Open Journal of Preventive Medicine
Vol.2 No.2(2012), Article ID:19537,4 pages DOI:10.4236/ojpm.2012.22032
Identification of the child with influenza A (H1N1): Clinical criteria versus the rapid influenza A-B test
![]()
Department of Pediatrics, High Specialty Central South Hospital, PEMEX, Mexico City, Mexico; *Corresponding Author: jesusreynaf@gmail.com
Received 6 January 2012; revised 18 February 2012; accepted 24 March 2012
Keywords: Diagnosis; Influenza H1N1; Efficacy; Criteria; Rapid Test
ABSTRACT
Introduction: During the influenza A (H1N1) pandemic infection, the Ministry of Health in Mexico recommended that it be considered as a suggestive clinical picture in patients with fever, cough and headache. In some places where the resource was available, the rapid seasonal influenza (A-B) test was used as an alternative identification strategy. Methods: From April 2009 to May 2010, patients under 18 years of age with acute respiratory tract symptoms were included in a retrospective study. They underwent the following procedures: 1) application of the clinical criteria recommended by the federal Ministry of Health during the A (H1N1) pandemic; 2) rapid test for seasonal influenza (A-B), and 3) search for the influenza A (H1N1) virus by means of the real time polymerase chain reaction (RT-PCR). The study was approved by the research and ethics committee at the Central South Hospital of the government-owned Mexican Petroleum Company (PEMEX). Results: One hundred and thirty pediatric patients with a median age of eight years and a range between one and 17 years were included. Taking into account the Ministry of Health’s criteria, we found the following: 50% sensitivity, 69% specificity, 80% positive predictive value and 27% negative predictive value versus the rapid test that displayed 82% sensitivity, 70% specificity, 80% positive predictive value and 27% negative predictive value. Conclusions: Clinical criteria display low sensitivity in the infected children studied, whereas the rapid influenza A-B test constitutes a better option to identify these patients.
1. INTRODUCTION
In 2009 Mexico was in the spotlight all over the world since it was considered to be the source of the pandemic due to the influenza A (H1N1) virus. Initial concern in the Mexican population in the face of the detection of the “new circulating virus” caused that at the slightest suspicion of respiratory disease, regardless of its seriousness and temporality, a significant number of people showed up at health service facilities to have the infection ruled out [1,2].
Preparing and responding to global risk such as the one entailed by the magnitude of a pandemic represents a challenge for any organization [3]. Therefore, health authorities in different localities had to come up swiftly with operational definitions, based on previous experiences, for the diagnosis of the infection by the influenza A (H1N1) virus [3,4]. Specially at the beginning of the pandemic, some health care sites, chiefly private ones, chose to use the rapid test for seasonal influenza as a detection method for infected people with influenza A (H1N1); at the time this strategy was not recommended since it was deemed useless [4,5]. In Mexico, the Ministry of Health recommended that influenza be considered as a suggestive clinical picture in patients with fever, cough and headache. In patients under five years of age, irritability was considered a cardinal sign, in place of the headache [3].
In particular, the government-owned Mexican Petroleum Company’s (PEMEX) health system adopted, as part of its protocol of care of patients with data of acute respiratory infection, the establishment of Respiratory Disease Clinics as a contingency measure. The rapid seasonal influenza (A-B) test and the real time polymerase chain reaction (RT-PCR) were used as auxiliary laboratory resources. PEMEX was one of the few institutions to analyze its own cases, besides resorting to the laboratory of national reference. There are few reports that evaluate the capacity of the non-specific rapid test for the influenza A (H1N1) virus and of the clinical criteria recommended for the identification of sick patients. Particularly in the pediatric age group there is a limitation in terms of an adequate interpretation of clinical data on the part of the parents, who are mostly responsible for reporting the children’s symptomatology [6]. Previous research had shown that the influenza A + B rapid test was an adequate screening tool, with high sensitivity, specificity, positive predictive value and negative predictive value, but these observations come from adult populations exclusively [4,7] .We aimed to determine whether this was also true for the pediatric population, which is more prone to upper airway disease, and to assess the diagnostic efficacy of the MH proposed screening criteria.
2. METHODS
From April 2009 to May 2010, patients under 18 years of age with acute respiratory tract symptoms that sought medical care at the Respiratory Disease Clinic, at the out-patient pediatric consultation facility or at the ER at PEMEX Central South Hospital (CSH) were included in a retrospective study. They underwent the following procedures: 1) guided interrogation in search of the clinical criteria recommended by the federal Ministry of Health during the pandemic; 2) rapid test for seasonal influenza A-B virus (QuickVue®) performed as indicated by the manufacturer [8], and 3) search for the influenza A (H1N1) virus by means of the real time polymerase chain reaction (RT-PCR) in a nasopharyngeal secretion sample, according to the protocol published by the World Health Organization (WHO) on May 21st, 2009 and the protocol recommended by the Robert Koch Institute in Germany as a gold standard [9].
In the case of every patient suspected of being infected with the influenza A (H1N1) virus, defined by the presence of fever, cough and headache (in subjects older that five years of age) or irritability in place of the headache (in children under five years of age), the results for the rapid test and for the RT-PCR were searched for by reviewing the institutional electronic medical record and the data-base from the CSH molecular biology laboratory.
Demographic variables were analyzed by means of descriptive statistics (median, range, percentages). The efficacy of the criteria was analyzed calculating sensitivity, specificity, positive predictive value and negative predictive value for the set of criteria and for each one individually. The difference in terms of efficacy between the tests was assessed by means of a difference between proportions with a 95% confidence interval.
3. RESULTS
Demographic Characteristics of the Population
During the study period, a total of 469 patients were sampled in search of the influenza A (H1N1) virus; 130 (27%) subjects were pediatric patients. The median age was eight years with a range between one and 17 years. Seventy four of these subjects were male and 56, female.
The median elapsed time between the onset of symptoms and the search for medical care was two days with a mean range of 20. The rest of patient characteristics are depicted in Table 1.
Ninety four out of 130 (72%) patients turned out to be RT-PCR-positive for the influenza A (H1N1) virus versus 36 out of 130 (28%) that were RT-PCR-negative. The most common symptoms in the population studied included: fever (85%), cough (82%), headache (55%) and rhinorrhea (55%).
Considering the Ministry of Health’s criteria (fever, cough, headache/irritability), versus RT-PCR we found a sensitivity of 50%, a specificity of 69%, a positive predictive value of 80%, and a negative predictive value of 27%; if we took into account the presence of at least two of the recommended symptoms (any combination of the following symptoms: fever, headache, cough and irritability in the place of headache in children under five
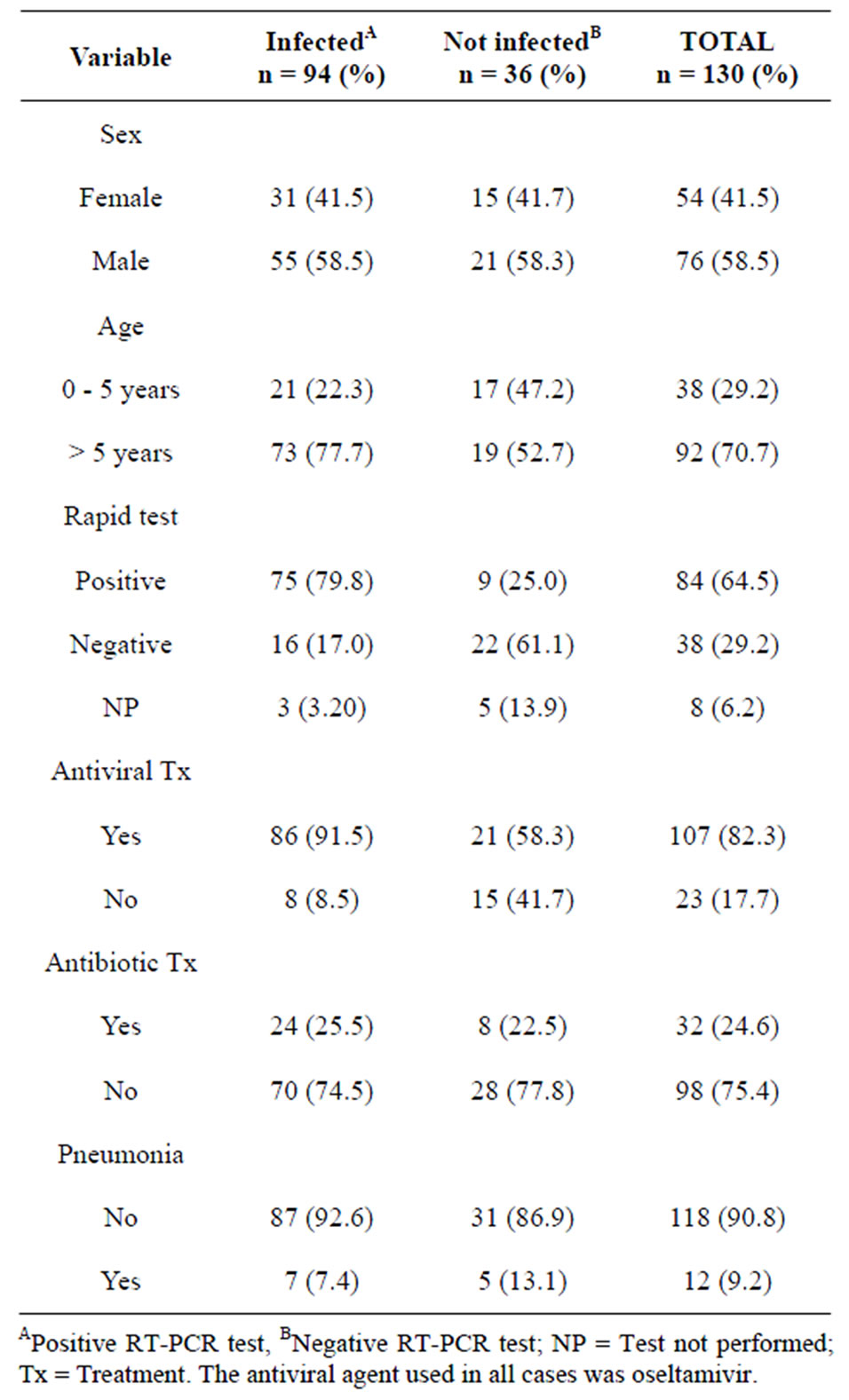
Table 1. Patient characteristics of children from the PEMEX health system with suspected influenza A (H1N1) virus infection.
years of age), sensitivity increased to 92%. When we compare the results of the rapid test as gold standard versus the MH criteria we found: sensitivity = 41%, specificity = 64%, positive predictive value = 72%, negative predictive value = 33%.
Considering each symptom individually, we found that fever, cough and headache displayed a sensitivity of over 80%, as it is shown in Table 2. The rapid test displayed a sensitivity of 82%, a specificity of 70%, a positive predictive value of 80%, and a negative predictive value of 27% when compared to RT-PCR.
The difference in sensitivity between the MH clinical criteria set and the rapid test (both against RT-PCR) was 32% (p < 0.05) and the area-under-the-ROC-curve for the clinical criteria was 0.67 versus 0.89% for the rapid test.
4. DISCUSSION
Rapid tests for seasonal influenza have been used increasingly in recent years due to their lack of difficulty,

Table 2. Test characteristics of different criteria set and of individual symptoms in the diagnosis of influenza A (H1N1) infection by RT-PCR standard.
their speed and their efficacy for the identification of infected patients with virus either A or B. A sensitivity of approximately 50% to 70% and a specificity of approximately 90% to 95% are accepted [10].
These tests were used in some locations as a tool for identifying patients infected with the influenza A (H1N1) virus during the pandemic that broke out in April 2009. Some people highlight their low sensitivity and specificity compared to other methodologies such as direct immunofluorescence, viral culture and RT-PCR. Nevertheless, during the 2009 emergency, it was testified that the rapid influenza tests, regardless of the commercial trademark, are useful to identify patients infected with the A (H1N1) virus [5]. They were used less than the clinical criteria recommended by the federal Ministry of Health on the basis of universally established data by WHO and the Pan American Health Organization (PAHO). These institutions globally justified actions such as the use of antiviral drugs or respiratory management and defined the course of action to be followed by health workers, based on the presence or absence of certain clinical criteria [3,4,6-8]. The primordial role of clinical data as initial referents to identify the disease is based on studies previously carried out in patients infected with the seasonal influenza virus. These studies showed sensitivities over 70%, which initially established that the extrapolation of these same data to the influenza A (H1N1) epidemic was very likely to be useful [4,10-15].
In Mexico the efficacy of the criteria was assessed retrospectively [4] in patients suspected of having been infected with the A (H1N1) virus; a sensitivity of 96% was found. The necessary comparison with our results shows a difference of almost 45% between the studies in terms of the figures. The explanation for these differences with respect to the diagnostic efficacy of the criteria between the studies can be established taking into account the characteristics of the population: 1) We included only pediatric-age patients who were taken to medical consultation in the face of the slightest indication of respiratory disease, which explains why the evolution of the disease did not entail complications for the most part (cases of pneumonia and influenza were established in only seven days) and no deaths were reported in our population. 2) From the perspective of the historical moment, when the circulation of the virus, its prevalence and the panic in the face of the disease were high, the resource came to be used indiscriminately (rapid test, RT-PCR); febrile patients with an acute evolution of the disease sought care two days after the onset of the symptoms. Fever was the most efficacious symptom in terms of identifying sensibilities over 80%; these subjects were the ones who mostly underwent the confirmatory test.
The following limitations have to be taken into account with respect to the results obtained: First, they are the product of a retrospective study and hence, there is the risk of an inadequate registration of the data (symptoms and evolution) in the clinical history, as well as of an inappropriate management of the information. Second, the fact that the test resulted positive does not mean that all the patients displayed the symptomatic complex, since the seriousness of the condition is multifactorial, as is the case in any respiratory disease. Third, since the study was carried out during a period of time characterized by a high degree of circulation of the virus, the study conclusions with respect to the diagnostic efficacy of the symptomatic complex or of the individual symptoms should be considered cautiously during periods when virus circulation is low. These conclusions are similar to those reported in our country with respect to the use of the seasonal influenza test to detect patients infected with the influenza A (H1N1) virus [7,16,17]. According to the results, the use of the rapid A-B test to identify seasonal influenza turned out to be more efficacious that the symptomatic complex to discriminate the pandemic influenza; this resource is conditioned in terms of availability and existence. It should also be noted that by now there are specific rapid tests for influenza A (H1N1), whose advantages do not seem to be greater in times of high viral circulation.
5. ACKNOWLEDGEMENTS
We are grateful to Pedro Zarate chief of Clinical Laboratory and to Guillermo Wakida and Juan Carlos Medina of the Department of Pediatrics for they assistance with this investigation.
REFERENCES
- The Members of the Novel Swine-Origin Influenza A (H1N1) Virus Investigation (2009) Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans. The New England Journal of Medicine, 360, 2605-2615. doi:10.1056/NEJMoa0903810
- Vidal, P., Reyna, J. and Richardson, V. (2011) Events temporarily associated with anti-influenza A (H1N1) vaccination in Mexico. Archives of Medical Research, Available on line 27 October 2011. http://www.sciencedirect.com/science/article/pii/S0188440911002104
- Hernández, M.A. (2009) Novel influenza [A (H1N1) 2009]; suspect case definition. Review of agreement on case definition criteria used in different Spanish regions. Revista Pediatría de Atención Primaria, 11, 383-98
- González, C.J., Iglesias, C.J.M., Romero, A.Y., Chávez, C.C., Gay, M.J.G. and Rivas, R.R. (2011) Cost-effectiveness in the detection on influenza H1N1;clinical data versus rapid tests. The Pan American Journal of Public Health, 29, 1-8.
- Figueroa, C., Nuñez, L., Aranda, D., Gómez, S., Osorio, E. and Vargas, H. (2009) Consistent analysis of four test for the detection of Influenza A in Bogotá. University of Colombia i Bogota, 50, 444-451
- Centers for Disease Control and Prevention (2010) Interin guidance on case definition to be used for investigations of novel influenza A (H1N1) cases. http://www.cdc.gov/h1n1flu/casedef.htm
- Castro, C.L.A., Llaca, D.J., Pérez, C.F.E., Gómez, E.A. and Flóres, A.A. (2011) Comparative study of a rapid testing with real time RT-PCR for diagnosis of influenza AH1N1 2009. Salud Publica de México, 53, 329-333. doi:10.1590/S0036-36342011000400007
- QuickVue Influenza A+B 25 Test Kit. Quidel Corporation, San Diego. Available in: http://www.quidel.com/libraries/pkginserts/RD/QVInfluenzaAB_ModComplex.pdf
- Suess, T., Buchholz, U., Dupke, S., Grunow, R., an der Heiden, M., Heider, A., et al. (2010) Shedding and transmission of novel influenza virus A/H1N1 infection in households—Germany 2009. American Journal of Epidemiology, 171, 1157-1164. doi:10.1093/aje/kwq071
- Centers for Disease Control and Prevention (2006) Rapid diagnostic testing for influenza: Information for clinical laboratory directors. US Department of Health and Human Services, Atlanta. http://www.cdc.gov/flu/professionals/diagnosis/rapidlab.htm
- Kasper, M.R., Wierzba, F.T., Sovann, L., Blair, J.P. and Putnam, D.S. (2010) Evaluation of an influenza-like illness case definition in the diagnosis of influenza among patients with acute febrile illness in Cambodia. BMC Infectious Diseases, 10, 320. doi:10.1186/1471-2334-10-320
- Boivin, G., Hardy, I., Tellier, G. and Maziade, J. (2000) Predicting influenza infections during epidemics with use of a clinical case definition. Clinical Infectious Diseases, 31, 1166-1169. doi:10.1086/317425
- Navarro, M.J.M., Pérez, R.M., Cantudo, M.P., Petit, G.C., Jiménez, V.M. and Rosa, F.M. (2005) Influenza-like illness criteria were poorly related to laboratory-confirmed influenza in a sentinel surveillance study. Journal of Clinical epidemiology, 58, 275-279. doi:10.1016/j.jclinepi.2004.08.014
- Friedman, M.J. and Attia, M.W. (2004) Clinical predictors of influenza in children. Archives of Pediatrics & Adolescent Medicine, 158, 391-394.
- Ohmit, S.E. and Monto, A.S. (2006) Symptomatic predictors of influenza. Clinical Infectious Diseases, 43, 564-568. doi:10.1086/506352
- Hawkes, M. (2010) Sensitivity of rapid influenza diagnostic testing for Swine-Origin 2009 A (H1N1) influenza virus in children. Pediatrics, 125, e639-e644. doi:10.1542/peds.2009-2669
- Cruz, A. (2010) Performance of a rapid influenza test in children during the H1N1 2009. Pediatrics, 125, e645-e650. doi:10.1542/peds.2009-3060

