+U
- H. Nowak, R. Kujava, R. Zadernowski, B. Roczniak and H. Kozlowska, “Antioxidative and Bactericidal Properties of Phenolic Compounds in Rapeseeds,” Fat Science and Technology, Vol. 94, No. 4, 1992, pp. 149-152.
- U. N. Wanasundara and F. Shahidi, “Canola Extract as an Alternative Natural Antioxidant for Canola Oil,” Journal of the American Oil Chemists’ Society, Vol. 71, No. 8, 1994, pp. 817-822.
- DGF, “Deutsche Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden und Verwandten Stoffen,” Wissenschaftliche Verlagsgesellschaft, Stuttgart, 1998.
- AOCS, “Official Methods and Recommended Practices of the AOCS,” 6th Edition, American Oil Chemists’ Society, Champaign, 2004.
- International Organization for Standardization, “Oilseeds— Determination of Hexane Extract (or Light Petroleum Extract), Called ‘Oil Content’,” International Organization for Standardization, Genève, 1988.
- FOSFA, “Determination of Oil Content in Oilseeds— Solvent Extraction, Reference Method,” FOSFA International Manual, Part 2, Standard Contractual Method 45- 50, 1998, pp. 283-288.
- L. A. Johnson and E. W. Lusas, “Comparison of Alternative Solvents for Oils Extraction,” Journal of the American Oil Chemists’ Society, Vol. 60, No. 2, 1983, pp. 181- 193.
- F. Temelli and N. T. Dunford, “The Effect of Processing Parameters on Extraction of Canola Oil and Phospholipids Using Supercritical Carbon Dioxide,” In: S. S. Koseoglu, K. C. Rhee and R. F. Wilson, Eds., Advances in Oils and Fats, Antioxidants, and Oilseed By-Product, AOCS Press, Champaign, 2004, pp. 25-31.
- D. Anderson, “A Primer on Oils Processing Technology,” In: Y. H. Hui, Ed., Bailey’s Industrial Oil and Fat Products, Wiley-Interscience, New York, 1996, pp. 124-125.
- J. P. Friedrich, G. R. List and A. J. Heakin, “PetroleumFree Extraction of Oil from Soybeans with Supercritical CO2,” Journal of the American Oil Chemists’ Society, Vol. 59, No. 7, 1982, pp. 288-292.
- S. L. Taylor, J. W. King and G. R. List, “Determination of Oil Content in Oilseeds by Analytical Supercritical Fluid Extraction,” Journal of the American Oil Chemists’ Society, Vol. 70, No. 4, 1993, pp. 437-439.
- L. Bruhl and B. Matthaus, “Extraction of Oilseeds by SFE—A Comparison with Other Methods for Determination of the Oil Content,” Fresenius Journal of Analytical Chemistry, Vol. 364, No. 7, 1999, pp. 631-634. HUdoi:10.1007/s002160051399U
- V. J. Barthet and J. K. Daun, “An Evaluation of Supercritical Fluid Extraction as an Analytical Tool to Determine Fat in Canola, Flax, Solin, and Mustard,” Journal of the American Oil Chemists’ Society, Vol. 79, No. 3, 2002, pp. 245-251.
- J. P. Friedrich and E. H. Pryde, “Supercritical CO2 Extraction of Lipid-Bearing Materials and Characterization of the Products,” Journal of the American Oil Chemists’ Society, Vol. 61, No. 2, 1984, pp. 223-228.
- M. Fattori, N. R. Bulley and A. Meisen, “Carbon Dioxide Extraction of Canola Seed: Oil Solubility and Effect of Seed Treatment,” Journal of the American Oil Chemists’ Society, Vol. 65, No. 6, 1988, pp. 968-974.
- M. A. Gomez and M. D. L. Ossa, “Quality of Wheat Germ Oil Extracted by Liquid and Supercritical Carbon Dioxide,” Journal of the American Oil Chemists’ Society, Vol. 77, No. 9, 2000, pp. 969-974.
- N. T. Dunford and F. Temelli, “Extraction of Phospholipids from Canola with Supercritical Carbon Dioxide and Ethanol,” Journal of the American Oil Chemists’ Society, Vol. 72, No. 9, 1995, pp. 1009-1015.
- R. Przybylski, Y. C. Lee and I. H. Kim, “Oxidative Stability of Canola Oils Extracted with Supercritical Carbon Dioxide,” LWT—Food Science and Technology, Vol. 31, No. 7-8, 1998, pp. 687-693.
- Z. Quancheng, S. Guihua, J. Hong and W. Moucheng, “Concentration of Tocopherols by Supercritical Carbon Dioxide with Co-Solvent,” European Food Research and Technology, Vol. 219, No. 4, 2004, pp. 398-402. HUdoi:10.1007/s00217-004-0968-4U
- E. Jenab, K. Rezaei and Z. Emam-Djomeh, “Canola Oil Extracted by Supercritical Carbon Dioxide and a Commercial Organic Solvent,” European Journal of Lipid Science and Technology, Vol. 108, No. 6, 2006, pp. 488-492. HUdoi:10.1002/ejlt.200600026U
- U. N. Wanasundara, R. Amarowicz and F. Shahidi, “Partial Characterization of Natural Antioxidants in Canola Meal,” Food Research International, Vol. 28, No. 6, 1995, pp. 525-530. HUdoi:10.1016/0963-9969(96)87362-5U
- U. Thiyam, H. Stockmann and K. Schwarz, “Antioxidant Activity of Rapeseed Phenolics and Their Interactions with Tocopherols during Lipid Oxidation,” Journal of the American Oil Chemists’ Society, Vol. 83, No. 6, 2006, pp. 523-528.
- J. Folch, M. Lees and G. H. Stanley, “A Simple Method for Isolation and Purification of Total Lipides from Animal Tissues,” Journal of Biological Chemistry, Vol. 226, No. 1, 1957, pp. 497-509.
- H. Li, J. Wu, C. Rembel and U. Thiyam, “Effect of Operating Parameters on Oil and Phenolic Extraction Using Supercritical CO2,” Journal of the American Oil Chemists’ Society, Vol. 87, No. 9, 2010, pp. 1081-1089.
- I. S. Saguy, A. Shani, P. Weinberg and N. Garti, “Utilization of Jojoba Oil for Deep-Fat Frying of Foods,” LWT—Food Science and Technology, Vol. 29, No. 5-6, 1996, pp. 573-577.
- W. R. Morrison and L. M. Smith, “Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride-Methanol,” Journal of Lipid Research, Vol. 5, No. 4, 1964, pp. 600-608.
- R. Khattab, M. Eskin, M. Aliani and U. Thiyam, “Determination of Sinapic Acid Derivatives in Canola Extracts Using High Performance Liquid Chromatography,” Journal of the American Oil Chemists’ Society, Vol. 87, No. 2, 2010, pp. 147-155.
- J. Shi, S. Kassama and Y. Kaduda, “Supercritical Fluid Extraction Technology,” In: J. Shi, Ed., Functional Food Ingredients and Nutraceuticals: Processing Technologies, CRC Press Taylor, Boca Raton, 2007, pp. 3-43.
- R. E. Timms, “Physical Properties of Oils and Mixtures of Oils,” Journal of the American Oil Chemists’ Society, Vol. 62, No. 2, 1985, pp. 241-248.
- R. G. Ackman and C. A. Eaton, “Specific Gravities of Rapeseed and Canbra Oils,” Journal of the American Oil Chemists’ Society, Vol. 54, No. 10, 1977, pp. 435-439.
- Canola Council of Canada, “Canola Oil: Physical and Chemical Properties,” 2009. https://canola-council.merchantsecure.com/uploads/Chemical1-6.pdf
- G. Knothe and R. O. Dunn, “A Comprehensive Evaluation of the Melting Points of Fatty Acids and Esters Determined by Differential Scanning Calorimetry,” Journal of the American Oil Chemists’ Society, Vol. 86, No. 9, 2009, pp. 843-856.
- O. O. Fasina, M. Craig-Schmidt, Z. Colley and H. Hallman, “Predicting Melting Characteristics of Vegetable Oils from Fatty Acid Composition,” LWT—Food Science and Technology, Vol. 41, No. 8, 2008, pp. 1501-1505. HUdoi:10.1016/j.lwt.2007.09.012U
- U. Riiner, “Investigation on the Polymorphism of Fats and Oil by Temperature Programmed X-Ray Diffraction,” LWT—Food Science and Technology, Vol. 3, 1970, pp. 101-106.
- R. Farhoosh, R. E. Kenari and H. Poorazrang, “Frying Stability of Canola Oil Blended with Palm Olein, Olive, and Corn Oils,” Journal of the American Oil Chemists’ Society, Vol. 86, No. 1, 2009, pp. 71-76.
- Canola Council of Canada, “Standards and Regulations,” 2009. http://www.canolacouncil.org/uploads/Standards1-2.pdf
- H. Kim and S. H. Yoon, “Effect of Extraction Solvents on Oxidative Stability of Crude Soybean Oil,” Journal of the American Oil Chemists’ Society, Vol. 67, No. 3, 1990, pp. 165-167.
- L. Brunetti, A. Daghetta, E. Fedeli, I. Kikic and L. Zanderighi, “Deacidification of Olive Oils by Supercritical Carbondioxide,” Journal of the American Oil Chemists’ Society, Vol. 66, No. 2, 1989, pp. 209-217.
- H. Rui, L. Zhang, Z. Li and Y. Pan, “Extraction and Characteristics of Seed Kernel Oil from White Pitaya,” Journal of Food Engineering, Vol. 93, No. 4, 2009, pp. 482- 486. HUdoi:10.1016/j.jfoodeng.2009.02.016U
- J. E. Kinsella, J. L. Shimp and J. Mai, “The Proximate and Lipid Composition of Several Species of Freshwater Fishes,” New York Food and Life Sciences Bulletin, No. 69, 1978, pp. 1-20.
- E. N. Frankel, “Chemistry of Autoxidation: Mechanism, Products and Flavor Significance,” In: D. B. Min and T. H. Smouse, Eds., Flavor Chemistry of Fats and Oils, AOCS, Champaign, 1985, pp. 1-37.
- R. Eggers and U. Sievers, “Processing Oilseed with Supercritical Carbon Dioxide,” Journal of Chemical Engineering of Japan, Vol. 22, No. 6, 1989, pp. 641-649. HUdoi:10.1252/jcej.22.641U
- B. Bozan and F. Temelli, “Supercritical CO2 Extraction of Flaxseed,” Journal of the American Oil Chemists’ Society, Vol. 79, No. 3, 2002, pp. 231-235.

