Advances in Biological Chemistry
Vol. 2 No. 3 (2012) , Article ID: 21819 , 8 pages DOI:10.4236/abc.2012.23039
Peculiarities of CO2 exchange in soybean genotypes contrasting in grain yield
![]()
1Research Institute of Crop Husbandry, Ministry of Agriculture of Azerbaijan Republic, Baku, Azerbaijan
2Institute of Botany, Azerbaijan National Academy of Sciences, Baku, Azerbaijan
Email: aliyev-j@botany-az.org
Received 7 June 2012; revised 13 July 2012; accepted 24 July 2012
Keywords: Photosynthesis; Photorespiration; Productivity; Soybean Genotypes
ABSTRACT
The peculiarities of leaf carbon dioxide gas exchange in soybean genotypes grown in field over a large area and contrasting in duration of vegetation, photosynthetic traits and productivity were studied. Varietal differences in the daily and ontogenetic changes in photosynthesis and photorespiration were identified. It was established that the period of the high activity of photosynthetic apparatus in high productive soybean genotypes lasts for a longer time. The photosynthetic rate and the rate of CO2 release in light due to photorespiration are higher in high productive genotypes. A value of photorespiration in contrasting soybean genotypes constitutes about 28% - 35% of photosynthetic rate. The ratio of gross photosynthesis to photorespiration in genotypes with different productivity is constant enough during ontogenesis, indicating a direct positive correlation between gross photosynthesis and photorespiration. Therefore, contrary to conception arisen during many years on the wastefulness of photorespiration, taking into account the versatile investigations on different aspects of photorespiration, it was proved that photorespiration is one of the evolutionarily developed vital metabolic processes in plants and the attempts to reduce this process with the purpose of increasing the crop productivity are inconsistent.
1. INTRODUCTION
Soybean belongs to the legume family (Fabaceae) originnally from East Asia and one of the oldest cultivated plants. The cultivation of the soybean is referred to the Chinese literature as early as the third millennium BC. It was recognized only in the XIX century, and since then it has been widely spread worldwide. Cultural soybean is widely grown in Asia, Southern Europe, North and South America, Central and Southern Africa, Australia, the islands in the Pacific and Indian oceans at latitudes from the equator to 55˚ - 60˚.
The soybean is often called “the miracle plant”, such interest is determined by a high quality of its grain, which contains 35% - 55% of easily digestible proteins, 17% - 27% of fats, 30% of carbohydrates, vitamins, etc., depending on variety and growing conditions. Among all worldwide cultivated agricultural crops the soybean is one of the most high-protein ones. Due to rich and varied chemical composition it is widely used as a food, forage and industrial crop, having a great agrotechnological importance as well [1-5]. The soybean has also the ability to assimilate air nitrogen [6] and, therefore, requires minimal costs for nitrogen fertilizers, which is often considered the single major energy contribution to agriculture.
World soybean production was about 210.9 million metric tons in 2009 [7]. The consumption of soy-based products increases worldwide due to the described beneficial effects, which include reduction of cholesterol level, prevention of cancer, diabetes and obesity, protection against intestinal and kidney diseases [8].
The soybean is an annual plant with a pivotal root system. All species of the soybean has trifoliate leaves with drooping leaflets and pinnate venation, occasionally leaves with 5-, 7- and 9-leaflets are found.
The process of photosynthesis is the main part of total plant productivity. The soybean, like most agricultural crops, belongs to the so-called C3-plants. A part of carbon dioxide assimilated during respiration in light is released from leaves simultaneously with photosynthesis [9]. This results in much less real value of CO2 assimilation in C3-plants than the realized photosynthesis.
Since 1970’s a concept on wastefulness of photorespiration has been formulated by many researches, and attempts to decrease or suppress it with the purpose to increase the crop productivity are still made [10-16]. The conception on possibility of significant increase in productivity of C3-plants through the selection of samples with low rate of photorespiration was developed. It was suggested to search the ways to eliminate or reduce photorespiration by genetic or chemical means [10-13,17-20]. However, chemicals which inhibit glycolate metabolism did not reduce photorespiration and increase photosynthetic efficiency [18]. In addition, on the basis of the theory about the relationship between photosynthesis and photorespiration based on the competition between CO2 and O2 for ribulose-1,5-bisphosphate carboxylase, which appears at the level of carboxylase-oxygenase function of this enzyme, the existence of a positive relationship between the processes of photosynthesis and photorespiration at a constant intracellular CO2 concentration has been demonstrated [21,22].
The results of long-term comprehensive study of components of leaf carbon dioxide gas exchange in soybean genotypes contrasting in productivity and photosynthetic traits under a natural growth conditions are presented in the paper.
2. MATERIALS AND METHODS
2.1. Plant Material
Experiments were performed on irrigated area at the Absheron Experimental Station of the Research Institute of Crop Husbandry. Research targets include different soybean (Glycine max (L.) Merr.) genotypes contrasting in height, architectonics, duration of vegetation, productivity and other morpho-physiological traits, Rannaya-10, Bystritsa, Volna, VNIIMK-3895, Komsomolka, Provar, VNIIMK-9, Plamya, Biyson and Visokoroslaya-3 were used (Table 1). The genotypes were short-stemmed (40 - 55 cm), medium-stemmed (60 - 70 cm), and high-stemmed (80 - 115 cm) with low productivity (2 - 2.3 t·ha–1), medium productivity (2.5 - 3.0 t·ha–1) and high productive (3.3 - 4.0 t·ha–1). The genotypes Provar and Biyson are introduced from the USA, the other genotypes were developed at the All-Union Research Institute of Oil and Essential Oil Crops (VNIIMK).
2.2. Growth Conditions
All genotypes were grown under identical field conditions over a large area in compliance with all requirements of cultivation agrotechnology and experimental work [23-27]. The record plot area was 54 m2, field experiments were repeated 4-times, and the optimal inter-row space was 60 cm. High agricultural background (optimal conditions for mineral nutrition) was used to determine the potential photosynthetic capacity of the studied soybean varieties [26,28].
Sowing was carried out at the end of April, under soil temperature no lower than 12˚C - 13˚C. Soil moisture was maintained at 70% - 75% of TAW (total available water capacity). During the growing season phenological observation of plant growth and development was carried out.
2.3. Experiment Arrangement
The rate of carbon dioxide gas exchange was measured using an infrared gas analyzer URAS-2T (“Hartman and Braun”, Germany) in an open air system [29,30]. The special brass made thermostatic leaf chamber with optical glass windows of 10 cm2 area was made. The limits of measurements were 0.005% - 0.05% CO2, error was ±0.5% of the upper limit of the scale [22,31]. CO2 concentration in the analyzed air was recorded using automatic recorder. The measurements were performed in an open air flow system connected in the differential mode [32]. The initial air flow was divided into two parts. One part passed through the air dehumidifier, filled with calcium chloride, through the filter, and then through the control cuvette of the gas analyzer.

Table 1. Characterization of soybean genotypes.
The other part passed through the leaf chamber, dehumidifier, filter, and then through the measuring cuvette. The air flow velocity through the entire system was adjusted using needle valves and rotometer. The gas analyzer recorded the difference in CO2 concentration at the inlet and outlet of the leaf chamber. The rate of gas exchange in leaves placed in the leaf chamber was determined by the difference in CO2 concentration and air velocity passing through the leaf chamber. For the measurements a hermetically sealed clip chamber with the area of 0.1 dm2, which has two inlets and outlets for air flow, separately surrounding the upper and lower leaf surface, was used.
During the measurements chamber was attached to leaves close to the stem maintaining their natural location and orientation, and exposed to sunlight until the gas exchange reached the steady-state level. The CO2 concentration in air was determined close to the leaf chamber before each gas exchange measurement. Night respiration was determined using the above mentioned equipment without use of thermostat, in a steady night temperature. In the heat of the day a light filter SZS-24 [22,31] was used to prevent overheating of leaves in the chamber. Photorespiration was determined using two methods, in atmosphere without СО2 and in atmosphere with reduced oxygen content (2%) [33,34]. In the first case, after photosynthesis had reached the steady-state level the СО2- lacking air was passed through the leaf chamber. The increase in СО2 concentration at the chamber outlet is an indicator for the estimation of photorespiration. In the second case, after photosynthesis had reached the steadystate level the air with a reduced content of oxygen was blown into the chamber, and the obtained values of photosynthesis were measured.
The rate of photorespiration was determined by difference between the values of CO2 release rate in light without CO2 and dark respiration.
The gas analyzer which was placed in a mobile laboratory allowed multiple measurements in the sowings of different genotypes to be performed in a short time while keeping the high sensitivity of the facility in the field and maintaining the natural course of physiological processes in entire plants (Figure 1).
Leaf assimilating area was measured using an automatic area meter “AAC-400” (“Hayashi” Denkoh Co. LTD, Japan). The specific leaf density (SLD) was calculated as the ratio of its dry weight to the area. Photosynthetically active radiation (PAR) was calculated according to Tooming and Gulyaev [35]. The obtained data were statistically processed by standard analysis methods [36].
3. RESULTS AND DISCUSSION
The analysis of morpho-physiological traits of the soybean

Figure 1. Measurement of the rates of photosynthesis and photores-piration in soybean genotypes in the field.
harvest showed that main factors of the yield are conditions for the functioning of all photosynthetic systems at the crop level determined by cultivation conditions, particularly mineral nutrition and irrigation. It was shown that high agricultural background provides the increase in yield and significant improvement of grain quality [28]. Intensive genotypes with optimum architectonics possess higher photosynthetic activity and provide high yield (3 - 4 t·ha–1) and high grain quality (40% protein).
The contribution of leaves to the total CO2 assimilation largely depends on their layer location and spatial orientation [26,27]. Physiologically active leaves of middle layers (9 - 11) with higher specific leaf density (0.44 - 0.51 g·dm−2) assimilate CO2 more intense than leaves of the other layers. Leaves of upper layers also have a maximal value of SLD and photosynthetic rate in comparison with that of the lower ones. Obviously, the increase in SLD of the leaves of upper and middle layers under a favorable luminosity keeps the lower layers under the luminosity insufficient for active photosynthesis.
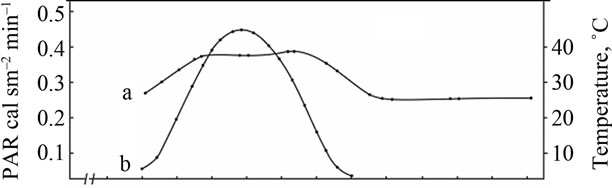
Comparative study of the rate of photosynthesis during the day showed that, regardless of genotypes, diurnal variations in leaf photosynthetic rate are characterized by double-peak curves with sharp increase in photosynthetic rate in the morning (9-11 a.m.) and the evening (4-6 p.m.) and midday depression (Figures 2-4). Leaf photosynthesis in the low productive genotype Bystritsa starts at approximately 7 a.m., increases rapidly at sunrise and reaches its maximum value at 11 a.m. Then the rate of photosynthesis sharply drops at 2-3 p.m., and the lowest value during the day is being observed. After 3 p.m. the second peak is observed. It should be noted that solar radiation at 2 p.m. was the highest and amounted to 0.44 cal·cm–2·min–1.
Change in ambient temperature and PAR during the day shows that their maximum value is achieved at 12 a.m.-4 p.m. (Figure 2(A)). Diurnal depression of photosynthesis occurs at this time. In the midday CO2 assimilation drop is caused by increase in temperature of leaves, resulting in increased respiration, water regime disturbance, weakening of assimilates outflow and changes in other physiological processes.
In the evening the rate of photosynthesis decreases and carbon dioxide compensation point is being observed. At nightfall the photosynthetic gas exchange turns into the respiratory and carbon dioxide is released as a result of respiration. At night the rate of dark respiration reaches its maximum value and then begins to decrease. After 5- 6 p.m. the CO2 release rate in the dark respiration decreases sharply, and after 7 a.m., at the sunrise the respiratory gas exchange turns into the photosynthetic, which increases dramatically within a short time.
High productive genotypes have a higher photosynthetic rate than low productive ones. A similar pattern is observed in the dynamics of the respiratory gas exchange. During the night period, high productive genotypes have relatively higher respiration rate.
The leaves of lower and middle layers of high productive genotypes during the branching stage assimilate rather more CO2 than leaves in similar layers of medium productive genotypes. Leaves of the middle layer assimilate more CO2 during this stage in all studied genotypes (Figure 5).
 (A)
(A)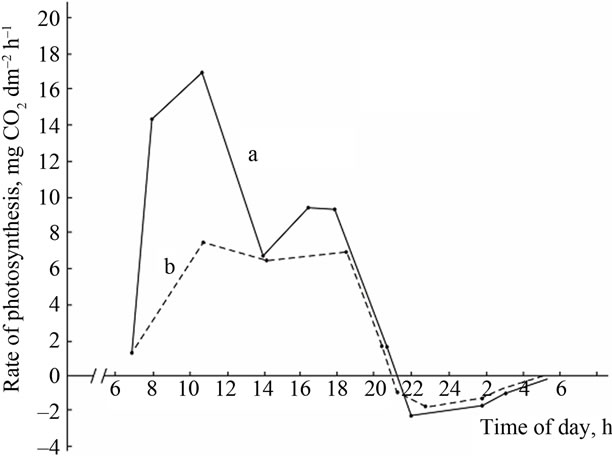 (B)
(B)
(A) a: Ambient temperature; (A) b: PAR; (B) a: The rate of leaf gas exchange in plants grown using mineral fertilizers; (B) b: The rate of leaf gas exchange in plants grown without mineral fertilizers (control).
Figure 2. The diurnal patterns of the leaf gas exchange rate in the low productive genotype Bystritsa at the grain filling stage.
 (A)
(A)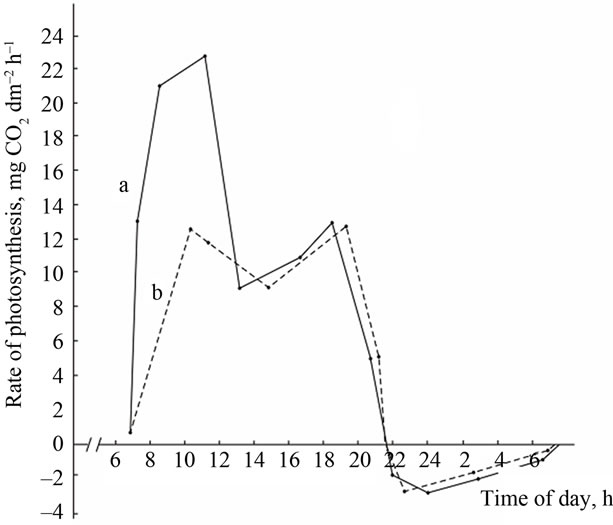 (B)
(B)
(A) a: Ambient temperature; (A) b: PAR; (B) a: The rate of leaf gas exchange in plants grown using mineral fertilizers; (B) b: The rate of leaf gas exchange in plants grown without mineral fertilizers (control).
Figure 3. The diurnal pattern of the leaf gas exchange rate in the high productive genotype Komsomolka at the grain filling stage.
During the flowering stage the rate of CO2 assimilation increases sharply in leaves of all layers in all genotypes, but the maximum value of CO2 assimilation is observed in leaves of the middle layer. In the period of od formation the intensity of lower layered leaves drops sharply. Throughout the growing season the leaves of the middle layer were distinguishing by the highest rate of photosynthesis. By the end of the growing season the activity of leaves of the upper layer remained high as well.
Soybean genotypes contrasting in genetic and phenoltypic peculiarities differ by maximum value of photosynthetic rate and duration of their highly active period as well during ontogenesis (Figure 6). Photosynthetic rate in leaves of different soybean genotypes gradually increases since the branching stage and reaches the maximum at the flowering—pod formation stages, and then decreases at the end of pod formation, and reaches a maximum value in high productive genotypes (on average 24 mg CO2 dm–2·h–1) during the periods from pod formation till grain filling. In the low productive genotypes, the greatest value of photosynthetic rate (21 mg CO2 dm–2·h–1) was observed at the initial stage of grain filling, and it lasted for a short period of time. Consequently, the duration of the periods from pod formation
 (A)
(A)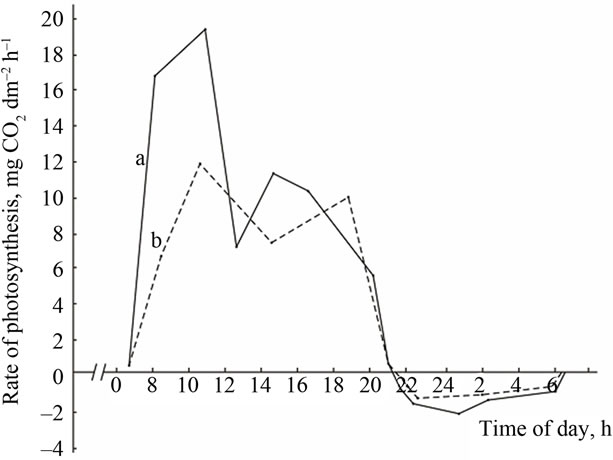 (B)
(B)
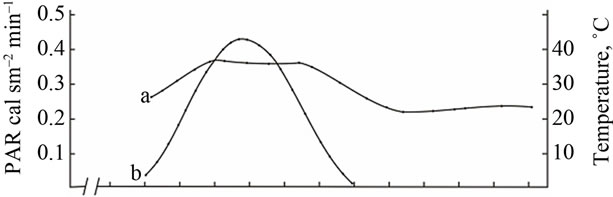
(A) a: Ambient temperature; (A) b: PAR; (B) a: The rate of leaf gas exchange in plants grown using mineral fertilizers; (B) b: The rate of leaf gas exchange in plants grown without mineral fertilizers (control).
Figure 4. The diurnal pattern of the leaf gas exchange rate in the medium productive genotype Provar at the grain filling stage.
till grain filling has a great importance for the grain yield [30,37-39]. Improvement of the growth conditions significantly contributes to increasing of photosynthetic activity of plants in field. And rate of photosynthesis increases by 30% - 50% [30].
At the same time, leaves of the high productive genotypes (VNIIMK-3895 and Komsomolka) at all developmental stages, especially during flowering and pod formation, assimilate CO2 more intensively and maintain high rate of photosynthesis for longer time. Pod formation stage in VNIIMK-3895 variety starts 5 - 8 days earlier and the rate of photosynthesis is maintained at a high level within 10 - 15 days. The longest flowering-pod formation period was observed in this variety (an average of 53 days over four years). Hence, the total longevity of the growing season does not play a major role in the grain yield but the duration of the period of pod formation and grain filling does [30,37-39].
In contrast to mediumand long-stemmed genotypes, the short-stemmed early maturing genotypes (Bystritsa and Volna) are characterized by a short period of high values of the photosynthesis rate. It suggests that early maturity and short stature are not always accompanied by a high value of the photosynthetic rate. The medium productive genotype Plamya with relatively low CO2 assimilation (23.3 mg CO2 dm–2·h–1) is characterized by longer period of photosynthetic activity, and is inferior to medium-stemmed genotypes in its yield (Figure 6).
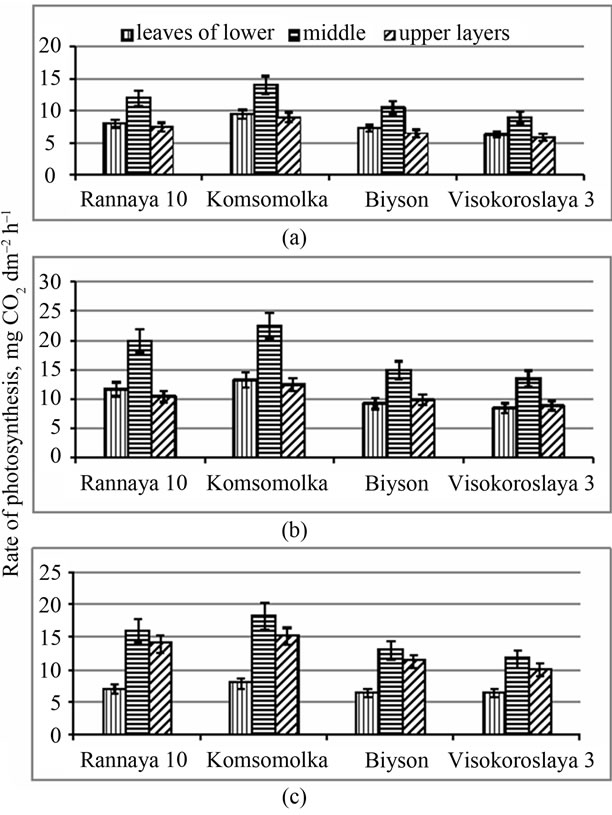
Figure 5. Seasonal dynamics of photosynthesis rate in leaves of different layers of the short-stemmed, high productive (Rannaya), medium-stemmed, high productive (Komsomolka) and longstemmed, medium productive (Biyson, Visokoroslaya) soybean genotypes: (a) Branching; (b) Flowering; (c) Beginning of pod formation.

Figure 6. Ontogenetic changes in the photosynthesis rate of CO2 assimilation in different soybean genotypes: 1: Volna; 2: VNIIMK-9; 3: VNIIMK-3895; 4: Plamya.
Improving the growing conditions significantly contributes to enhance the photosynthetic activity of plants in the cultivated area. Herewith, the rate of photosynthesis increases by 30% - 50%.
Like most major agricultural crops related to C3-plants, soybean has active photorespiration that consumes the part of photosynthetic products.
Change in carbon dioxide gas exchange components, except dark respiration, occurs proportionally in all studied genotypes during ontogenesis (Figure 7). The maximum value of these components is observed in low productive varieties (Bistritsa, Volna) at 60th day of age, in high productive (VNIIMK-3895 and Komsomolka) and medium productive ones (Provar and VNIIMK-9) at 80th day of age, while in the Plamya—at 90th day of age.
The ratio of true photosynthesis and photorespiration in the leaf ontogenesis is considerably constant and constitutes on average 29% for low productive varieties, 35% for high and 28% for medium productive ones [27,30, 37,38].
This suggests that about a third of the carbon assimilated in photosynthesis is being consumed during photorespiration.
The identical pattern of change in rates of true photosynthesis and photorespiration during the growing season suggests the existence of a positive relationship between them.
Quantitative characteristics of carbon dioxide gas exchange components demonstrates that if we consider the true value of photosynthesis as 100%, then the average value of the net photosynthesis in low productive wheat plants will be 65%, photorespiration—29%, dark respiration—6%, in high productive—60%, 35%, 5%, and in medium productive ones—66%, 28% and 6%, respectively. The data showed that the main role in the process of CO2 release in light belongs to photorespiration that is greater in high productive soybean genotypes in comparison with low productive ones.
On the basis of these results, we can conclude that the attempts to find or create high productive genotypes with high photosynthesis and low photorespiration rates have no future and it is appropriate in breeding programs to focus on genotypes that have higher rates of both photosynthesis and photorespiration.
The following parameters are suggested for the purposeful selection of high productive soybean genotypes: compact leaf shape, medium-sized leaves, which are located mainly in the middle layer, high rates of photosynthesis and photorespiration, high specific leaf density and longer period of pod formation-grain filling.
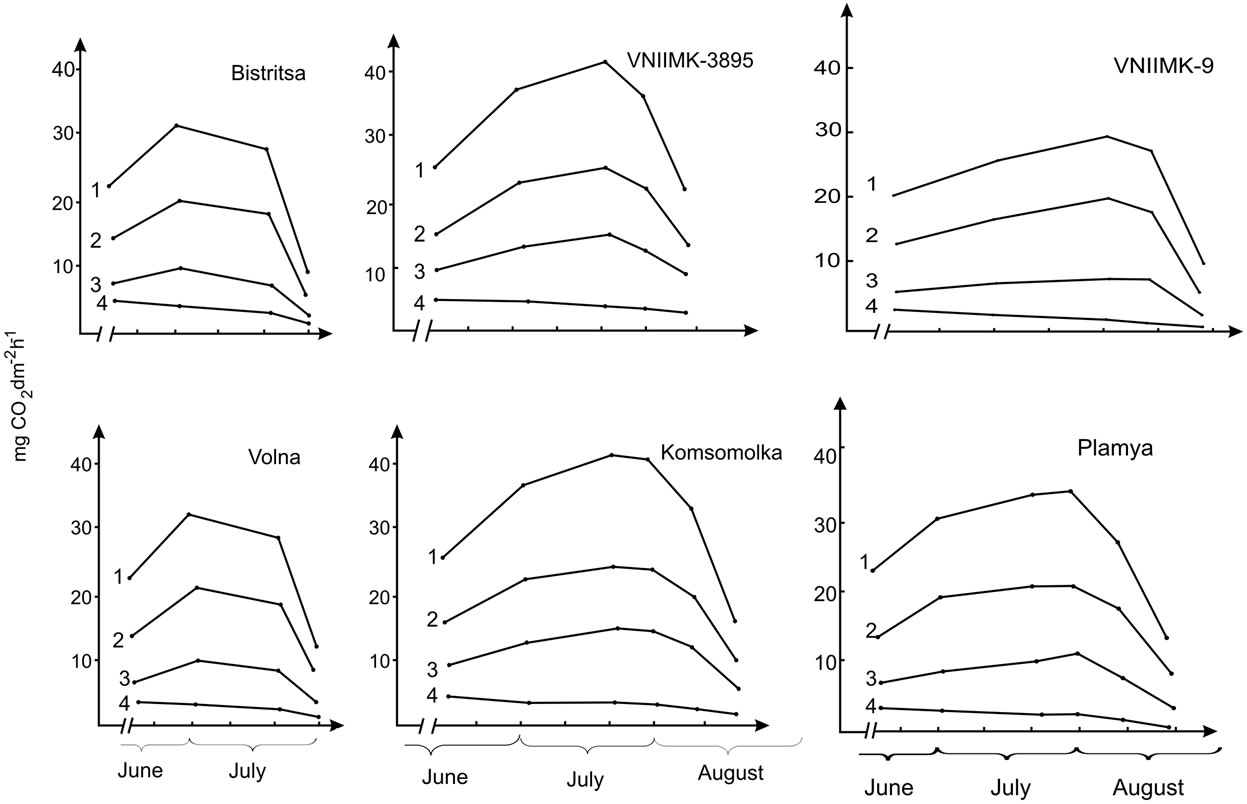
Figure 7. Components of CO2 exchange in the leaves of the low productive (Bystritsa, Volna), high productive (VNIIMK-3895, Komso-molka) and medium productive (VNIIMK-9, Plamya) soybean genotypes: 1: Gross photosynthesis; 2: Net photosynthesis; 3: Photorespiration; 4: Dark respiration.
Thus, the high rates of photosynthesis and photorespiration in conjunction with the favorable photosynthetic traits, an optimum leaf area index and the best architectonics, define the high productivity of the soybean genotypes. Therefore, contrary to conception on the wastefulness of photorespiration, proposed in the many years by different authors, our comprehensive investigations on the different aspects of photorespiration indicate that photorespiration is one of the evolutionarily developed vital metabolic processes in plants. The attempts to reduce this process with the purpose of increasing the crop productivity are inconsistent [29,30,40-44].
![]()
![]()
REFERENCES
- Aliyev, J.A. and Akperov, Z.I. (1995) Photosynthesis and soybean grain yield. Rodnik, Moscow, Baku (in Russian).
- Aliyev, J.A. and Akperov, Z.I. (1998) Fotosinteza şi recolta de soia. Ştininta, Chişina.
- Ososki, A.L. and Kennelly, E.J. (2003) Phytoestrogens: A review of the present state of research. Phytotherapy Research, 17, 84-869. doi:10.1002/ptr.1364
- Pimentel, D. and Patzek, T. (2008) Ethanol production using corn, switchgrass and wood; biodiesel production using soybean. In: Pimentel, D., Ed., Biofuels, Solar and Wind as Renewable Energy Systems, Springer, New York, 373-394. doi:10.1007/978-1-4020-8654-0_15
- Sakai, T. and Kogiso, M. (2008) Soy is of flavones an immunity. Journal of Medical Investigation, 55, 176-173. doi:10.2152/jmi.55.167
- Burris, R.H. and Roberts, G.P. (1993) Biological nitrogen fixation. Annual Review of Nutrition, 13, 317-335. doi:10.1146/annurev.nu.13.070193.001533
- Soy Stats (2010) http://www.soystats.com/2010/Default-frames.htm
- Friedman, M. and Brandon, D.L. (2001) Nutritional and health benefits of soy proteins. Journal of Agricultural and Food Chemistry, 49, 1069-1086. doi:10.1021/jf0009246
- Sharkey, T.D. (1988) Estimating the rate of photorespiration in leaves. Physiologia Plantarum, 73, 147-152. doi:10.1111/j.1399-3054.1988.tb09205.x
- Zelitch, I. (1971) Photosynthesis, photorespiration, and plant productivity. Academical Press, New York, London.
- Zelitch, I. (1975) Improving the efficiency of photosynthesis. Science, 188, 626-633. doi:10.1126/science.188.4188.626
- Ogren, W.L. (1975) Control of photorespiration in soybean and mаize. In: Marchelle, R., Ed., Environmental and Biological Control of Photosynthesis, W. Junk, The Hague, 45-52. doi:10.1007/978-94-010-1957-6_5
- Ogren, W.L. (1976) Search for higher plants with modifications of the reductive pentose phosphate pathway of CO2 assimilation. In: Burris, R.H. and Black, C.C., Eds., CO2 Metabolism and Plant Productivity, University Park Press, Baltimore, 19-29.
- Chollet, R. and Ogren, W.L. (1975) Regulation of photorespiration in C3 and C4 species. Botanical Review, 41, 137-179. doi:10.1007/BF02860828
- Holaday, A.S. and Chollet, R. (1984) Photosynthetic/ photorespiratory characteristics of C3-C4 intermediate species. Photosynthesis Research, 5, 307-323. doi:10.1007/BF00034976
- Peterhansel, C. and Maurino V.G. (2011) Photorespiration redesigned. Plant Physiology, 155, 49-55. doi:10.1104/pp.110.165019
- Zelitch, I. (1992) Control of plant productivity by regulation of photorespiration. BioScience, 42, 510-516. doi:10.2307/1311881
- Servaites, J.C. and Ogren, W.L. (1977) Chemical inhibition of the glycolate pathway in soybean leaf cells. Plant Physiology, 60, 461-466. doi:10.1104/pp.60.4.461
- Kebeish, R., Niessen, M., Thiruveedhi, K., Bari, R., Hirsch, H.-J., Rosenkranz, R., Stäbler, N., Schönfeld, B., Kreuzaler, F. and Peterhansel, C. (2007) Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nature Biotechnology, 25, 593-599. doi:10.1038/nbt1299
- Maurino, V.G. and Peterhansel, C. (2010) Photorespiration: Current status and approaches for metabolic engineering. Current Opinion in Plant Biology, 13, 249-256. doi:10.1016/j.pbi.2010.01.006
- Aliev, J.A., Guliev, N.M., Kerimov, S.Kh. and Hidayatov, R.B. (1988) Enzymes of the primary СО2 fixation in flag leaf ontogenesis of wheat genotypes. Izvestiya Akademii Nauk Azerbaijanskoj SSR, 4, 12-20 (in Russian).
- Aliev, J.A., Guliev, N.M., Kerimov, S.Kh. and Hidayatov, R.B. (1996) Photosynthetic enzymes of wheat genotypes differing in productivity. Photosynthetica, 32, 77-85.
- Anonym (1978) Guidelines on soybean cultivation on irrigated lands in the Northern Caucasus. Krasnodar, 19 (in Russian).
- Aliyev, J.A., Akperov, Z.I. and Nabiyev, M.H. (1981) Soybean cultivation under irrigation conditions of Azerbaijan SSR (Guidelines). Baku (in Russian).
- Aliyev, J.A., Akperov, Z.I. and Nabiyev, M.H. (1982) Soybean cultivation in irrigated lands of Azerbaijan SSR. Azerneshr, Baku (in Russian).
- Aliyev, J.A. and Akperov, Z.I. (1985) Dinamics of sowing structure and photosynthetical traits of soybean genotypes. Izvestiya Akademii Nauk Azerbaijanskoj SSR, 3, 3-10 (in Russian).
- Mirzoyev, R.S. (1990) CO2 gas exchange of soybean genotypes different in photosynthetic traits and productivity. Ph.D. thesis, Baku (in Russian).
- Aliyev, J.A. and Akperov, Z.I. (1986) Conception on ideal soybean. Izvestiya Akademii Nauk Azerbaijanskoj SSR, 2, 3-11 (in Russian).
- Aliev, D.A., Kerimov, S.Kh., Guliev, N.M. and Akhmedov, A.A. (1996) Carbon metabolism in wheat genotypes with contrasting photosynthetic characteristics. Russian Journal of Plant Physiology, 43, 42-48.
- Aliyev, J.A., Akhmedov, A.A. and Mirzoyev, R.S. (1992) Dinamics of CO2 gas exchange in leaves of soybean in field. Izvestiya Akademii Nauk Azerbaijanskoj SSR, 1-6, 76-82 (in Russian).
- Voznesensky, V.L. (1977) Photosynthesis of desert plants. Nauka, Leningrad (in Russian).
- Karpushkin, L.T. (1971) The use of infrared gas analyser to study CO2 gas exchange in plants. In: Molotkovskiy, Yu.G., Ed., Biophysical Methods in Plant Physiology, Nauka, Moscow, 44-71 (in Russian).
- Akhmedov, G.A. (1986) СО2 exchange in wheat ontogenesis depending on phenotypic traits, growth conditions and photosynthetic productivity. Ph.D. Thesis, Baku (in Russian).
- Šesták, Z., Jarvis, P.G. and Catsky, J. (1971) Criteria for the selection of suitable methods. In: Šesták, Z., Jarvis, P.G. and Catsky, J., Eds., Plant Photosynthetic Production. Manual of Methods, W. Junk Publishing Co, The Hague, 1-48.
- Tooming, H.G. and Gulyayev, B.I. (1967) Methods of measurement of photosynthetically active radiation. Nauka, Moscow (in Russian).
- Dospekhov, B.A. (1985) Methods of field experience. Agropromizdat, Moscow (in Russian).
- Mirzoyev, R.S. (1988) Seasonal variations of photosynthesis intensity of various soybean genotypes. Proceedings of IV Republic Conference, Baku, Azerbaijan, 78 (in Russian).
- Mirzoyev, R.S. (1988) CO2 gas exchange and photosynthetic traits of various soybean genotypes. Proceedings of Republic Conference of Young Scientists, Tbilisi, Georgia, 38 (in Russian).
- Akperov, Z.I. and Mirzoyev, R.S. (1990) Photosynthetic traits of various soybean genotypes contrast in grain yield. Vestnik selskokhozaystvennoy nauki AzSSR, 2, 6-9 (in Russian).
- Aliyev, J.A. (1998) Importance of photosynthesis of various organs in protein synthesis in grain of wheat genotypes under water stress. Proceedings of the XIth International Congress on Photosynthesis, 4, 3171-3174.
- Aliyev, J.A. (2001) Diversity of photosynthetic activity of organs of wheat genotypes and breeding of highyielding varieties tolerant to water stress. Proceedings of the 12th International Congress on Photosynthesis, Brisbane. http://www.publish.csiro.au/ps2001
- Aliyev, J.A. (2004) CO2 assimilation, architectonics and productivity of wheat genotypes in sowing. Proceedings of the 13th International Congress of Photosynthesis, 2, 1047-1048.
- Aliyev, J.A. (2007) The intensity of CO2 assimilation, photorespiration and productivity of wheat genotypes Triticum L. Abstracts of the 14th International Congress on Photosynthesis, 91, 278.
- Aliyev, J.A. (2012) Photosynthesis, photorespiration and productivity of wheat and soybean genotypes. Physiologia Plantarum, 145, 369-383. doi:10.1111/j.1399-3054.2012.01613.x

