 Vol.2, No.3, 253-261 (2010) doi:10.4236/health.2010.23036 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ Health Antioxidant attenuation of ROS-involved cytotoxicity induced by Paraquat on HL-60 cells Wen-Hua Zhang, Yang Yang, Chang-Jun Lin, Qin Wang School of Life Sciences, Lanzhou University, Lanzhou, China; qwang@lzu.edu.cn Received 23 December 2009; revised 10 January 2010; accepted 14 January 2010. ABSTRACT The cytotoxic effects of Paraquat, an herbicide refractory to treatment after intentional or acci- dental contact, were investigated on the human leukemia HL-60 cells. With the establishment of Paraquat injury model of HL-60 cells, trypan blue exclusion assaying was performed to have determined the effects of Paraquat-induced cy- totoxicity on HL-60 cells in a concentration- dependent manner. Upon treatmen t with v arious concentrat ions of Paraqua t, pronounced increase on the levels of intracellular production of O2•- and H2O2 was detected with employment of fluorescent probes. Indicative of the oxidative stress, levels of MDA and T-AOC were quanti- tated to have determined the causal role for Paraquat in subjecting HL-60 cells to oxidative damage. Based on this finding, effects of anti- oxidant enzymes including GSH, NAC, CAT and SOD on attenuating the Paraquat-induced oxi- dative damage on HL-60 cells were examined, aiming to identify the most effective antioxidant enzyme for alleviating the cytotoxicity induced by Paraquat. In conjunction with the determina- tion of cytotoxicity exerted by all the antioxidant enzymes on HL-60 cells, GSH-with its least in- herent cytotoxicity on HL-60 cells-was identified as a promising candidate ingredient for extenu- ating the Paraqua t -in duced cytotoxicity. Keywords: Paraquat; HL-60 Cells; Oxidative Damage; Cytotoxicity; Antioxidant Enzymes 1. INTRODUCTION As response to increasingly more environmentally- associated outbreaks of diseases, Paraquat (PQ) had al- ready gained tremend ous attention since 1966, fo llowing the determination of the toxicity of PQ for a number of animal species [1] and a report on two cases of acci dental poisoning by PQ in human [2]. Herein, PQ is the trade name of the dic hloride salt of the ra dical 1, 1’-dimet hyl-4, 4’-dipyridy liuim , whose herbi cidal pr operty was reported in 1958. Since its toxicity is dosage-dependent, a wide spectrum of studies on PQ-induced toxicity had been conducted at various levels, aiming to standardize the safe dosage to minimize its toxicity to other untargeted organisms. Although an in vivo experiment upon the toxicity of PQ, which set up the rats and mouse as study objects and factored into the acute and chronic toxicity as well as teratogenicity and mutagenicity, was conducted to have claim ed t hat PQ w o ul d be safe if used foll o wi n g the recommended use instruction, the hard-to-control ma- nipulation of use in practice and differentiated suscepti- bility of animal species necessitate the investigations of underlying mechanism of PQ-induce cytotoxicity. More- over, advanced techniques had already been employed to identify effective candidate molecules to reduce PQ- induced toxici ty [3]. PQ is biologically active after it has been sprayed in the field. It’s either strongly bound to soil particles or de- composed into a non-toxic form by soil bacterial and sunlight [4]. However, active form of PQ is highly toxic to humans and many cases of acute poisoning and death had been reported over the past few decades [5-9]. The most frequent routes of exposure to PQ, either acciden- tally or intentionally, in humans and animals are by in- gestion or through direct skin contact. Regardless of the well-tuned administration of circulation and body-defense systems, PQ could be rapidly distributed in most tissues, with the highest concentration in the lungs and kidneys [10], where the compound accumulates and causes great damage in Cl ar a cel l s and alveolar type I an d II epithelial cells [11]. Mechanisms involving the generation of Re- active Oxygen Species (ROS) were implicated in the PQ-induced cell apoptosis [12-16]. Although the infor- mation on the possible mechanism of human carcino- genesis associated with PQ exposure is limited, several papers have suggested a possible link to Non-Hodgkin’s Lymphoma by induction of chromosomal aberrations, gene mutations in human lymphocytes, and apoptosis in human B lymphocytes [17,18]. ROS is hypothesized to induce cell damage or initiate a cascade of signaling mechanisms that ultimately lead to cell adaptation, apoptosis or necrosis [19]. The mecha-  W. H. Zhang et al. / HEALTH 2 (2010) 253-261 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 254 nisms of PQ toxicity include the formation of ROS me- diated by cytochrome P450 reductases and subsequent damage of ROS to cellular macromolecules [20]. The dysfunction of pulmonary microvascu lar endothelial cell membrane and high oxidative potential in pulmonary microvascular endothelial cells, which resulted from the overproduction of hydrogen peroxide, had been well- documented to be involved in PQ-induced cytotoxicity [21,22]. Additionally, EPR techniques had been em- ployed to have confirmed that PQ could have triggered the intracellular production of ROS during which the formation of oxygen radicals (O2•-) and hydroxyl radical (OH.) involved the m echanism of hydrogen t ransfer [23]. As one of the PQ-induced toxicity modes, mechanism of ROS-induced neuronal damage by PQ had been proposed [24]. Thus, Cellular Reactive Oxygen Species metabo- lism has been the focus of intense interest regarding physiological activities, due in part to the evidence that over expression of antioxidant enzymes confers resis- tance to oxidative stress, improvement in disease and increased lifespan, respectively [25,26]. ROS including superoxide, hydrogen peroxide and hydroxyl radicals are commonly generated through cellular metabolism and their physiological balance is achieved through neutrali- zation of cellular antioxidant enzymes, which include Glutathione (GSH), Superoxide Dismutase (SOD), Cata- lase (CAT) and Glutathione Peroxidase (GPx) [27,28]. Oxidative stress occurs as a consequence of the imbalance between the production of ROS and cellular antioxidant capacity. Antioxidant enzymes, whi ch are biologically and functionally coupled with ROS, have been evolved to maintain the high-efficiency performance of cell functions. A putative illustration of how a cell responds at risk of oxidative damage had been proposed [2 9]. Here, based on the establishment of in vitro PQ injury model of HL- 60 cell s, we ha ve studie d the R OS-invol ved cytotoxicity induced by PQ in HL-60 cells through measuring the contents and time-resolved changes of malondialdehyde (MDA), total anti-oxidation compe- tence (T-AOC), O2•- and H2O2. A concentration- and time-dependent inhibition of cell growth has also been quantified to determine whether the release of O2•- and H2O2 into cytosol could potentially induce the cytotovic- ity on HL-60 cells. In addition, we have explored that whether the treatment with antioxidant enzymes could attenuate the PQ-induced cytotoxicity on HL-60 cells. Inspired by the PQ-detoxication effects of multiple emulsions [30], we hope the GSH could be developed as an ingredient in attenuating the PQ-induced to xicity. 2. MATERIALS AND METHODS 2.1. Cell Line and Culture Medium Human Promyelocytic Leukemia HL-60 cell line was obtained from the cell library of Institute of Cancer Mo- lecular biology and Drug Screening at Lanzhou Univer- sity. RPMI-1640 cell culture medium was purchased from Gibco (Santa Clara, USA). Mycoplasma-free Neo- natal Bovine Serum was purchased from Hangzhou Si- jiqing Biological Engineering Materials. 2.2. Reagents, A ssay Ki t s and Othe r Materia ls Paraquat was purchased from Sigmal-Aldrich. Assay kits of MDA and T-AOC were purchased from Promega (Madison, WI, USA). Culture dishes and 24-well plates were purchased from Hangzhou Sijiqing Biological En- gineering Materials (Hangzhou, China). 2’, 7’-Di- chlorofluorescein diacetate (DCF-DA) and Dihydro- ethidium (DHE) probes were purchased from Molecular Probes (Eugene, OR, USA). Other common experiment materials were of analytically pure. 2.3. Culture of Human Promyelocytic Leukemia HL-60 Cells and Analysis of Cells Proliferation by Trypan Blue Exclusion HL-60 cells were grown in RPMI medium supplemented with 2g/L NaHCO3, 100U/ml penicillin, 100μg/ml streptomycin and 10% fetal calf serum in a humidified atmosphere of 95% air and 5% CO2. Cultures were initi- ated at a density of 104/ml at 37℃ and grown exponen- tially to about 106/ml in 72 hours. Under the hypothesis that the imbalance between oxidative stress and antioxi- dant capacity could be restored if the antioxidant en- zymes are replenished subsequently to the oxidative damage [31], cells were treated with various concentra- tions of PQ and desired concentrations of antioxidant enzymes for assigned time to determine both the PQ- induced cytotoxicity and antioxidant-mediated cytopro- tection. To perform the trypan blue exclusion, cells were washed and harvested with sterile PBS. The cell suspen- sion was mixed at the ratio of 4:1 with 0.4% trypan blue solution and incubated for 5 min. The viable cells were counted in a hemocytometer with an inverted- phase contrast microscope (Olympus IX81, Japan). 2.4. Detection of Intracellular Production of O2•- and H2O2 by Inverted Fluorescent Microscope For assessment of intracellular ROS produced by treat- ment with PQ in the HL-60 cells, the DCF-DA and DHE assay were performed [32,33]. The production of H2O2 was measured with a non-polar compound 2’, 7’- dichlorofluorescein diacetate (DCF-DA) that readily enter the cells, where it is cleaved to form non-fluorescent 2’, 7’-dichlorofluorescein (DCFH) by endogenous es- terases. DCFH reacts with H2O2 to produce a fluorescent compound 2’, 7’-dichlorofluorescein, which is trapped inside the cells and indicates the intracellular level of H2O2 [34,35]. To detect the intracellular production of O2•-, DHE probe was used where DHE is oxidized to 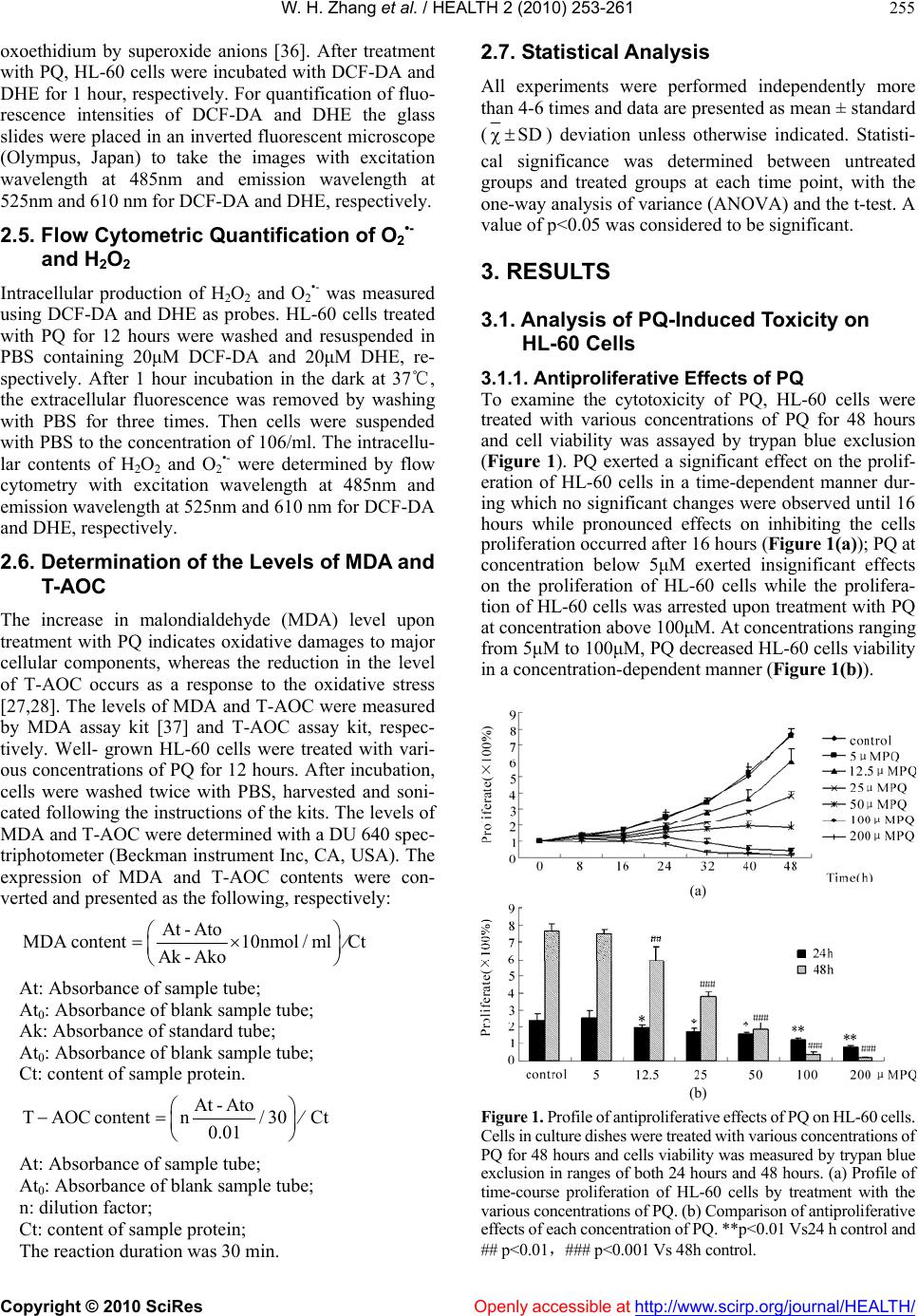 W. H. Zhang et al. / HEALTH 2 (2010) 253-261 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 255 oxoethidium by superoxide anions [36]. After treatment with PQ, HL-60 cells were incubated with DCF-DA and DHE for 1 hour, respectively. For quantification of fluo- rescence intensities of DCF-DA and DHE the glass slides were placed in an inverted fluorescent microscope (Olympus, Japan) to take the images with excitation wavelength at 485nm and emission wavelength at 525nm and 610 nm for DCF-DA and DHE, resp ect i vely. 2.5. Flow Cytometric Quantification of O2•- and H2O2 Intracellular production of H2O2 and O2•- was measured using DCF-DA and DHE as probes. HL-60 cells treated with PQ for 12 hours were washed and resuspended in PBS containing 20μM DCF-DA and 20μM DHE, re- spectively. After 1 hour incubation in the dark at 37, ℃ the extracellular fluorescence was removed by washing with PBS for three times. Then cells were suspended with PBS to the concen tration of 106/ml. The intracellu- lar contents of H2O2 and O2•- were determined by flow cytometry with excitation wavelength at 485nm and emission wavelength at 525nm and 610 nm for DCF-D A and DHE, respectively. 2.6. Determination of the Levels of MDA and T-AOC The increase in malondialdehyde (MDA) level upon treatment with PQ indicates oxidative damages to major cellular components, whereas the reduction in the level of T-AOC occurs as a response to the oxidative stress [27,28]. The levels of MDA and T-AOC were measured by MDA assay kit [37] and T-AOC assay kit, respec- tively. Well- grown HL-60 cells were treated with vari- ous concentrations of PQ for 12 hours. After incubation, cells were washed twice with PBS, harvested and soni- cated following the instructions of the k its. The levels of MDA and T-AOC were determined with a DU 6 40 sp ec- triphotometer (Beckman instrument Inc, CA, USA). The expression of MDA and T-AOC contents were con- verted and presented as the following, respectively: At- Ato MDAcontent10nmol /mlCt Ak- Ako At: Absorbance of sample tube; At0: Absorbance of blank sample tube; Ak: Absorbance of standard tube; At0: Absorbance of blank sample tube; Ct: content of sample protein. At- Ato C contentn/ 30Ct 0.01 At: Absorbance of sample tube; At0: Absorbance of blank sample tube; n: dilution factor; Ct: content of sample protein; The reaction duration was 30 min. 2. ed independently more 7. Statistical Analysis All experiments were perform than 4-6 times and data are presented as mean ± standard (SD ) deviation unless otherwise indicated. Statisti- cal significance was determined between untreated groups and treated groups at each time point, with the one-way analysis of variance (ANOVA) and the t-test. A value of p<0.05 was considered to be significant. 3. RESULTS 3.1. Analysis of PQ-Induced Toxicity on 3.1.1tive Effects of PQ 0 cells were HL-60 Cells . Antiprolifera To examine the cytotoxicity of PQ, HL-6 treated with various concentrations of PQ for 48 hours and cell viability was assayed by trypan blue exclusion (Figure 1). PQ exerted a significant effect on the prolif- eration of HL-60 cells in a time-dependent manner dur- ing which no significan t changes were observed until 16 hours while pronounced effects on inhibiting the cells proliferation occurred after 16 hours (Figur e 1(a) ); PQ at concentration below 5μM exerted insignificant effects on the proliferation of HL-60 cells while the prolifera- tion of HL-60 cells was arrested upon treatmen t with PQ at concentration above 100μM. At concentrations ranging from 5μM to 100μM, PQ decreased HL-60 cells viability in a concentration-dependent manner (Figure 1(b)). (a) (b) Figure 1. Profile of antiprolifereffects of P Q on HL-60 cells. ative Cells in culture dishes were treated with various concentrations of PQ for 48 hours and cells viability was measured by trypan blue exclusion in ranges of both 24 hours and 48 hour s. (a) Profile of time-course proliferation of HL-60 cells by treatment with the various concentrations of PQ. (b) Comparison of antiproliferative effects of each concentration of PQ. **p<0.01 Vs 24 h control and ## p<0.01,### p<0.001 Vs 48h control. 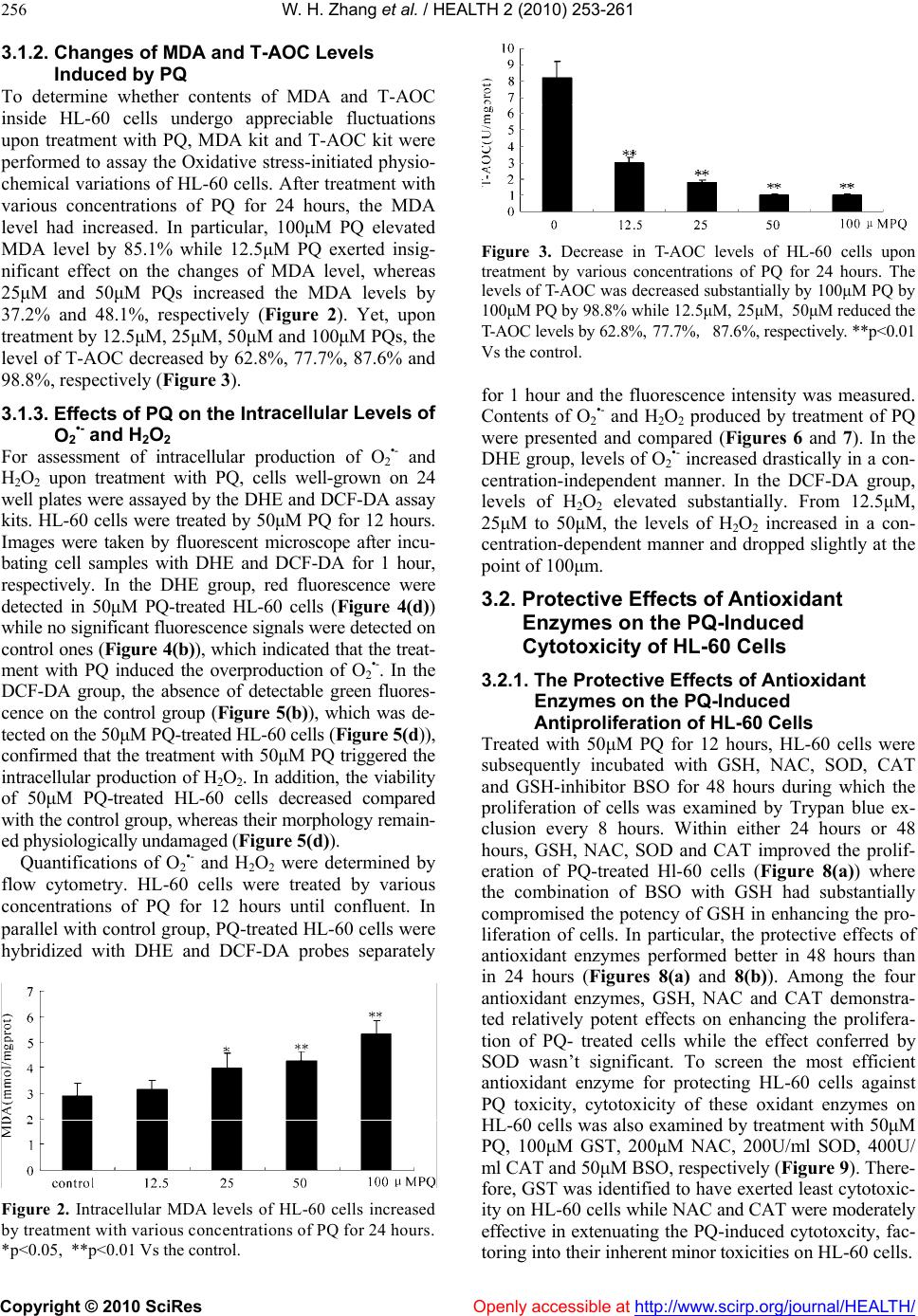 W. H. Zhang et al. / HEALTH 2 (2010) 253-261 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 256 C Levels To detontents of MDA and T-AOC tracellular Levels of For asacellular production of O2 and ined by flo 3.1.2. Changes of MDA and T-AO Induced by PQ ermine whether c inside HL-60 cells undergo appreciable fluctuations upon treatment with PQ, MDA kit and T-AOC kit were performed to assay the Oxidative stress-in itiated physio- chemical variations of HL-60 cells. After treatment with various concentrations of PQ for 24 hours, the MDA level had increased. In particular, 100μM PQ elevated MDA level by 85.1% while 12.5μM PQ exerted insig- nificant effect on the changes of MDA level, whereas 25μM and 50μM PQs increased the MDA levels by 37.2% and 48.1%, respectively (Figure 2). Yet, upon treatment by 12.5μM, 25μM, 50μM and 100μM PQs, the level of T-AOC decreased by 62.8%, 77.7%, 87.6% and 98.8%, respectively (Figure 3). 3.1.3. Effects of PQ on the In O2•- and H2O2 sessment of intr•- H2O2 upon treatment with PQ, cells well-grown on 24 well plates were assayed by the DHE and DCF-DA assay kits. HL-60 cells were treated by 50μM PQ for 12 hours. Images were taken by fluorescent microscope after incu- bating cell samples with DHE and DCF-DA for 1 hour, respectively. In the DHE group, red fluorescence were detected in 50μM PQ-treated HL-60 cells (Figure 4(d)) while no significant fluorescence signals were detected on control ones (Figure 4(b)), which indicated that the treat- ment with PQ induced the overproduction of O2•-. In the DCF-DA group, the absence of detectable green fluores- cence on the control group (Figure 5(b)), which was de- tected on the 50μM PQ-treated HL-60 cells (Figure 5(d)), confirmed that the treatmen t with 50μM PQ triggered the intracellular production of H2O2. In addition, the viability of 50μM PQ-treated HL-60 cells decreased compared with the control group, whereas their morphology remain- ed physiologicall y undam aged (Figure 5(d)). Quantifications of O2•- and H2O2 were determ w cytometry. HL-60 cells were treated by various concentrations of PQ for 12 hours until confluent. In parallel with control group, PQ-treated HL-60 cells were hybridized with DHE and DCF-DA probes separately Figure 2. Intracellular MDA levels of HL-60 cells increased by treatment with various concentrations of PQ for 24 hours. *p<0.05, **p<0.01 Vs the control. Figure 3. Decrease in T-AOC levels of HL-60 cells upon r 1 hour and the fluorescence intensity was measured. s of Antioxidant 3.2.1oxidant Treate cells were treatment by various concentrations of PQ for 24 hours. The levels of T-AOC was decreased substantially by 100μM PQ by 100μM PQ by 98.8% while 12.5μM, 25μM, 50μM reduced the T-AOC levels by 62.8%, 77.7%, 87.6%, respectively. **p<0.01 Vs the control. fo Contents of O2•- and H2O2 produced by treatment of PQ were presented and compared (Figures 6 and 7). In the DHE group, lev els of O2•- increased drastically in a co n- centration-independent manner. In the DCF-DA group, levels of H2O2 elevated substantially. From 12.5μM, 25μM to 50μM, the levels of H2O2 increased in a con- centration-dependent manner and dropped slightly at the point of 100μm. 3.2. Protective Effect Enzymes on the PQ-Induced Cytotoxicity of HL-60 Cells . The Protective Effects of An ti Enzymes on the PQ-Induced Antiproliferation of HL-60 Cells d with 50μM PQ for 12 hours, HL-60 subsequently incubated with GSH, NAC, SOD, CAT and GSH-inhibitor BSO for 48 hours during which the proliferation of cells was examined by Trypan blue ex- clusion every 8 hours. Within either 24 hours or 48 hours, GSH, NAC, SOD and CAT improved the prolif- eration of PQ-treated Hl-60 cells (Figure 8(a)) where the combination of BSO with GSH had substantially compromised the potency of GSH in enhancing the pro- liferation of cells. In particular, the protective effects of antioxidant enzymes performed better in 48 hours than in 24 hours (Figures 8(a) and 8(b)). Among the four antioxidant enzymes, GSH, NAC and CAT demonstra- ted relatively potent effects on enhancing the prolifera- tion of PQ- treated cells while the effect conferred by SOD wasn’t significant. To screen the most efficient antioxidant enzyme for protecting HL-60 cells against PQ toxicity, cytotoxicity of these oxidant enzymes on HL-60 cells was also examined by treatment with 50μM PQ, 100μM GST, 200μM NAC, 200U/ml SOD, 400U/ ml CAT and 50μM BSO, respectively (Figure 9). There- fore, GST was identified to have exerted least cytotoxic- ity o n HL - 6 0 ce ll s while NAC and CAT were moderately effective in extenuating the PQ-induced cytotoxcity, fac- toring into their inherent minor toxicities on HL-60 cells. 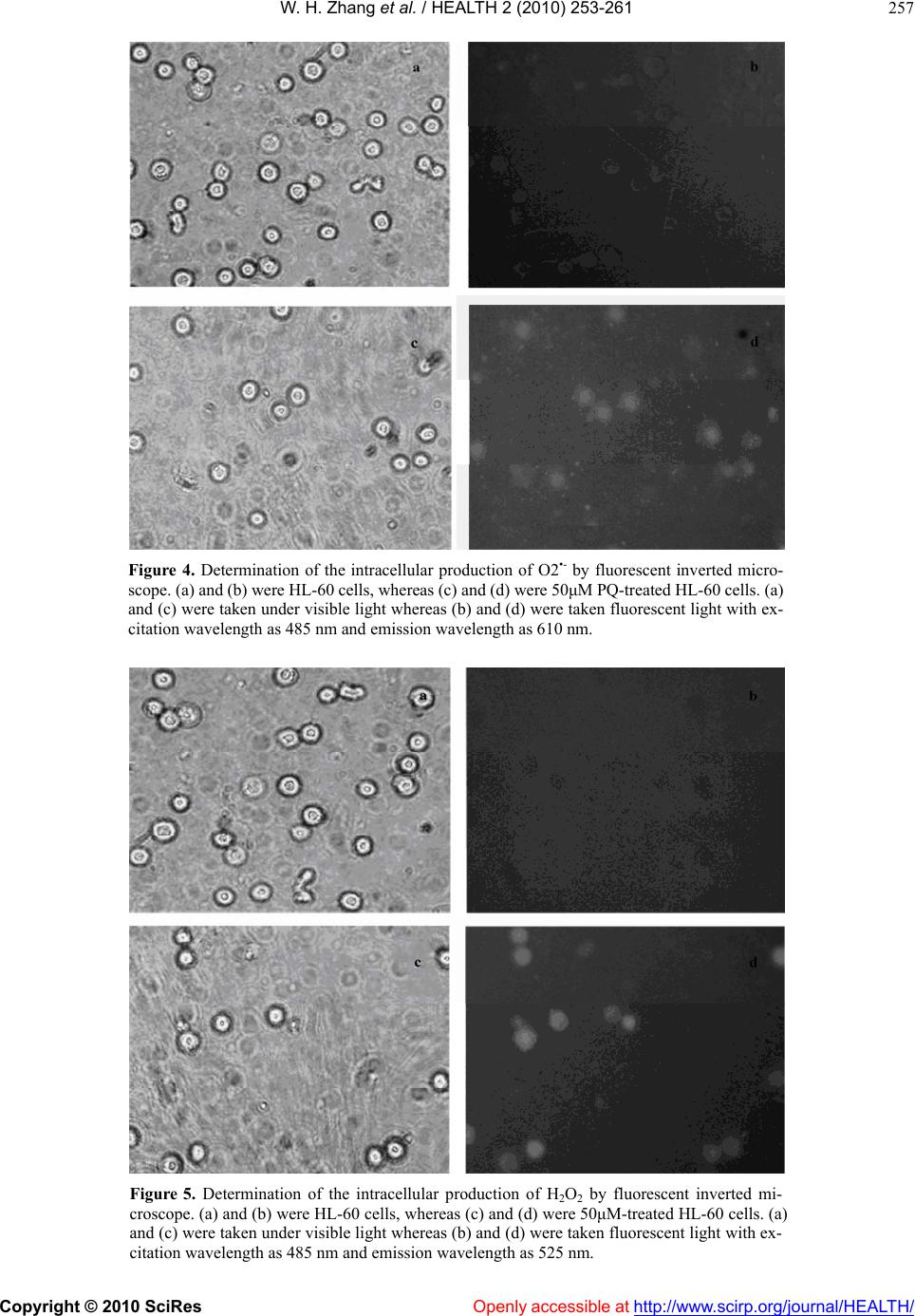 W. H. Zhang et al. / HEALTH 2 (2010) 253-261 Copyright © 2010 SciRes http://www.scirp.org/journal/HEALTH/Openly accessible at 257 Figure 4. Determination of the intracellular production of O2•- by fluorescent inverted micro- scope. (a) and (b) were HL-60 cells, whereas (c) and (d) were 50μM PQ-treated H L-60 cells. (a) and (c) were taken under visible light whereas (b) and (d) were taken fluorescent light with ex- citation wavelength as 485 nm and emission wavelength as 610 nm. Figure 5. Determination of the intracellular production of H2O2 by fluorescent inverted mi- croscope. (a) and (b) were HL-60 cells, whereas (c) and (d) were 50μM-treated HL-60 cells. (a) and (c) were taken under visible light whereas (b) and (d) were taken fluorescent light with ex- citation wavelength as 485 nm and emission wavelength as 525 nm. 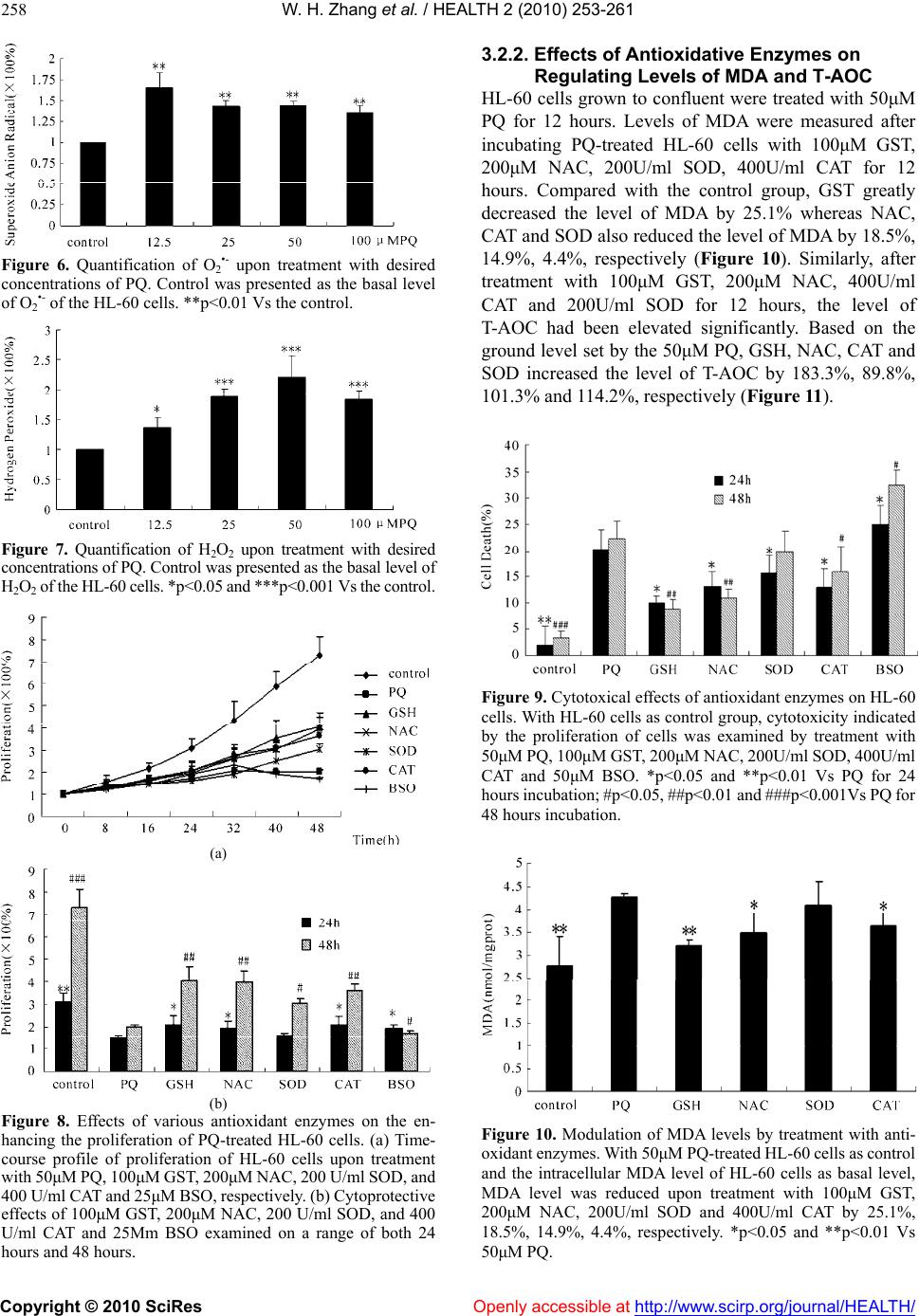 W. H. Zhang et al. / HEALTH 2 (2010) 253-261 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 258 Figure 6. Quantification of O2•- upon treatment with desired concentrations of PQ. Control was presented as the basal level of O2•- of the HL-60 cells. **p<0.01 Vs the control. Figure 7. Quantification of H2O2 upon treatment with desired concentrations of PQ. Control was presented as the basal level of H2O2 of the HL-60 cells. *p<0.05 and ***p<0.001 Vs the control. (a) (b) Figure 8. Effects of variousoxidant enzymes on the en- f Antioxidative Enzymes on 50μM anti hancing the proliferation of PQ-treated HL-60 cells. (a) Time- course profile of proliferation of HL-60 cells upon treatment with 50μM PQ, 100μM GST, 200μM NAC, 200 U/ml SOD, and 400 U/ml CAT and 25μM BSO, r espectivel y. (b) Cytoprotectiv e effects of 100μM GST, 200μM NAC, 200 U/ml SOD, and 400 U/ml CAT and 25Μm BSO examined on a range of both 24 hours and 48 hours. Regulating 3.2.2. Effects o Levels of MDA an d T- AOC HL-60 cells grown to confluent were treated with r 12 hours. Levels of MDA were measured PQ foafter incubating PQ-treated HL-60 cells with 100μM GST, 200μM NAC, 200U/ml SOD, 400U/ml CAT for 12 hours. Compared with the control group, GST greatly decreased the level of MDA by 25.1% whereas NAC, CAT and SOD also reduced the level of MDA by 18.5%, 14.9%, 4.4%, respectively (Figure 10). Similarly, after treatment with 100μM GST, 200μM NAC, 400U/ml CAT and 200U/ml SOD for 12 hours, the level of T-AOC had been elevated significantly. Based on the ground level set by the 50μM PQ, GSH, NAC, CAT and SOD increased the level of T-AOC by 183.3%, 89.8%, 101.3% and 114.2%, respectively (Figure 11). Figure 9. Cytotoxical effects of antioxidant enzymes on HL-60 cells. With HL-60 cells as control group, cytotoxicity indicated by the proliferation of cells was examined by treatment with 50μM PQ, 100μM GST, 200μM NAC, 200U/ml SOD, 400U/ml CAT and 50μM BSO. *p<0.05 and **p<0.01 Vs PQ for 24 hours incubation; #p<0.05 , ##p<0.01 and ###p<0.001Vs PQ for 48 hours incubation. Figure 10. Modulation of MDA levels by treatment with anti- oxidant enzymes. With 50μM PQ-treated HL-60 cells as control and the intracellular MDA level of HL-60 cells as basal level, MDA level was reduced upon treatment with 100μM GST, 200μM NAC, 200U/ml SOD and 400U/ml CAT by 25.1%, 18.5%, 14.9%, 4.4%, respectively. *p<0.05 and **p<0.01 Vs 50μM PQ.  W. H. Zhang et al. / HEALTH 2 (2010) 253-261 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 259 Figure 11. Increase of T-AOC levels upon treatment with an- tioxidant enzymes. With 50μM PQ-treated HL-60 cells as con- ented to be able to cause a range of progressive and irreversible diseases to animal species PQ avenging overproduced am lls w can be determined for the in- volvement of oxidative damage in the PQ-induced cyto- trol and the intracellular T-AOC level of HL-60 cells as basal level, T-AOC levels were elevated by treatment with 100μM GST, 200μM NAC, 200U/ml SOD and 400U/ml CAT by 183.3%, 114.2%, 89.8% and 101.3%, respectiv ely. *p<0.05 and **p<0.01 Vs 50μM PQ. 4. DISCUSSION PQ has been well-docum and humans which are refractory to treatment [38-42]. As it used worldwide in agriculture, the toxicities of PQ on its untargeted organism are supposed to be well-administered. A wide spectrum of investigations pivoting on the treat- ment of PQ-induced diseases on humans have been con- ducting to either quantify the safe dosage [43,44], or to clinically monitor the long term evolution of PQ-caused disease [45]. Although its pathological mechanism re- mains mainly elusive, the contribution of oxidative stress in the pathogenic process has been evidenced [32]. How- ever, the inadequacy to elucidate the oxidative stress- initiated pathway at the tissue level with different animal models necessitates relevant studies of that pathway at the cellular level. Toward this end, the PQ-induced injury mode l of H L60- cells w as es tab lished to stu dy th e ROS- mediated cytotoxicit y on HL-60 cel ls. Antiproliferative effects of PQ on HL-60 cells and pro- liferation-enhancing effects of antioxidant enzymes on -treated HL-60 cells were examined by Trypan blue exclusion assaying, respectively. The threshold of PQ concen tration w ith phys iologica l relevan ce was dete rmined with finding that PQ concentration below 5μM conferred no pronounced effect on the cell growth and proliferation while PQ concentration above 200μM led directly to the death of all HL-60 cells. With concentration ranging from 12.5μM to 100μM, the antiproliferative effect of PQ was observed to be concentration-dependent, which was in agreement with a recent finding that production of hydro- gen peroxide may contribute to the induction of apoptosis on HL-60 cells [46]. Subsequently, 50μM PQ was ex- perimentally chosen to treat HL-60 cells for studying other physiochemical rele vance. Indicative of the ROS-induced damage to cellular components and ability of sc ounts of ROS, MDA and T-AOC were examined quantitatively to determine the cytotoxicity of PQ on HL-60 cells, respectively. Relatively high concentrations of PQ (50-100μM) caused an appreciable increase in the MDA level while the T-AOC level dropped accordingly in a concentration-dependent manner. It is generally con- sistent with the finding that various con cen tration s of PQ increased peroxidation in PC12 cells [24]. Thus, the in- volvement of imbalance between the oxidative compe- tence and antioxidant capacity was determined in the PQ-induced cytotoxicity. Furthermore, the intracellular production of O2•- and H2O2 was confirmed by fluores- cent inverted microscope through hybridizing cell sam- ples with DHE and DCF-DA probes and quantified by employment of flow cytometric analysis. Determination of the intracellular production by treatment with various concentrations of PQ suggested that production of O2•- wasn’t sensitive to the concentration s of PQ, whereas the intracellular production of H2O2 was in a concentra- tion-dependent manner. That finding was in part consis- tent with the report that PQ (0.01-1mM) induced the intracellular burst of ROS in rat cortical neurons [47]. In our study, effects of GSH, NAC, SOD and CAT on extenuating PQ-induced cytotoxicity on HL-60 ce ere further examined, following the treatment with 50μM PQ. In explicit agreement with the finding that levels of antioxidant enzymes increased in inverse pro- portion to the levels of ROS [48,49]. Among 100μM GST, 200μM NAC, 400U/ml CAT and 200U/ml SOD which were experimentally administered to examine their potency, 100μM GSH was determined to reduce the MDA level by 25.1% and increase the T-AOC lev el sig- nificantly by 183.3%. 200μM NAC functioned well with its performance on reducing MDA level and enhancing T-AOC level by 18.5% and 89.8%, respectively. As a parallel, BSO, which was the inhibitor of GSH, was also used to exclude the false positive results of the efficacy conferred by antioxidant enzymes. Hopefully, 100μM GSH was identified as the most effective antioxidant enzymes for attenuating the PQ-induced cytotoxicity, in combination with examining the inherent cytotox icity of all antioxidant enzymes on the HL-60 cells. 5. CONCLUSIONS In this study, a causal role toxic effects on HL-60 cells. Upon treatment with various concentrations of PQ, the compromised capacity of HL-60 cells to scavenge the intracellular overproduced O2•- and H2O2 was responsible for the cytotoxicity of HL-60 cells on grounds that the levels of O2•-, H2O2, MDA, T-AOC were altered unfavorably in the physiological level. Fur-  W. H. Zhang et al. / HEALTH 2 (2010) 253-261 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 260 MENTS a and Mr. Ximing Xu ssion. This work was REFERENCES ligott, T.F. and Hurst, E.W. (1966) at. British Journal of Industrial Medi- ccumulation: Tissue and species specificity. t into rat lung. , G. (1998) Intrahippocampal in- esses of nervous system development: A Martin, A., Moran, J.M., Garcia-Rubio, L., Fran- Catalytic metallopor- al manufacturing and -pharmaco-redox ed com- mbrane dysfunction in pul- ed oxidative stress and dysfunction ed free radicals in Paraquat-treated hemical Research, 23, 1387- thermore, effects of antioxidant enzymes on attenuating the PQ-induced cytotoxicity of HL-60 cells were also examined to have identified the GSH as the most effective one for extenuating the PQ-induced cytotoxicity. How- ever, whether it could be used as an ingredient in devel- oping PQ safener remains to be settled. Moreover, further characterizing the underpinning mechanisms involved in PQ cytotoxicity might contribute substantially to a com- prehensive understanding of how environmental toxicants including pesticides and herbicides lead to the increas- ingly more frequent outbreaks of a plethora of environ- ment-a sso ciat ed di sea se s. 6. ACKNOWLEDGE The authors thank their colleagues, Mr. Peng Ji for their technical assistance and results discu financially supported by the Science and Technology Support Projects of Gansu Province (No.2007GS01641) and the State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Insti- tute, Chinese Academy of Agricultural Sciences. [1] Clark, D.G., McEl toxicity of ParaquThe cine, 23, 126-132. [2] Bullivant, C.M. (1966) Accidental poisoning by Paraquat : Report of two cases in man. British Medical Journal, 1, 1272-1273. [3] Somayajulu-Nitu, M., Sandhu, J.K., Cohen, J., Sikorska, M., Sridhar, T.S., Matei, A., Borowy-Borowski, H. and Pandey, S. (2009) Paraquat induces oxidative stress, neu- ronal loss in substantia nigra region and Parkinsonism in adult rats: Neuroprotection and amelioration of s ymptoms by water-soluble formulation of Coenzyme Q10. BMC Neuroscience, 10, 88. [4] Roberts, T.R., Dyson, J.S. and Lane, M.C. (2002) Deacti- vation of the biological activity of Paraquat in the soil en- vironment: A review of long-term environmental fate. Journal of Agricultural and Food Chemistry, 50, 3623- 3631. [5] Onyon, L.J. and Volans, G.N. (1987) The epidemiology and prevention of Paraquat poisoning. Hum Toxicol, 6, 19-29. [6] Bismuth, C., Garnier, R., Baud, F.J., Muszynski, J. and Keyes, C. (1990) Paraquat poisoning: An overview of the current status. Drug Safety, 5, 243-251. [7] Botella de Maglia, J. and Belenguer Tarin, J.E. (2000) Paraquat poisoning: A study of 29 cases and evaluation of the effectiveness of the “Caribbean scheme”. Medicina Clinica (Barc), 115, 530-533. [8] Luo, Z.Y. (2007) Treatment 16 cases of acute Paraquat poisoning. Chinese Journal of Labour Hygiene and Oc- cupational Diseases, 25, 330. [9] Zhao, F.L., Wang, J. and Guo, W. (2009) Clinical obser- vation on 22 cases of acute Paraquat poisoning. Chinese Journal of Labour Hygiene and Occupational Diseases, 27, 56-57. [10] Rose, M.S., Lock, E.A., Smith, L.L. and Wyatt, I. (1976) Paraquat a Biochemical Pharmacology, 25, 419-423. [11] Rose, M.S., Smith, L.L. and Wyatt, I. (1974) Evidence for energy-dependent accumulation of Paraqua Nature, 252, 314-315. [12] Melchiorri, D. , Del Duca, C., Picc irilli, S. , Trombetta, G., Bagetta, G. and Nistico jection of Paraquat produces apoptotic cell death which is prevented by the lazaroid U74389G, in rats. Life Scien ces, 62, 1927-1932. [13] Barone, S., Das, K.P. , Lassiter , T.L. and White, L.D. (2000) Vulnerable proc review of markers and methods. Neurotoxicology, 21, 15-36. [14] Gonzalez-Polo, R.A., Niso-Santano, M., Ortiz-Ortiz, M.A., Gomez- cisco-Morcillo, J., Zaragoza, C., Soler, G. and Fuentes, J.M. (2007) Relationship between autophagy and apoptotic cell death in human neuroblastoma cells treated with Paraquat: Could autophagy be a “brake” in Paraquat-induced apop- totic death? Autophagy, 3, 366-367. [15] Chen, P., Chen, Z., Li, A., Lou, X.C., W u, X.K., Zh ao, C.J., Wang, S.L. and Liang, L.P. (2008) phyrin protects against Paraquat neurotoxicity in vivo. Biomedical and Environmental Sciences, 21, 233-238. [16] Drechsel, D.A. and Patel, M. (2008) Role of reactive oxygen species in the neurotoxicity of environment agents implicated in Parkinson’s disease. Free Radical Biology & Medicine, 44, 1873-1886. [17] Kuo, M.L. and Lin, J.K. (1993) The genotoxicity of the waste water discharged from Paraquat its pyridyl components. Mutation Resear ch, 300, 223-229. [18] Takeyama, N., Tanaka, T., Yabuki, T. and Nakatani, T. (2004) The involvement of p53 in Paraquat-induced apoptosis in human lung epithelial-like cells. Interna- tional Journal of Toxicology, 23, 33-40. [19] Haddad, J.J. (2004) Redox and oxidant-mediated regulation of apoptosis signaling pathways: Immu no conception of oxidative siege versus cell death commitment. International Immunopharmacology, 4, 475-493. [20] Farrington, J.A., Ebert, M., Land, E.J. and Fletcher, K. (1973) Bipyridylium quaternary salts and relat pounds V. Pulse radiolysis studies of the reaction of Paraquat radical with oxygen: Implications for the mode of action of bipyridyl herbicides. Biochimica et Bio- physica Acta, 314, 372-381. [21] Tsukamoto, M., Tampo, Y., Sawada, M. and Yonaha, M. (2000) Paraquat-induced me monary microvascular endothelial cells. Pharmacol Toxicol, 86, 102-109. [22] Tsukamoto, M., Tampo, Y., Sawada, M. and Yonaha, M. (2002) Paraquat-induc of the glutathione redox cycle in pulmonary microvascu- lar endothelial cells. Toxicology and Applied Pharma- cology, 178, 82-92. [23] Zang, L.Y., van Kuijk, F.J. and Misra, H.P. (1995) EPR studies of spin-trapp lung microsomes. Biochemistry & Molecular Biology International, 37, 255-262. [24] Yang, W.L. and Sun, A.Y. (1998) Paraquat-induced cell death in PC12 cells. Neuroc 1394.  W. H. Zhang et al. / HEALTH 2 (2010) 253-261 Copyright © 2010 SciRes http://www.scirp.org/journal/HEALTH/Openly accessible at 261 n in heart mitochondria exposed to ischaemia and g brain. 49-671. Implications for therapeutics of neurode- Quarterly, 3, 1-6. in- re of n(II)-induced apopto sis in duced cell viability loss .P., Kimura, S., Yukimura, T., Kiyomoto, c acid eard, B. (1970) Paraquat poisoning , B. and Sun, X.L. (2008) Treatment of Paraquat raquat concentrations athna, L., Eddleston, M., W ilks, M .F., Woollen, B.H., ang, J.O., Lee, E.Y. artolomeo, A. E., Tran-Thi, Q.H., Kahl, R. and , J.M., Perez-Gomez , C., Rosado, R., 000) [25] Solaini, G. and Harris, D.A. (2005) Biochemical dys- functio reperfusion. Biochemical Journal, 390, 377-394. [26] Blomgren, K. and Hagberg, H. (2006) Free radicals, mi- tochondria, and hypoxia-ischemia in the dev elopin Free Radical Biology & Medicine, 40, 388-397. [27] Dringen, R. (2000) Metabolism and functions of glu- tathione in brain. Progress in Neurobiology, 62, 6 [28] Dringen, R. (2005) Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal, 7, 1223-1233. [29] Floyd, R.A. and Hensley, K. (2002) Oxidative stress in brain aging: generative diseases. Neurobiology of Aging, 23, 795-807. [30] Frasca, S., Couvreur , P., Seiller , M ., Pareau, D., Lacour , B., Stambouli, M. and Grossiord, J.L. (2009) Paraquat de- toxication with multiple emulsions. International Journal of Pharmaceutics, 380, 142-146. [31] Hays, P. G. (1995) VIP interview: Patrick G. Hays. Interview by Maria R. Traska. Managed Car e [32] LeBel, C.P., Ischiropoulos, H. and Bondy, S.C. (1992) Evaluation of the probe 2’, 7’-dichlorofl uorescin as an dicator of reactive oxygen species formation and oxidative stress. Chemical Resear ch in Toxicology, 5, 227-231. [33] Benov, L., Sztejnberg, L. and Fridovich, I. (1998) Critical evaluation of the use of hydroethidine as a measu superoxide anion radical. Free Radical Biology & Medi- cine, 25, 826-831. [34] Oubrahim, H., Stadtman, E.R. and Chock, P.B. (2001) Mitochondria play no roles in M HeLa cells. Proceedings of the National Academy of Sciences USA, 98, 9505-9510. [35] Lee, C.S., Han, E.S. and Lee, W.B. (2003) Antioxidant effect of phe nelzine on MPP+-inin S differentiated PC12 cells. Neurochemical Research, 28, 1833-1841. [36] Miyata, K., Rahman, M., Shokoji, T., Nagai, Y., Zhang, G.X., Sun, GH., O Kohno, M., Abe, Y. and Nishiyama, A. (2005) Aldosterone stimulates reactive oxygen species production through ac- tivation of NADPH oxidase in ra t mesangial cells. Journal of American Society of Nephrology, 16, 2906-2912. [37] Ohkawa, H., Ohishi, N. and Yagi, K. (1979) Assay for lipid peroxides in animal tissues by thiobarbituri reaction. Analytical Biochemistry, 95, 351-358. [38] Matthew, H., Logan, A., Woodruff, M.F. and H (1968) Paraquat poisoning-lung transplantation. British Medical Journal, 3, 759-763. [39] McDonagh, B.J. and Martin, J. in children. Ar chives of Disease in Childhood, 45, 425-427. [40] Wesseling, C., Hogstedt, C., Picado, A. and Johansson, L. (1997) Unintentional fatal Paraquat poisonings among agricultural workers in Costa Rica: Report of 15 cases. American Journal of Industrial Medicine, 32, 433-441. [41] Hantson, P. (2008) Paraquat poisoning. Rev Prat, 58 866-867. [42] Zheng, X. poisoning. Chinese Journal of Labour Hygiene and Oc- cupational Diseases, 26, 635-637. [43] Ikebuchi, J. (1987) Evaluation of Pa in Paraquat poisoning. Archives of Toxicology, 60, 304- 310. [44] Senar Tomenson, J.A., Roberts, D.M. and Buckley, N.A. (2009) Prediction of outcome after Paraquat poisoning by meas- urement of the plasma Paraquat concentration. Oxford Journal of Medicine, 102, 251-259. [45] Lee, K.H., Gil, H.W., Kim, Y.T., Y and Hong, S.Y. (2009) Marked recovery from Paraquat- induced lung injury during long-term follow-up. Korean Journal of Internal Medicine, 24, 95-100. [46] Fabiani, R., Fu ccelli, R., Pi eravanti, F., De B and Morozzi, G. (2009) Production of hydrogen peroxide is responsible for the induction of apoptosis by hy- droxytyrosol on HL60 cells. Molecular Nutrition & Food Research, 53, 887-896. [47] Schmuck, G., Rohrdanz, chluter, G. (2002) Oxidative str ess in rat cor tical neu rons and astrocytes in duced by P araqu at in v itro . Neurotoxicity Research, 4, 1-13. [48] Mates, J.M., Se gura lalla, L., Blanca, M. and Sanchez-Jimenez, F.M. (1999) Antioxidant enzymatic activities in human blood cells after an allergic reaction to pollen or house dust mite. Blood Cells, Molecules, and Diseases, 25, 103-109. [49] Mates, J.M., Perez-Gomez, C. and Blanca, M. (2 Chemical and biological activity of free radical ‘scaven- gers’ in aller gic diseases. Clini ca Chimica Acta, 296, 1-15.
|