Health
Vol.10 No.10(2018), Article ID:88251,10 pages
10.4236/health.2018.1010111
The Level of CD4+ T Cell Count among Reproductive Age Women Coinfected with Human Immune Virus, Hepatitis Surface Antigen and Herpes Simplex Virus in Kogi State, Nigeria
Babatunde Ishola Gabriel Adejumo1*, Francis Enifo Oronsaye1, Uteno Itanyi Drisu2, Margaret Oyarazi Adebowale3, Ojo Moses Oke4, Uchechukwu Dimkpa5, Kingsley Ifeanyichukwu Omosor6, Oladimeji Nasiru Abdulrahman7, Esmond Nwanbunneze Ukatu8, Emmanuel Alaba Michael9
1Medical Laboratory Science Department, University of Benin, Benin City, Nigeria
2Department of Medical Services, Federal Polytechnic, Idah, Nigeria
3Medical Laboratory Science Department, Federal School of Medical Laboratory Sciences and Health Technology, Jos, Nigeria
4Medical Laboratory Science Department, School of Health Sciences and Technology, Akure, Nigeria
5Physiology Department, Nnewi Campus, Nnamdi Azikiwe University, Awka, Nigeria
6Shalom Diagnostic Medical Laboratory, Warri, Nigeria
7Medical Laboratory Science Department, College of Health Technology, Offa, Nigeria
8Gulin Pharmaceuticals Nigeria Limited, Sango Ottaa, Nigeria
9Medical Laboratory Science Department, College of Health Sciences and Technology, Idah, Nigeria

Copyright © 2018 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: August 9, 2018; Accepted: October 28, 2018; Published: October 31, 2018
ABSTRACT
Background: There are pockets of evidence to show the existence of co-infections of viral particles in humans. Aim: The study aimed at evaluating the CD4+ T cell count among women of reproductive age co-infected with human immune virus, hepatitis surface antigen and herpes simplex virusin Kogi state, Nigeria. Methodology: 342 females of reproductive age within the ages of 15 - 49 years participated in this study. They were recruited from various local government areas of three Senatorial districts of Kogi state. Blood samples were collected from participants and analyzed for HSV1/HSV2, HIV, HBsAg and CD4 using different scientific methods and procedures. Results: There was no significant differences in mean CD4+ T cell counts between subjects who tested positive and those who tested negative for only HSV1 (p = 0.61), HSV2 (p = 0.95), HIV (p = 0.48) and co-infection for HSV1, HSV2, HIV (0.68). In contrast, mean CD4+ T cell count was significantly higher in those who tested positive compared with those who tested negative for HBsAg alone (p = 0.03) and those co-infected with HSV1, HSV2, HBsAg (p = 0.01). Analysis of variance (ANOVA) indicated no significant differences in CD4+ T cell counts among the different classes of infections. Conclusion: This study shows no decline in the count of CD4+ T cell on the co-infections of HSV1, HSV2 and HIV, but higher significant difference in those co-infected with HSV1, HSV2 and HBsAg was recorded among the women of child bearing age in Kogi state.
Keywords:
HSV1/HSV2, CD4, HIV, HBsAg Coinfections, Women, Kogi State, Nigeria

1. Introduction
HSV1 and HSV2 infections are common among HIV1 positive individuals, with prevalence that is equal or more than those in the general population. In recent years, many studies have examined the prevalence of HSV2 among HIV1 infected individuals, finding seroprevalences of 50% - 90% in some populations, significantly higher than those negative to HIV1 [1] . Sharing of sexual transmission route between the 2 viruses may explain this finding to increased plasma HIV load [2] [3] . However, relationship between HSV2 seropositivity and longitudinal measurement of HIV1 disease progression has been quantified by few studies.
Shedding of oral or genital secretions of HSV occurs in HSV2-infected individuals, regardless of HIV1serostatus, and most of the shedding is asymptomatic. Prospective studies have also shown that both HSV1 and HSV2 infected individuals shed oral and genital HSV which occurs more frequently among those who are infected with HIV1 than among HSV-infected/HIV1-negative persons [4] [5] [6] .
HSV mucosal shedding occurs more frequently among HIV1-positive persons; the higher HSV shedding quantity occurs among those with lower CD4+ T cell counts [5] [7] [8] . Worthy of note are people with intermediate or high CD4+ T cell counts who may also shed HSV2 frequently, though there is substantial variability in how often is HSV2 shed and quantity among individuals [8] .
Co-infections of hepatitis B virus (HBV) and HIV is common; 70% - 90% of HIV-positive individuals have one time or the other been infected with HBV in the United States [9] [10] . 5% - 10% of HIV-infected individuals who are exposed to HBV have chronic infection, a rate 10 times higher than that of the general population [11] [12] . HIV/HBV co-infection rates are highest among homosexuals and injection drug users in the United States. In contrast, in Asia and sub-Saharan Africa, the modes of transmissions are vertical and early childhood exposure respectively, and overall HBV prevalence is higher; the prevalence of HBV among HIV-infected individuals also is higher, at an estimated 20% - 30% [13] [14] .
Opportunistic infections and reactivation of latent pathogens occurs when HIV1 compromises human immunity mainly by destroying CD4+ T cells. Many infectious pathogens including HBsAg, HSV share a common route of transmission with HIV. The HSV2 pandemic is further reinforced by the HIV pandemic and vice versa; and it is becoming clearer that effective control of spread of HIV will only yield a positive result if control of HSV2 infection along with other sexually transmitted diseases (STD) is integrated [15] [16] .
To the best of our knowledge, there is no documentary evidence of co-infections of HIV, HBsAg and HSV (either type 1 (HSV1) or type 2 (HSV2) or combined (HSV1/HSV2) in Nigeria. The present study therefore was aimed at evaluating the level of CD4+ T cell count in co-infections of HSV, HBsAg and HIV among women of reproductive age in Kogi state of Nigeria.
2. Methodology
2.1. Study Population
Three hundred and thirty (330) blood samples were obtained with informed consent from adults aged between 15 - 49 years that accepted a voluntary counseling before testing. Women who have attained the reproductive age (≥18 years) and who were without known previous HBV, HIV or HbsAg status were eligible for the study. Participants who have been vaccinated against HBsAg were excluded from this study (n = 12). One hundred and ten (110) samples each from the three Senatorial districts of Kogi state were collected. The Ethics committee of Ministry of Health, Kogi state approved this study. A well-structured questionnaire was administered to every participant of this study.
2.2. Sample Collection and Processing
An aliquot of 10mls venous blood obtained from each subject by peripheral venepuncture, 5 millilitres of the blood was emptied carefully into an ethylenediaminetetra-aceticacid (EDTA) container immediately for the estimation of CD4+ T cell, while the remaining 5 mls was emptied into a dry sterile plain bottle carefully to avoid lysis. The sample was allowed to clot and spun using a bench centrifuge at 3000 rpm at room temperature for 10 minutes. The serum sample was then separated from the cells within 1 hour into a clean sterile tube and stored at −20˚C prior to the analysis of HSV1/HSV2, HIV and HBsAg antibodies respectively.
2.3. Sample Analysis
The following investigations were carried out on the sample to ascertain the status of the participants.
2.4. HBsAg
Hepatitis B surface antigen of the participants was determined with MonolisaAgHBsPlus ELISA kits from BIO RAD according to the manufacturers’ instructions.
2.5. HIV1
HIV status of the participants was ascertained using determine, statpak and unigold test kits respectively. The kits were commercially purchased and manufacturers’ instructions were strictly followed.
2.6. CD4+ T Cell Count
The CD4+ T cell was done immediately after sample collection using Flowcytometry (Partec Germany). The blood sample was placed on a roller mixer for 25 minutes for proper mixing. Briefly, 20 µl of CD4+ T cell easy count was pipetted into a partec test tube (CD4+ T cell mAbPE), and 20 µl of EDTA blood sample was added. The mixture was incubated for 15 minutes in the dark at room temperature mixing at intervals. 800 µl of CD4+ T cell easy count was added. The tube was then placed in the Flowcytometry for counting and the CD4+ T cell value was obtained by a programmed computer connected to instrument.
2.7. HSV1/HSV2 Antibody Determinations
Antibody concentrations of all the participants to HSV1/HSV2 were determined using ELISA kits which were commercially purchased from Calbioteck Company, USA by following the procedure given in the kit protocol.
2.8. Data Analysis
Descriptive data was expressed as mean ± standard deviation for continuous variables and percentages for categorical variables. Comparative analysis was done using independent sample t-test and analysis of variance (ANOVA). Test of significance was set at p < 0.05. All statistics were done using SPSS/IBM software, version 20.
3. Results
Demographic information obtained from the participants (Table 1) show that out of the 330 subjects, 257 (77.9%) were young adults and 73 (22.1%) were middle-aged; 130 (39.4%) were married, 200 (60.6%) were single; 19 (5.7) were exposed to sex early (<15 years), while 192 (58.2%) were exposed to sex from 15 years and above.
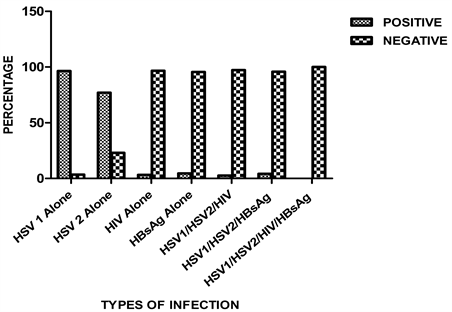
In the overall cohort (n = 330), the incidence of HSV1, HSV2, HIV and hepatitis B infection was 96.4%, 77%, 3.3% and 4.5% respectively (Figure 1). Co-infection for HSV1, HSV2 and HIV was found to occur in 2.7% persons, while co-infection for HSV1, HSV2, and HBsAg was detected in 4.2% of the cohort. There was no incidence of HSV1/HSV2/HIV/HBsAg co-infection.
Table 1. Distribution of the study population according to their demographic characteristics and sexual behaviours.
Figure 1. Seroprevalence of HSV1/HSV2, HIV and HBsAg and co-infections.
Table 2 shows lack of significant differences in mean CD4+ T cell counts between subjects who tested positive and those who tested negative for only HSV1 (p = 0.61), HSV2 (p = 0.95), HIV (p = 0.48) and co-infection for HSV1, HSV2, HIV (0.68). In contrast, mean CD4+ T cell count was significantly higher in those who tested positive for HBsAg alone (p = 0.03) and those co-infected with HSV1, HSV2, HBsAg (p = 0.01) compared with those who tested negative for these infections.
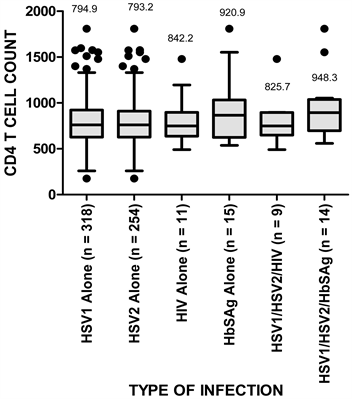
Figure 2 shows boxplot indicating the mean,median, interquartile ranges, minimum and maximum valuesof CD4+ T cell counts of patients with seropositive HSV1, HSV2, HIV and HBsAg infections as well as co-infection for HSV1, HSV2, HIV and HSV1, HSV2, HBsAg. Analysis of variance (ANOVA) indicated no significant (p = 0.088) differences in CD4+ T cell counts among the different classes of infections.
4. Discussion
Pre-existing co-infections, such as Herpes Simplex Virus (HSV1/HSV2), and
Table 2. Mean CD4+ T cell count according to the statuses of the HSV1, HSV2, HIV and HBsAg infections and co-infections.
Figure 2. Boxplot showing the distribution of CD4+ T cell count in participants with seropositive HSV1, HSV2, HIV and HbSAg infections and co-infections. Boxes indicate the median and interquartile ranges. Vertical lines above and below boxes indicate the minimum and maximum values. The numbers above whiskers show mean values. Each outlier is shown by an asterisk.
HBV do not prevent the acquisition of HIV1 infection. Though the clinical course of HIV1 infection may vary and complex depending on the co-infecting agents that are involved. Many studies have suggested an increase in the risk of HSV2 infected individuals in acquiring HIV1 as reviewed by [8] , with recent works suggesting possible molecular mechanism [17] .
In some studies, HIV1 replication has shown to increase in the presence of HSV infection [18] , but in other works, it has shown no relationship [19] . This work therefore examines the coexistence of viral particles in HIV, HBsAg and HSV1/HSV2-infected participants and the level of the CD4+ T cell counts in these co-infections.
In the overall cohort (n = 330), the incidence of HSV1, HSV2, HIV and HBsAg infections was 96.4%, 77%, 3.3% and 4.5% respectively. Co-infection for HSV1, HSV2 and HIV was found to occur in 2.7% persons, while co-infection for HSV1, HSV2, and HBsAg was detected in 4.2% of the cohort. There was no incidence of HSV1/HSV2/HIV/HBsAg co-infection. This is in disagreement with work of Jason et al., 2007 [20] , in which they did not observe any relationship between HIV1/HSV2 co-infections and plasma HIV RNA level overtime in early HIV1 infection. However, a previous study carried out among pregnant women in Ukraine between 2009 and 2013 [21] , reported higher prevalence of herpes simplex virus types 2, but no increased HIV mother to child transmission risk. To the best of our knowledge, there is limited study done on the co-infections of HSV1/HSV2/HBsAg and HSV1/HSV2/HIV1/HBsAg respectively in Nigeria. This may be due to the high level of advocacy and awareness campaign by government on the danger which HIV1 acquisition poses on the populace.
We observed nosignificant differences in mean CD4+ T cell counts between subjects who tested positive and those who tested negative for only HSV1, HSV2, HIV and co-infection for HSV1, HSV2, and HIV. In contrast, mean CD4+ T cell count was significantly higher in those who tested positive for HBsAg alone and those co-infected with HSV1, HSV2, HBsAg compared with those who tested negative for these infections. This agrees with the work of [22] , who observed that herpes simplex virus type-2 co-infection does not accelerate CD4+ T cell count decline in untreated HIV infection. However, HSV1/HSV2/HBsAg co-infection with elevated CD4+ T cell count agrees with the work of Jason et al., 2007 [20] , which demonstrated elevated CD4 cell counts in adults with HIV/HSV2 early infection. We may not be able to categorically state here the exact time the HIV positive participants in this study contacted the infection because the participants were not aware of their infections.
Our study also evaluated the mean CD4+ T cell counts of patients with seropositive HSV1, HSV2, HIV and HBsAg infection as well as co-infection for HSV1, HSV2, HIV and HSV1, HSV2, HBsAg. Analysis of variance (ANOVA) indicated no significant differences in CD4+ T cell counts among the different classes of infections. The complexity of the mechanism by which the human immune system counters other infections such as HIV1 in the presence of HSV2 infection has been previously reported [23] . However, it is thought that HSV2 changes the function and phenotype of monocytes [24] [25] [26] , delaying their maturation, which may in turn change the phenotype and function of interacting CD4+ and CD8+ T cells respectively. HSV2 serves as a ligand for certain TLR molecules [27] , and may mediate the effect on dendritic cells. HSV2 has the capacity to alter innate immune system, with subsequent impacts on the adaptive immune system.
5. Conclusion
The present study suggests that co-infections of viral particles (HSV1, HSV2, HIV and HbSAg) exist among women of reproductive age in Kogi state. The CD4+ T cell count was not affected in HSV1/HSV2/HIV co-infection but appeared altered in HSV1/HSV2/HBsAg co-infection. The unawareness of these viral infections may be attributed to either the slow nature of their progression or their asymptomatic nature or both. High level of orientation and awareness is therefore required from the government to protect this vulnerable group. Furthermore, early sex education among teenagers and continuous sex education should be encouraged among the adults.
Funding
The research was privately funded. No grant or sponsorship from anywhere.
Acknowledgements
We acknowledge the Ministry of Health, Kogi state for ethical approval, and the participants in all the local Government areas of Kogi state.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Adejumo, B.I.G., Oronsaye, F.E., Drisu, U.I., Adebowale, M.O., Oke, O.M., Dimkpa, U., Omosor, K.I., Abdulrahman, O.N., Ukatu, E.N. and Michael, E.A. (2018) The Level of CD4+ T Cell Count among Reproductive Age Women Coinfected with Human Immune Virus, Hepatitis Surface Antigen and Herpes Simplex Virus in Kogi State, Nigeria. Health, 10, 1449-1458. https://doi.org/10.4236/health.2018.1010111
References
- 1. Strick, L.B., Wald, A. and Celum, C. (2006) Management of Herpes Simplex Virus Type 2 Infection in HIV Type 1-Infected Persons. Clinical Infectious Diseases, 43, 347-356. https://doi.org/10.1086/505496
- 2. Gray, R.H., Li, X., Wawer, M.J., et al. (2004) Determinants of HIV1 Load in Subjects with Early and Later HIV Infections, in a General-Population Cohort of Rakai, Uganda. The Journal of Infectious Diseases, 189, 1209-1215. https://doi.org/10.1086/382750
- 3. Mole, L., Ripich, S., Margolis, D. and Holodniy, M. (1997) The Impact of Active Herpes Simplex Virus Infection on Human Immunodeficiency Virus Load. The Journal of Infectious Diseases, 176, 766-770. https://doi.org/10.1086/517297
- 4. Schacker, T., Zeh, J., Hu, H., Shaughnessy, M. and Corey, L. (2002) Changes in Plasma Human Immunodeficiency Virus Type 1 RNA Associated with Herpes Simplex Virus Reactivation and Suppression. The Journal of Infectious Diseases, 186, 1718-1725. https://doi.org/10.1086/345771
- 5. Augenbraun, M., Feldman, J., Chirgwin, K., Zenilman, J., Clarke, L., DeHovitz, J., Landesman, S. and Minkoff, H. (1995) Increased Genital Shedding of Herpes Simplex Virus Type 2 in HIV-Seropositive Women. Annals of Internal Medicine, 123, 845-847. https://doi.org/10.7326/0003-4819-123-11-199512010-00006
- 6. Kim, H.N., Harrington, R.D., Crane, H.M., et al. (2009) Hepatitis B Vaccination in HIV-Infected Adults: Current Evidence, Recommendations and Practical Considerations International Journal of STD & AIDS, 20, 595-600. https://doi.org/10.1258/ijsa.2009.009126
- 7. Wright, P.W., Hoesley, C.J., Squires, K.E., Croom-Rivers, A., Weiss, H.L. and Gnann Jr., J.W. (2003) A Prospective Study of Genital Herpes Simplex Virus Type 2 Infection in Human Immunodeficiency Virus Type 1 (HIV1)—Seropositive Women: Correlations with CD4 Cell Count and Plasma HIV1 RNA Level. Clinical Infectious Diseases, 36, 207-211. https://doi.org/10.1086/345440
- 8. Corey, L., Wald, A., Celum, C.L. and Quinn, T.C. (2004) The Effects of Herpes Simplex Virus-2 on HIV1 Acquisition and Transmission: A Review of Two Overlapping Epidemics. Journal of Acquired Immune Deficiency Syndromes, 35, 435-445. https://doi.org/10.1097/00126334-200404150-00001
- 9. Rodríguez-Méndez, M.L., González-Quintela, A., Aguilera, A., et al. (2000) Prevalence, Patterns, and Course of Past Hepatitis B Virus Infection in Intravenous Drug Users with HIV1 Infection. The American Journal of Gastroenterology, 95, 1316-1322. https://doi.org/10.1111/j.1572-0241.2000.01981.x
- 10. Scharschmidt, B.F., Held, M.J., Hollander, H.H., et al. (1992) Hepatitis B in Patients with HIV Infection: Relationship to AIDS and Patient Survival. Annals of Internal Medicine, 117, 837-838. https://doi.org/10.7326/0003-4819-117-10-837
- 11. Bodsworth, N., Donovan, B. and Nightingale, B.N. (1989) The Effect of Concurrent Human Immunodeficiency Virus Infection on Chronic Hepatitis B: A Study of 150 Homosexual Men. The Journal of Infectious Diseases, 160, 577-582. https://doi.org/10.1093/infdis/160.4.577
- 12. Alter, M.J. (2006) Epidemiology of Viral Hepatitis and HIV Co-Infection. Journal of Hepatology, 44, S6-S9. https://doi.org/10.1016/j.jhep.2005.11.004
- 13. Hoffmann, C.J. and Thio, C.L. (2007) Clinical Implications of HIV and Hepatitis B Co-Infection in Asia and Africa. The Lancet Infectious Diseases, 7, 402-409. https://doi.org/10.1016/S1473-3099(07)70135-4
- 14. Uneke, C.J., Ogbu, O., Inyama, P.U., et al. (2005) Prevalence of Hepatitis-B Surface Antigen among Blood Donors and Human Immunodeficiency Virus-Infected Patients in Jos, Nigeria. Memórias do Instituto Oswaldo Cruz, 100, 13-16. https://doi.org/10.1590/S0074-02762005000100002
- 15. Corey, L. and Handsfield, H.H. (2000) Genital Herpes and Public Health: Addressing a Global Problem. JAMA, 283, 791-794. https://doi.org/10.1001/jama.283.6.791
- 16. Kolawole, O.M., Adu, F.D., Agbede, O.O., Oni, A.A. and Bakare, R.A. (2008) Epidemiological Patterns of Human Immunodeficiency Virus and Herpes Simplex Virus Co-Infection in Ibadan Nigeria. African Journal of Biomedical Research, 11, 23-26.
- 17. Rebbapragada, A., Pettengell, C., Sunderji, S., Huibner, S., Sheung, A., et al. (2006) Potential Mucosal Immune Mechanisms for Increased HIV Susceptibility in Women Infected by Herpes Simplex Type 2. AIDS 2006—XVI International AIDS Conference, Toronto, Canada, August 2006.
- 18. Nagot, N., Ouedraogo, A., Foulongne, V., Konate, I., Weiss, H.A., et al. (2007) Reduction of HIV1 RNA Levels with Therapy to Suppress Herpes Simplex Virus. The New England Journal of Medicine, 356, 790-799. https://doi.org/10.1056/NEJMoa062607
- 19. Cachay, E.R., Frost, S.D., Richman, D.D., Smith, D.M. and Little, S.J. (2007) Herpes Simplex Virus Type 2 Infection Does Not Influence Viral Dynamics during Early HIV1 Infection. The Journal of Infectious Diseases, 195, 1270-1277. https://doi.org/10.1086/513568
- 20. Barbour, J.D., Sauer, M.M., Sharp, E.R., Garrison, K.E., Long, B.R., Tomiyama, H., Bassichetto, K.C., Oliveira, S.M., Abbate, M.C., Nixon, D.F., Kallas, E.G., et al. (2007) HIV1/HSV2 Co-Infected Adults in Early HIV1 Infection Have Elevated CD4+ T Cell Counts. PLoS ONE, 2, e1080. https://doi.org/10.1371/journal.pone.0001080
- 21. Aebi-Popp, K., Bailey, H., Malyuta, R., Volokha, A. and Thorne, C. (2016) High Prevalence of Herpes Simplex Virus (HSV)-Type 2 Co-Infection among HIV-Positive Women in Ukraine, but No Increased HIV Mother-to-Child Transmission Risk. BMC Pregnancy Childbirth, 16, 94. https://doi.org/10.1186/s12884-016-0887-y
- 22. Tan, D.H.S., Raboud, J.M., Kaul, R., Brunetta, J., et al. (2013) Herpes Simplex Virus Type 2 Coinfection Does Not Accelerate CD4 Count Decline In Untreated HIV Infection. Clinical Infectious Diseases, 57, 448-457.
- 23. Cunningham, A.L. and Dwyer, D.E. (2004) The Pathogenesis Underlying the Interaction of HIV and Herpes Simplex Virus after Co-Infection. HIV Therapy, 9, 9-13.
- 24. Bosnjak, L., Miranda-Saksena, M., Koelle, D.M., Boadle, R.A., Jones, C.A., et al. (2005) Herpes Simplex Virus Infection of Human Dendritic Cells Induces Apoptosis and Allows Cross-Presentation via Uninfected Dendritic Cells. The Journal of Immunology, 174, 2220–2227. https://doi.org/10.4049/jimmunol.174.4.2220
- 25. Jones, C.A., Fernandez, M., Herc, K., Bosnjak, L., Miranda-Saksena, M., et al. (2003) Herpes Simplex Virus Type 2 Induces Rapid Cell Death and Functional Impairment of Murine Dendritic Cells in Vitro. Journal of Virology, 77, 11139-11149. https://doi.org/10.1128/JVI.77.20.11139-11149.2003
- 26. Mikloska, Z., Bosnjak, L. and Cunningham, A.L. (2001) Immature Monocyte-Derived Dendritic Cells Are Productively Infected with Herpes Simplex Virus Type 1. Journal of Virology, 75, 5958-5964. https://doi.org/10.1128/JVI.75.13.5958-5964.2001
- 27. Mark, K.E., Corey, L., Meng, T.C., Magaret, A.S., Huang, M.L., et al. (2007) Topical Resiquimod 0.01% Gel Decreases Herpes Simplex Virus Type 2 Genital Shedding: A Randomized, Controlled Trial. The Journal of Infectious Diseases, 195, 1324-1331. https://doi.org/10.1086/513276