Open Journal of Pediatrics
Vol.3 No.4(2013), Article ID:40155,4 pages DOI:10.4236/ojped.2013.34066
The use of remifentanil in ex utero intrapartum treatment procedures
![]()
Duke University Hospital, Durham, USA
Email: *Chad.w.whited@gmail.com
Copyright © 2013 Chad Whited, Eileen Raynor. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 5 October 2013; revised 2 November 2013; accepted 10 November 2013
Keywords: EXIT; Ex Utero Intrapartum Treatment Procedure; Remifentanil; Airway
ABSTRACT
Purpose: We propose that using remifentanil in ex utero intrapartum treatment (EXIT) procedures reduces the need for maternal exposure to general anesthesia. Using remifentanil along with spinal anesthesia eliminates the fetal and maternal risks associated with inhalational general anesthesia, allows the mother to be awake, and obviates the need for and costs associated with general anesthesia and a second anesthesia team. Materials and Methods: We performed a retrospective review of all sequential patients undergoing ex utero intrapartum treatment procedure at our hospital from 1/1/2009 to 11/1/2010. All procedures were performed under regional neuraxial analgesia, using nitroglycerine as a tocolytic agent and remifentanil for analgesia. Variables included indication, time to secured fetal airway, complications, estimated blood loss, need for additional anesthetics, participating personnel, and survival. Results: All five of our ex utero intrapartum treatment procedures were successfully completed with combined spinal epidural remifentanil anesthetic. No patient was required additional alternative anesthetic. There were no complications with mother or fetus. Indications for procedure were arthyrogryposis (n = 3), fetal goiter, and micrognathia. Average time to secured airway was 10.25 minutes. Average estimated blood loss was 1010 ml. All five mothers were conscious during their procedure. Conclusions: We report the largest series of ex utero intrapartum treatment procedures performed with remifentanil regional anesthesia. We found that the combined use of nitroglycerin and regional remifentanil anesthesia is a safe alternative to the pediatric otolaryngologist for performing ex utero intrapartum treatment procedures without the risks of general anesthesia, allowing the mother to be awake for the delivery, and reducing the cost of providing care.
1. INTRODUCTION
Ex utero intrapartum treatment (EXIT) procedures are effective means for evaluating and securing the fetal airway in prenatally identified head and neck anomalies. It involves obstetrics partially delivering the infant while maintaining uteroplacental circulation, so that the pediatric otolaryngologist can evaluate and secure a threatened airway. First described in 1992 for a fetus with obstructing epignathus [1], the EXIT procedure was refined during its use for the reversal of tracheal occlusion in patients with congenital diaphragmatic hernias [2,3].
Most centers use general anesthesia for both the mother and the fetus, relying on inhaled halogenated anesthetic agents for uterine relaxation. This potentially exposes the mother to the risks associated with general anesthesia, requires the mother to be asleep for the procedure, and can require two anesthesia teams with their associated costs. Our series examines the surgical outcomes of EXIT procedures performed at Duke University Hospital using combined neuraxial regional anesthesia with remifentanil, thereby avoiding general inhalational anesthetics.
Remifentanil is currently the most suitable systemic opioid for obstetric use, providing adequate analgesia to the parturient and the neonate with the fewest adverse effects. It is an ultra-short acting µ-receptor agonist that crosses the placental barrier and is rapidly metabolized by the neonate with minimal accumulation [4].
2. MATERIALS & METHODS
This study was approved by Duke University Institutional Review Board. We reviewed our experience with all ex utero intrapartum treatment procedures at Duke University Hospital between January 2009 and November 2010. Data retrospectively collected from the records of these patients included time to secured airway, need for additional anesthetic, indication for EXIT procedure, complications, participating medical personnel, and survival rates.
Each patient was counselled preoperatively with a multidisciplinary EXIT Team including pediatric otolaryngology, social work, maternal fetal medicine, obstetrics, and anesthesia. The plan for securing the neonatal airway was reviewed preoperatively with the team prior to placement of the maternal epidural. Succinyl choline was available if necessary to facilitate neonatal intubation.
Each patient had a combined spinal epidural placed and remifentanil administered intravenously for analgesia. Nitroglycerin bolus and continuous infusion maintained uterine relaxation and maternal-fetal blood flow. A lower uterine incision was made and the fetal head and torso were partially delivered keeping the maternal-fetal circulation intact. The pediatric Otolaryngologist-Head and Neck Surgeon (OHNS) team then evaluated the airway and secured it if necessary. The umbilical cord was then cut and the newborn delivered to neonatology.
3. RESULTS
There were a total of 5 EXITs, and all were for evaluating and securing the fetal airway. All were successfully performed with combined spinal epidural regional anesthesia, nitroglycerine for uterine relaxation, phenylephrine for hemodynamic stability, and remifentanil for maternal-fetal analgesia. Zero parturients required general anesthesia, and no additional fetal analgesia was needed. Only one anesthesia team was required for each of the procedures.
Prenatal ultrasonography findings demonstrated hyperextended neck, multiple joint contractures, polyhydramnios, micrognathia, and thyroid mass. This correlated with the indications of one for fetal goiter, three for arthrogryposis, and one for micrognathia.
One required tracheostomy, one required only direct laryngoscopy with no airway compromise, and three were successfully intubated. No patients required administration of succinyl choline. Time to secured airway was 21 minutes for tracheostomy and averaged 6.67 minutes for intubation. Average maternal blood loss during the obstetrics portion of the case was 1010 ml, and there was minimal blood loss during the fetal, OHNS, portion (Table 1). APGAR scores ranged from 3 - 9 and 7 - 9 and weight averaged 2.698 kg (2.25 kg - 3.09 kg). There were no operative complications.
4. DISCUSSION
The ex utero intrapartum treatment procedure entails obstetrics partially delivering the head and torso of a fetus with suspected airway obstruction through a lower uterine incision while maintaining utero-placental circulation. This allows time for the surgical team to stabilize the fetal airway before interruption of maternal circulation and delivering the neonate to the neonatology team. First described in 1992 for a fetal epignathus [1], and later refined for the reversal of tracheal clippings in the treatment of patients with congenital diaphragmatic hernias [2,3], the indications for EXIT procedures are expanding for stabilizing fetal cardio-pulmonary statuses.
Historically, general anesthesia with inhaled halogenated anesthetics has been used to maintain uterine relaxation. Maintaining uterine relaxation is critical to EXIT procedures to avoid anoxia with a compromised fetal airway. This preserves maternal-fetal circulation and creates time for fetal interventions. However, with medical trends of increasing patient safety, avoiding unnecessary risks, reducing costs of care, and allowing the mother to be awake during the delivery, our EXIT Team began performing procedures under regional neuraxial anesthesia.
Nitroglycerine has proven to be an effective tocolytic while providing uterine relaxation. This negates the need for inhaled volatile agents, and allows EXITs to be performed under regional anesthesia. Its onset of uterine relaxation is under one minute with effect duration of 1 - 2 minutes. This short duration minimizes the risks of maternal hemorrhage or uterine atony [5]. Hemodynamic stability was achieved with phenylephrine.
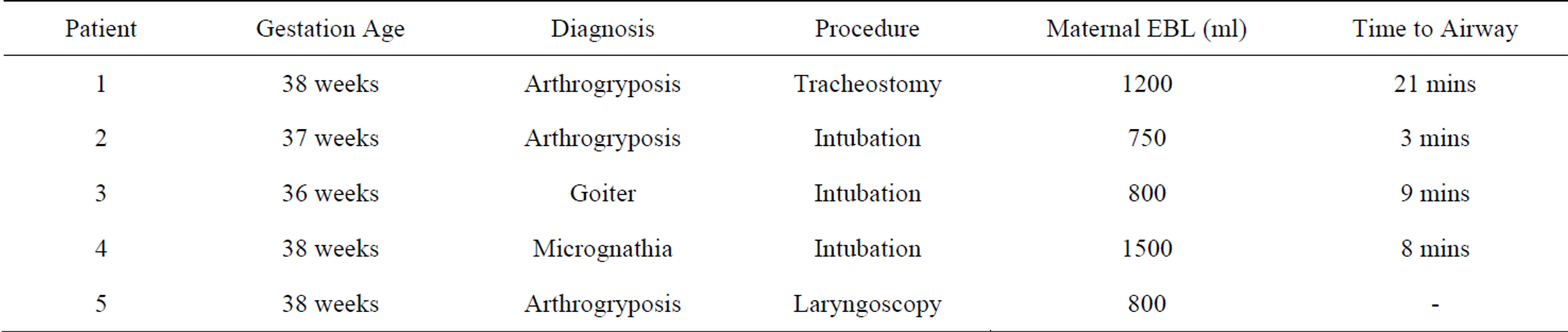
Table 1. EXIT procedures performed at Duke University Hospital (1/1/2009-11/1/2010).
Remifentanil was chosen for the analgesic because it is an ultra-short acting opiate [4]. Its onset is within one minute and half-life is 3 minutes [6]. It readily crosses the placental barrier with an umbilical vein to maternal artery ratio of 0.82. Remifentanil has rapid neonatal metabolism with an umbilical vein to umbilical artery ratio of 0.29. These confer the added benefit of fetal analgesia and immobilization [7]. There have been no documented reports where naloxone was necessary, thus indicating low fetal accumulation [6]. Maternal sedation on remifentanil is minimal and desaturations are transient. Neonatal APGAR scores do not deviate from the expected range. For our cases there was no need for additional fetal analgesic. However, we find it prudent to have an additional fetal analgesic cocktail available for pain or agitation during the procedure.
When comparing our surgical outcomes of EXITs performed with remifentanil to those reported in the literature for EXITs under general anesthesia (GA), the results are similar. Looking at the two largest case series of EXITs under GA, Hirose et al. and Bouchard et al., there were more procedures for congenital diaphragmatic hernias which likely produced younger than average gestational ages and longer placental circulation times than our series. However, between Hirose et al., Bouchard et al., and our series, live birth percentage was comparable with 98.7%, 96.77%, and 100%, respectively (Table 2). The GA series’s combined average estimated maternal blood loss was 967 ml which is comparable to ours under remifentanil analgesia of 1010 ml. Time on placental support was an expectedly longer average of 39.6 minutes (2 - 150 min) for the GA series [2,3]. Though our average placental circulation time was 6.67 minutes for intubation, we safely demonstrated a placental circulation time of 21 minutes using regional anesthesia when we performed the tracheostomy.
EXIT procedures are still relatively uncommon and indications greatly vary, making direct data comparisons less than ideal. However, it is remarkable that there were no maternal or fetal complications in our series. Our data is limited, especially when compared to the larger studies mentioned. As we continue to perform EXITs using our remifentanil protocol we will continue to prospectively collect and compare our outcomes to those in the literature. It is important to note that our experiences have been for securing fetal airways and did not mandate prolonged mass resection at the time of delivery.
5. CONCLUSION
We have reported the largest series of patients undergoing EXIT procedures under remifentanil and regional anesthesia. We found that the combined use of remifentanil and neuraxial analgesia provides effective anesthe-

Table 2. EXIT outcomes comparison.
sia to both the mother and the fetus while avoiding the maternal risks of general anesthesia and negates the need for additional anesthesia teams. This also allows the mother to be awake for the delivery of her child. In the correctly selected patients, this technique is a safe and efficient alternative available to the pediatric otolaryngologist for performing EXIT procedures.
6. ACKNOWLEDGEMENTS
Terrence K Allen, MBBS, Duke University Medical Center, Division of Women’s Anesthesiology, Walter T Lee, MD, Duke University Medical Center, Otolaryngology-Head & Neck Surgery, Dr. Eileen Raynor had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- Catalano, P., Urken, M.L., Alvarez, M., et al. (1992) New approach to the management of airway obstruction in “High Risk” neonates. Archives of Otolaryngology—Head and Neck Surgery, 118, 306-309. http://dx.doi.org/10.1001/archotol.1992.01880030094019
- Bouchard, S., Johnson, M., Flake, A., et al. (2002) The EXIT procedure: Experience and Outcome in 31 cases. Journal of Pediatric Surgery, 3, 418-426. http://dx.doi.org/10.1053/jpsu.2002.30839
- Hirose, S., Farmer, D., Lee, H., Nobuhara, K.K. and Harrison, M.R. (2009) The ex utero intrapartum treatment procedure: Looking back at the EXIT. Journal of Pediatric Surgery, 39, 375-380. http://dx.doi.org/10.1016/j.jpedsurg.2003.11.011
- Hill, D. (2008) The use of remifentanil in obstetrics. Anesthesiology Clinics, 26, 169-182.
- Clark, K., Viscomi, C., Lowell, J. and Chien, E.K. (2004) Nitroglycerin for relaxation to establish a fetal airway (EXIT procedure). Obstetrics & Gynecology, 103, 1113- 1115. http://dx.doi.org/10.1097/01.AOG.0000125158.61232.b3
- Egan, T.D. (2000) Pharmacokinetics and pharmacodynamics of remifentanil: An update in the year 2000. Current Opinion in Anaesthesiology, 13, 449-455. http://dx.doi.org/10.1097/00001503-200008000-00009
- Kan, R.E., Hughes, S.C., Rosen, M.A., Kessin, C., Preston, P.G. and Lobo, E.P. (1998) Intravenous remifentanil: Placental transfer, maternal and neonatal effects. Anesthesiology, 88, 1467-1474. http://dx.doi.org/10.1097/00000542-199806000-00008
NOTES
*Corresponding author.

