Food and Nutrition Sciences
Vol.5 No.4(2014), Article ID:42787,15 pages DOI:10.4236/fns.2014.54049
High Fibre Diets and Alzheimer’s Disease
1Centre of Excellence for Alzheimer’s Disease Research and Care, School of Medical Sciences, Edith Cowan University, Joondalup, Australia; 2School of Psychiatry and Clinical Neurosciences, The University of Western Australia, Nedlands, Australia.
Email: *i.martins@ecu.edu.au
Copyright © 2014 Ian James Martins, W. M. A. D. Binosha Fernando. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Ian James Martins, W. M. A. D. Binosha Fernando. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received July 30th, 2013; revised August 30th, 2013; accepted September 8th, 2013
KEYWORDS: Cholesterol; Phytosterols; Fatty Acids; Amyloidosis; Liver; Neurodegeneration
ABSTRACT
The understanding of cholesterol and its pathogenesis to Alzheimer’s disease (AD) pathogenic process is important for the possible prevention of AD. High fibre diets that contain phytosterols have been shown to lower LDL and increase HDL cholesterol and are implicated in membrane cholesterol and amyloid beta (Aβ) homeostasis. The convergence of diet and AD may be related to the effects of phytosterols since plasma cholesterol is closely linked and regulated by phytosterols. Dietary fibre modifications that are low in fat and glucose reduce the risk for AD by not only effecting cell membranes and nutrient sensing G coupled receptors but also by regulating number of nuclear receptors such as histone deacetylases (HDAC) and peroxisome proliferator activated receptors (PPAR) that control glucose, fatty acids and cholesterol and have significant effects on the brain cholesterol homeostasis and amyloidosis. The peripheral sink Aβ hypothesis indicates that the peripheral clearance of Aβ and its regulation by dietary phytosterols is of substantial interest since it may delay hypercholesterolemia and the early onset of amyloid plaque development. Liver disease has been of central importance with aging and programmed cell death pathways. Nutritional therapy has emerged as a novel approach to control appetite and the role of nutrigenomics as an early nutritional therapy may assist genes to delay liver and brain diseases such as Parkinson’s disease (PD) and Huntington’s disease (HD) that are associated with aging. The understanding of phytosterols and the role of these lipids in drug therapy such as cholesterol lowering drugs may provide molecular mechanisms that are involved in the regulation of cell Aβ clearance and metabolism. High fibre diets also contain various fatty acids such as the short chain fatty acids (SCFA) and the understanding of synergistic effects of SCFA and phytosterols in glucose regulation and cholesterol homeostasisis important to our understanding of diet, lifestyle and drugs in relation to peripheral amyloidosis and gene expression that play an early role in the development of AD.
1. Introduction
Environmental factors such as exercise, circadian rhythms abnormalities, oxidative stress and aspects of various diets in Western countries are now of considerable importance when considering the risk for Alzheimer’s disease (AD). The consumption of high fat and high cholesterol diets (HFHC) has clearly been associated with increased plasma cholesterol and oxidative stress in various tissues. In various animal models of AD, a strong correlation has been found with HFHC diets and increased brain amyloid beta (Aβ) levels. The molecular mechanisms underlying the AD cholesterol connection are important in the prevention of the disease since considerable evidence indicates that cellular cholesterol levels, intracellular cholesterol transport and cholesterol esterification play an important role in Aβ generation and programmed cell death pathways. Specific diets and extending lifespan with avoidance of programmed cell death pathways have become an urgent therapeutic intervention for anti-aging related diseases [1]. In Western countries, age related diseases such as obesity and diabetes have become common as risk factors for appetite dysregulation, atherosclerosis and AD [2]. The Western diet is known to be high in fat and cholesterol with effects on the liver and brain lipid homeostasis and marked effects on peripheral amyloidosis [2].
The key element of this review is to improve the understanding of cholesterol metabolism and its role in the prevention of AD. The role of therapeutic lipids such as phytosterols and fatty acids will be discussed that reduce hypercholesterolemia and improve the liver clearance of Aβ (peripheral sink Aβ hypothesis). The significance of nutritional therapy has emerged as a novel approach to control appetite and improve nutrigenomics that may activate genes involved in Aβ clearance and delay liver and brain diseases that are associated with aging. Molecular mechanisms that are involved in the regulation of cell Aβ clearance and metabolism require appropriate changes to diets such as high fibre diets that promote caloric restriction and allow consumption of diets that are low in fat and high in antioxidants, trace minerals and fish that are associated with appetite control and decreased risk for AD. The aim of these dietary strategies will accelerate liver and brain cholesterol metabolism with improvements and reversal of non alcoholic fatty liver disease (NAFLD) and maintenance of peripheral Aβ metabolism (Figure 1 ref [2]).
Assessment of AD risk indicates that diet apart from genetic factors may provide a useful model for the prevention and management of the disease. Risk factors indicating that liver and metabolic health is central to AD have become important since memory loss and neurodegenerative damage become essentially irreversible. Therefore, nutritional research has concentrated on the identification of nutrient sensing diets that provide regulation of histone deacetylases (HDAC) that are involved in epigenetic control of gene expression that controls metabolic and tissue glucose and cholesterol homeostasis [3,4]. In calorie restriction epigenetic regulation of genes that control lifespan [5,6], fatty acid acid [7] and aging is associated with an increase in the Sirtuin 1 (Sirt 1) protein which is a member of the HDAC family [8]. HDAC and their dietary regulation are essential as therapy for glucose and cholesterol maintenance and reversal of neurodegeneration at early stages of AD [9-11].
Interests in cholesterol diets and sedentary lifestyles have become a major concern in Western countries and diets rich in fibre which may allow appropriate changes in calorie restriction and HDAC regulation with effects on liver cholesterol and glucose metabolism that main-
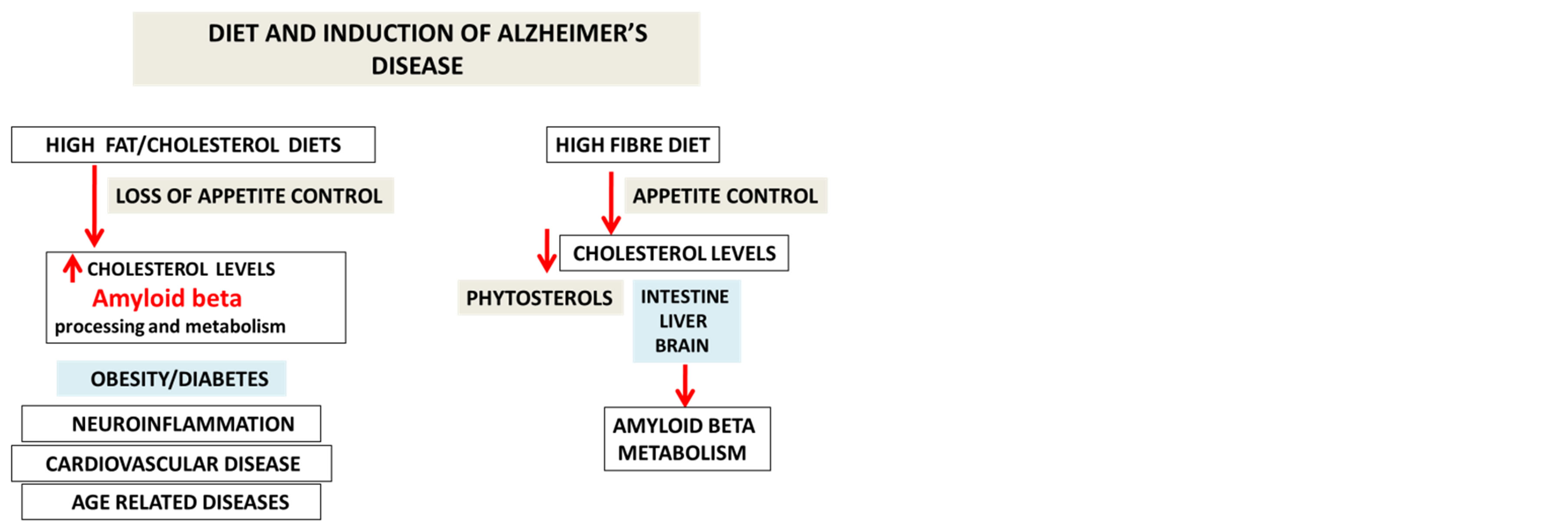
Figure 1. Diets control appetite and cholesterol metabolism with the regulation of amyloid beta metabolism.
tain active HDAC gene expression for peripheral Aβ metabolism and homeostasis [12,13]. Dietary fibre contains phytosterols, short chain fatty acids (SCFA) and other nutritional agents essential for reversing the effects of HFHC diets on down regulation of HDAC genes involved in cholesterol and Aβ homeostasis (Figure 1). Diets that are high in fat have been associated with hyperphagia and appetite associated hormones such as insulin, glucagon, ghrelin and leptin which have been found to be altered with these diets and exercise [14-18]. Interests in nutritional therapy by consumption of high fibre diets have important effects on appetite control [19- 23] and activation liver nutrient receptors. NAFLD has become the most important chronic disease in first and third world countries and effects approximate 40% of the global population [24]. The effects of SCFA, phytosterol and phytostanols in high fibre diets and their actions on cholesterol lowering drugs such as statins on Aβ metabolism have not been addressed and have become important to NAFLD [25-27] and AD treatment. The role of fatty acids [28-30] and phytosterols on liver nutrient sensing genes such as Sirtuin 1 (Sirt 1) and G coupled protein receptors (GPCR) needs to be further addressed with relevance to the treatment of appetite dysregulation, NAFLD and the metabolic syndrome in obesity and diabetes that are chronic diseases associated with the increased risk for AD [31].
2. Cholesterol and AD Pathogenesis
The main constituent of senile plaques associated with AD, namely Ab [32] is a proteolytic product of a larger protein, the amyloid precursor protein (APP). The Ab (1-40) is synthesized in the early secretory and endocytic cellular pathways and the Ab (1-42) is generated mainly in the secretory pathway [33]. APP is cleaved by three proteases, classified as a, b and g secretases and formation of Ab from APP is thought to occur via a two step process involving the b-site cleaving enzyme (BACE) and the putative g-secretases [34-36]. The APP protein is cleaved into bAPPs (amino acids 18-671 of APP) and Ab (amino acids 672-711/713 of APP). Cholesterol has been shown to be directly involved in membrane APP/Ab interactions and alterations in these interactions may be involved in the early stages of amyloidogenesis [37,38].
Detailed studies have previously shown that plasma high density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterol levels in AD patients when compared to age matched individuals [39-41] were significantly correlated to cognitive decline [41]. The liver and membrane cholesterol homeostasis has been shown to be involved with HDL levels and the ATP-binding cassette transporter (ABCA1) plays an important role in APP processing and peripheral cholesterol homeostasis with maintenance of central nervous system and neuronal Aβ and cholesterol homeostasis [42-45]. Interests in peripheral cholesterol metabolism have accelerated since clearance and metabolism of Aβ oligomers are important factors involved in the progression of amyloid plaque development. In the brain, cholesterol levels can also be regulated by sterol regulatory element-binding proteins (SREBP) that belong to the family of transcription factors that regulate intracellular cholesterol and lipid metabolism [46].
The role of cholesterol in modulating the expression of APP and the levels of Aβ has been reported [47-50]. Cholesterol modulates Aβ levels and Aβ acts on lipid metabolism by inhibiting cholesterol synthesis by inhibition of 3 hydroxy 3 methylglutaryl coenzyme A (HMGCoA) and plays an important role in sphingomyelin metabolism to form ceramide [51]. The enzyme acyl-coenzyme A: cholesterol acyltransferase 1 (ACAT) esterifies cholesterol and long chain fatty acids and the enzyme controls Aβ and cholesteryl ester generation in the intestine and liver [52-54]. In the liver, the cholesteryl esters have a profound effect on APP processing and inhibition of the ACAT1 enzyme may reduce Aβ with effects on Aβ plaque development [54]. Both enzymes are closely involved in LDL receptor regulation and several reviews have shown that LDL receptors (LDLr) are involved in cholesterol homeostasis, APP processing and AD pathogenesis [55-57]. In LDL receptor deficient mice, elevated LDL levels were associated with cerebral amyloidosis and in human, polymorphisms in the LDL receptor gene were associated with AD [55-56]. In the brain and liver, the LDL receptors play an important role in the metabolism of cholesterol and Aβ with the LDL receptor related protein 1 (LRP1) closely linked to AD. LRP1 also acts on blood brain barrier for the transport of Aβ to the periphery from the brain [58,59]. LRP1 is involved in the clearance of apolipoprotein E (apo E), alpha 2 macroglobulin, transforming growth factor-beta, and tissue plasminogen activator (tPA). Other members of the LDL receptor family such as apo ER2, LRP1B, and sorting protein related receptor containing LDL receptor class A repeats (SORLA) are involved in APP and Aβ metabolism [60, 61]. The receptor for advanced glycation end products (RAGE) may allow transport of Aβ from the plasma into the brain but LRP1 in man predominates for the major pathway for transport of Aβ from the brain into the plasma (Figure 2).
Interests in lowering peripheral cholesterol levels to reduce the risk of AD have been the focus of many research studies with particular impact in the regulation of brain Aβ metabolism. The interests in low HDL and high LDL in the plasma of AD patients have increased research in food and nutrition to assess the role of atherosclerosis in obesity and diabetes. Diets and brain cholesterol homeostasis has gained central interest to AD since cholesterol has not been reported to cross the BBB [62-64]. The brain must obtain cholesterol from de novo synthesis with astrocytes and oligodendrocytes mainly involved in cholesterol synthesis and neurons account for a small amount of the brain cholesterol [65,66]. Diets that promote neuron and maintain synapse are vital in the treatment of AD since there is a gradual loss of nerve synapses in brain regions in AD which is associated with disturbed cholesterol homeostasis [67,68]. Brain cholesterol homeostasis is maintained by cholesterol excretion in the form of 24Shydroxysterol (24 S OHC) accomplished by the cytochrome P450 species [69] and in man the brain releases approx. 6 mg of 24S OHC into the periphery each day that is removed predominantly by the liver [70]. In AD patients, studies have shown that cholesterol metabolism is disturbed with elevated 24S OHC levels possibly related to neuronal death and neurodegeneration [71-73]. The release of 24 S OHC from the brain is critical to the maintenance of neurons and synapses and it cannot be excluded that with aging the elevated release of oxysterols from phytosterols in the brain may also be involved in abnormal neuron survival and synapse maintenance.
Drugs such as statins are inhibitors of HMG-CoA reductase and up-regulate LDL receptor levels with reduc-
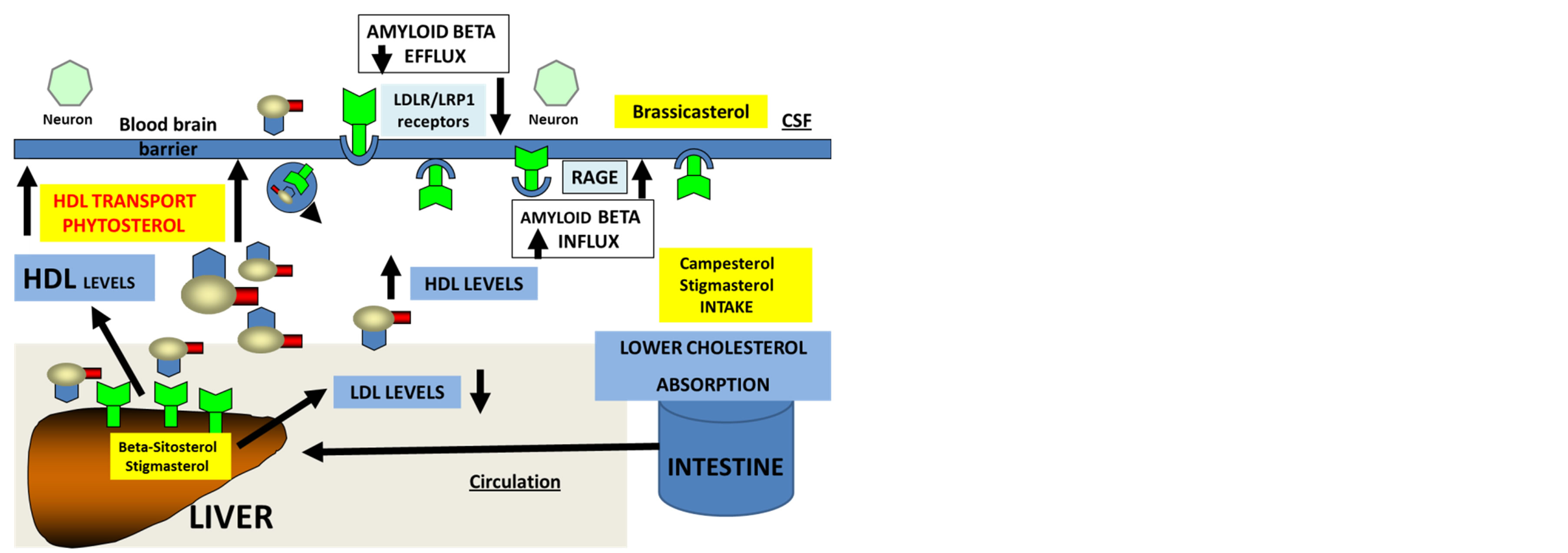
Figure 2. Phytosterols regulate intestine, liver and brain cholesterol and amyloid beta metabolism.
tion in LDL levels [74,75]. Cholesterol lowering drugs have been shown to have marked effects on Aβ levels with statins involved in the reduction of Aβ levels [76-80]. The mechanisms of actions of statins on lowering the risk of AD may involve membrane interactions by adjusting cholesterol and other specific sterols sensitive to Aβ homeostasis. The role of diets and drug therapy may allow maintenance of membrane cholesterol levels that maintain proper synaptic function and abnormal events from drugs such as statins in relation to injury to mitochondria and muscles could be avoided with specific high fibre diet interventions that allow long term use of drug therapy.
3. Phytosterols and Cholesterol Homeostasis
In large scale studies in man Western diets that contain high fat and high cholesterol contents have been associated with increased risk for AD and in experimental studies in rodents HFHC diets lead to increased brain Aβ levels and abeta plaque like deposits in the brain [81,82]. In studies with mediterranean diets high in fibre low in fat content, high in antioxidant, trace mineral and fish content with active lifestyles was associated with decreased risk for AD [81,82]. Interests in high fibre diets and their effects on other neurodegenerative disease such as Parkinson’s disease (PD) and Hungtington’s disease (HD) have been reported with the higher caloric intake associated with the increased risk for PD and HD [83-90]. Nutritional therapy in neurodegeneration has become important with effects on nutrient sensing receptors such as Sirt1 and GPCR in the liver and brain with particular interests in pharmacotherapy with GPCR targets in PD and HD [91, 92]. GPCRs or fragments may bind second messengers that directly initiate or regulate transcriptional events in the nuclear domain [93-95] and GPCR regulation of nuclear events are possibly associated with nutritional regulation by dietary cholesterol or low calorie high fibre diets. Elevated cholesterol levels and oxidative stress induced by HFHC diets modify membrane cholesterol [96] with the alteration in membrane GPCR function or transport of GPCR to the nucleus.
In neurodedgeneration, neuronal dysfunction that leads to death plays a pivotal role in neuronal loss and the hypothesis that phytosterols are involved in the regulation of neuronal cholesterol and Aβ metabolism has focused on the role of these phytonutrients that may underline the AD disease process. Foods that maintain brain health are essential for the aging communities in various countries. The ability to alter dietary composition of fibre, fat and cholesterol allows the nutritionist to increase the survival of neurons and to withstand programmed cell death and maintain cognition and brain function. In Western communities, individuals synthesize approx 1 gram of cholesterol per day and consume 400 mg from their diet. Phytosterol consumption and its ability to maintain plasma cholesterol and membrane cholesterol are important to the pathogenicity of neuronal Aβ with consumption of approx. 2 g per day associated with reduction in cholesterol absorption with effects on membrane cholesterol molecular mechanisms [97-99].
Brain cholesterol homeostasis and phytosterol intake are closely interlinked and diets with specific composition of phytosterols are possibly important in the maintenance of liver, brain and oxysterol metabolism [97-106]. Phytosterols such as campesterol and stigmasterol inhibit intestinal cholesterol absorption and beta-sitosterol and stigmasterol act on the liver to reduce cholesterol levels (Figure 2, ref [97,99,101]). In the cerebrospinal fluid, brassicasterol has been detected and is reported to be a significant biomarker for AD with indication of altered transport of this phytosterol to the brain [102]. In aging and senescence, phytosterols accumulate in the brain with loss of control of cholesterol and oxysterol metabolism and transport within the brain [105-108]. Specific phytosterols such as beta sitosterol that regulate cholesterol levels also control APP processing and Aβ production in platelets [109,110] with current interest of specific phytosterols to nutrition science and AD (Figure 2). The role of phytosterols in the regulation of intestine, liver and brain cholesterol is critical to Aβ metabolism and metabolism of cerebral oxysterols which is closely related to neurodegenerative diseases [111-117].
The interest in phytosterols in AD has accelerated since the incidence of liver disease, obesity and diabetes has increased with the incidence of AD. Interests in the peripheral sink Aβ hypothesis in relation to phytosterol metabolism and their regulation of peripheral cholesterol homeostasis (vice versa) have been the focus of dietary fibre and relevance to AD. In Western countries, nutritional therapy has targeted the therapies for liver diseases and NAFLD is common in these countries [118]. Phytosterols have clear effects on the liver X receptors (LXR) and ATP-binding cassette transporters (ABCA1) pathways unravelling the mechanisms for the control of cell and membrane cholesterol homeostasis [119-123]. The LXRs are nuclear receptors that are found in the brain and influence a variety of genes involved in cellular cholesterol efflux and cells of the CNS [81,82]. The target gene for LXR is ABCA1 and activation of LXR has been shown to stimulate ABCA1 levels and decrease Aβ levels [81,82].
Sirt1 belongs to the HDAC family and the role of this anti-aging protein is linked to cholesterol and Aβ metabolism with links to obesity, diabetes and AD [2,124]. Sirt1 positively regulates the nuclear receptor LXR and in vivo reduces the expression of LXR targets such as ABCA1. In obesity and diabetes, Sirt 1 is switched off and the effects of phytosterols may be ineffective on cholesterol homeostasis via LXR and ABCA1 pathways that provide important dietary modulation of the nuclear receptor control of cholesterol and Aβ homeostasis in the liver and brain. Sirt 1 is involved in fatty acid metabolism, appetite and circadian control with involvement of peroxisome proliferator-activated receptors (PPAR) that are affected by fatty acids with significance to the PPAR alpha-Sirt 1 complex and peripheral amyloidogenesis [2]. Phytosterols regulate gene expression and cell cholesterol homeostasis and determine the LDL receptor membrane expression with effects on LDL concentrations in man. Phytosterols are closely involved in nutrigenomics with control and regulation of the nuclear receptor Sirt 1 actions that control cholesterol and Aβ levels (Figure 3 (ref [2,119-123, 124]).
Diet and cholesterol therapy using drugs has been the central focus for the treatment of AD. Caloric restriction and exercise do not only affect cholesterol metabolism but also phytosterol metabolism with prevention of the accumulation of oxysterols and phytosterols in the liver and brain membranes [115,116]. A major focus on the use of diet, cholesterol lowering drugs and lifestyles is to increase neuron number and decrease the size of plaques in the brain with aging. The membrane interactions of Aβ require cholesterol for clearance and metabolism with the increase in the membrane cholesterol involved in the regulation of Aβ assemblies [125-127]. The role of caloric restriction in activation of HDAC genes which control membrane cholesterol and the role of phytosterols and phytostanols (Figure 4 [99-102] have become of central interest since membrane interactions with cholesterol and Aβ may be required for the clearance and metabolism of Aβ. Increasing membrane phytosterol contents may control unstable protein/lipid complexes with the composition of phytosterols essential for neuron number and survival. Membrane phospholipids have been shown to be important for insertion of Aβ and the role of phytosterols (beta-sitosterol) in the determination of cholesterol/phospholipid stability has been reported [128-130]. Furthermore, the displacement of cholesterol by phytosterols or phytostanols may have implications for Aβ insertion and aggregation [131-133].
In various species, phytosterols are absorbed with different rates with low phytosterol absorption rates in rabbits and high phytosterol absorption rates in man. The role of diet and membrane phytosterols in cell membranes has attracted interest since the important role of cholesterol in ion channel regulation has been reported. Membrane lipids are an important part of the structure of neurons and conduct electrical impulses in association with membrane proteins [134-136]. Lipids are an integral component of bilayer membranes and maintain ion channels that are involved in intercellular communication [134,135]. Lipids within membranes are involved in
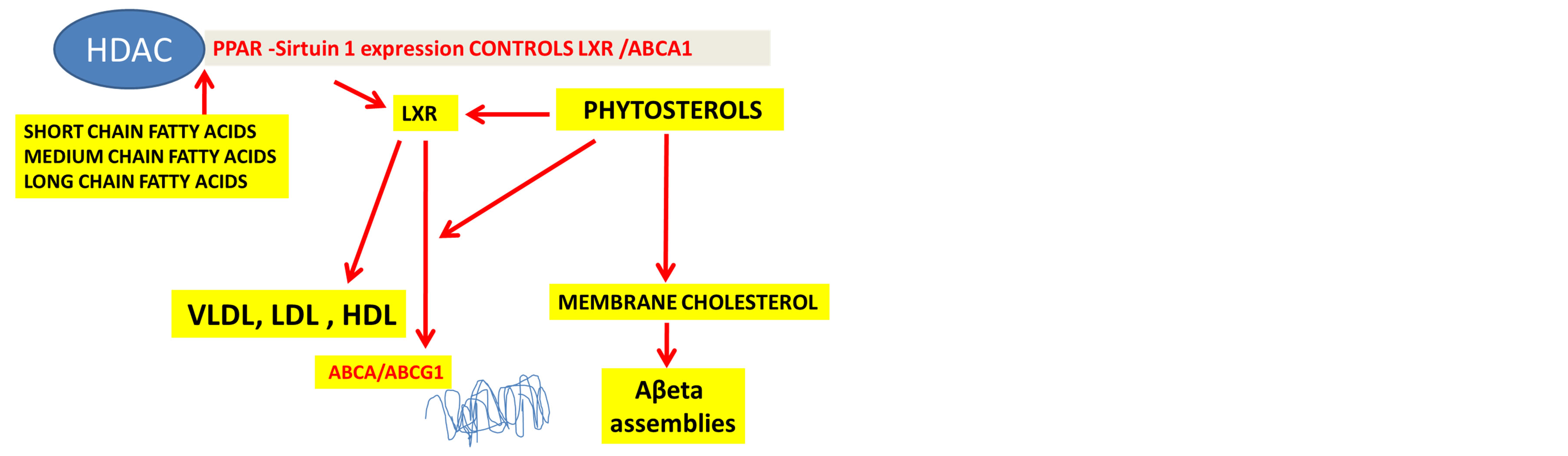
Figure 3. Fatty acids and phytosterols control PPAR-HDAC by caloric regulation of nuclear receptors with effects on cell cholesterol metabolism via liver X receptors and ATP binding cassette transporters.
maintaining membrane potentials by regulating the movement of ions across neuronal membranes, maintain synaptic function and produce lipid messengers for signal transduction [137]. The association of phospholipids, glycosphingolipids such as ceramide or gangliosides, glycerophospholipids (plasmalogen) and sterols makes up the membrane bilayers in neurons and packing of cholesterol within membranes may have marked effects on membrane protein structure and function and regulation of ion pumps [138]. Lipid rafts containing sphingomyelin and cholesterol form micro-domains for the recruitment of lipid modified proteins and ion channels [134]. Cholesterol has been shown to be involved in the regulation of ion channels and Aβ has been shown to closely regulate various ion channels [139-142]. In particular, Aβ oligomer formation is cytotoxic and leads to neuronal death with alterations in membrane ion channel activity and synaptic plasticity [143,144]. The role of specific phytosterols in the brain and their effects on the regulation of cholesterol and ion channels are poorly understood and diets that contain specific phytosterols and their relationship to Aβ and ion channels may have important implications for aging and AD.
4. Synergistic Effects of Phytosterol and Fatty Acids
Phytosterols and SCFA are natural food components with potential health benefits. Both compounds are mainly derived from high fibre diet and have properties such as anti-cancer [145] anti-atherosclerotic, anti-inflammatory [146-149] and anti-oxidant [150,151]. Phytosterol reduce cholesterol levels and an increase in the level of butyric acid, propionic and acetate in the blood is associated with a significant reduction in plasma cholesterol levels [152, 153]. Low molecular size, short carbon chain length and a high water solubility of SCFA have facilitated its rapid absorption in the portal vein and metabolism [154]. Higher metabolism rate of butyric acid has reduced its



Figure 4. Phytosterols and phytostanols found in high fibre diets.
clinical potential. Thus it is reasonable to suggest that combination of phytosterol and SCFA may further reduce cholesterol and increase its efficacy as an anti cancer, anti inflammatory and anti-oxidant agent. Esterification of phytosterol with short chain fatty acids may improve its integration into a variety of foods without disturbing the efficiency of phytosterol as with omega 3 fatty acids and phytosterol.
Phytosterol esters with fish oil have been found to lower the plasma cholesterol levels than conventional phytosterol esters [155]. Furthermore, this synergistic supplement has a more hypotriglyceridemic effect than the conventional fish oil [155]. Esterification of phytosterol with oleic acid and esterification of beta sitosterol with conjugated linoleic acid have been previously reported [156]. Esterification of phytosterol with SCFA or especially with butyric acids has been poorly reported. Recent studies have shown formation of phytosterol esters via direct esterification with butyric acid and via trans esterification with tributyrin and ethyl butyrate [157]. Animal studies will be helpful to understand this combination while human studies are required to identify its long term effects. On the other hand, aliphatic and steroid esters of gamma-amino [U-14C] butyric acid (GABA) which is a synthesized compound have proved to have a high penetration capacity across the blood brain barrier [158].
The combination of SCFA and phytosterols on cholesterol homeostasis indicate that high fibre diets are regulators of amyloidosis and may prove effective as AD therapy. Studies have shown that SCFA are formed as a result of fibre fermentation could be affected by the consumption of phytosterol. Consumption of margarine enriched with phytosterol esters did not show any effect on the micro flora profile or to their metabolic activities or SCFA content [159]. SCFA have effects on appetite control and are considered to be important to HDAC inhibition (Figure 3) which is currently an important therapy for AD. SCFA exhibit marked effects on free fatty acid metabolism on binding to G coupled receptors that are involved in appetite control and play an important role in obesity and AD [160-162]. SCFA have been shown to have marked effects on insulin sensitivity and lipid metabolism and are associated to the field of insulin resistance syndrome and AD. Apart from synergistic effects of phytosterol and SCFA as AD therapy in particular butyric acid may have independent effects on AD. Interest in the effects of butyric acid on liver has increased since NAFLD has become common in Western countries with close association with obesity and diabetes that are high risk factors for AD. Sodium butyrate contains anti-inflammatory and cancer chemo-preventive properties in colon [163-165]. Acetate, propionate and butyrate have the ability to transport through the blood brain barrier and could be utilized as the energy source by short-chain L-3-hydroxyacyl-CoA dehydrogenase [166]. This enzyme is highly important to Aβ and known as endoplasmic reticulum amyloid beta-peptide-binding protein and as a multifunctional protein has the capacity to oxidise oxidize 17β-estradiol [166]. The anti-cancer and anti-proliferative features of butyrate are not important to energy metabolism but may be vital to the modulation of gene expression [167] via the inhibition of histone deacetylases [165] and NF-κB signaling [168]. Studies have shown that butyrate could effect gene expression in microglial cells, as in colonocytes [167].
Butyrate could induce the adaptive responses in NF- κB signaling and has a capacity to regulate the responsiveness of microglial cells to lipopolysaccahrides (LPS). Research studies on colonocytes [164], intestinal epithelial cells [169] and murine macrophages [168] has demonstrated that butyrate could down-regulate NF-κB signalling induced by cytokines or LPS. Yin et al. (2001) have shown that butyrate could inhibit proteasome activity and has the ability to up-regulate the level of inhibitory IκB proteins and thus prevents the cytosolic NF-κB activation. In other studies, butyrate has been shown to [170] down-regulate the expression of Toll-like receptor 4 (TLR4) that transmits LPS signals to activate NF-κB system and suggests that butyrate could suppress NF-κB activation. It may be interesting to understand the effect of sodium butyrate on the inhibition of NF-κB signaling in microglial cells. Discharge of proinflammatory and/or cytotoxic factors, including tumor necrosis factor-alpha (TNF-α), interleukin 1-beta (IL-1β), interleukin-6 (IL-6), interleukin-12 (IL-12), andnitric oxide (NO) could cause gradual damage in neurodegenerative diseases such as stroke, trauma, AD, multiple sclerosis, and human immunodeficiency virus (HIV)-associated dementia.
Dietary fibre also contains other fatty acids such as medium chain fatty acids and long chain fatty acids and also affects nuclear receptors with particular relevance to the PPARγ involved in an increase in insulin sensitivity and diabetes. LCFA activates nuclear receptor PPAR α and gene transcription with control of lipid and glucose homeostasis. Diets rich in fibre lead to synergistic effects of phytosterols and SCFA, MCFA and LCFA on nuclear receptor activity (PPAR-Sirt1) expression and activation that allow control of glucose and cholesterol homeostasis involved in prevention of obesity and diabetes being the risk factors for AD.
5. Conclusion
Phytosterols and phytostanols and their esters are found in oils, fats, nuts, seeds, cereal, vegetables and fruits and their effects on reducing cholesterol absorption and cholesterol lowering therapies have been reported. High levels of SCFA have been found in barley, oats, brown rice, bran, green beans, legumes, leafy greens, apples, kiwi and oranges. Medium chain such as lauric acid and long chain fatty acids has been found in coconut oil. Interest in high fibre diets as anti-Alzheimer’s or anti-diabetic and their effects on drug therapy such as statins have attracted considerable interest although plasma cholesterol is clearly reduced in the drug therapy and effects on phytosterol interactions on membranes need to be further addressed in relation to promotion of Aβ binding, clearance and metabolism. The peripheral clearance of Aβ and its relationship to high fibre diets is of particular interest to therapy in AD because high fibre diets are particularly effective in NAFLD treatment and assist in the rapid brain transport of Aβ to the liver. The role of SCFA and phytosterols on nutrient sensing genes such as Sirt1 and G coupled protein receptors indicates the therapeutic role of high fibre diets on appetite regulation and the metabolic syndrome in chronic diseases such as obesity and diabetes and their increased risk for AD, PD and HD. Dietary fibre intake may improve the actions of statins by activation of nutrient sensing genes with synergistic effects of cholesterol lowering drugs and diet on improving peripheral Aβ metabolism in AD individuals.
REFERENCES
- J. Shen and J. Tower, “Programmed Cell Death and Apoptosis in Aging and Life Span Regulation,” Discovery Medicine, Vol. 8, No. 43, 2009, pp. 223-226.
- I. J. Martins, R. Creegan, W. L. F. Lim and R. N. Martins, “Molecular Insights into Appetite Control and Neuroendocrine Disease as Risk Factors for Chronic Diseases in Western Countries,” Open Journal of Endocrine and Metabolic Diseases, Vol. 3, No. 5A, 2013, pp. 11-33.
- M. Plank, D. Wuttke, S. van Dam, S. A. Clarke and J. Pde Magalhães, “A Meta-Analysis of Caloric Restriction Gene Expression Profiles to Infer Common Signatures and Regulatory Mechanisms,” Molecular Biosystems, Vol. 8, No. 4, 2012, pp. 1339-1349. http://dx.doi.org/10.1039/c2mb05255e
- Y. Li, M. Daniel and T. Tollefsbol, “Epigenetic Regulation of Caloric Restriction in Aging,” BMC Medicine, Vol. 9, No. 98, 2011, pp. 1-12.
- J. F. Trepanowski, R. E. Canale, K. E. Marshall, M. M. Kabir and R. J. Bloomer, “Impact of Caloric and Dietary Restriction Regimens on Markers of Health and Longevity in Humans and Animals: A Summary of Available Findings,” Nutrition Journal, Vol. 10, No. 107, 2011, pp. 1-13.
- S. Ribaric, “Diet and Aging,” Oxidative Medicine and Cellular Longevity, Vol. 2012, 2012, pp. 1-20. http://dx.doi.org/10.1155/2012/741468
- M. D. Bruss, C. F. Khambatta, M. A. Ruby, I. Aggarwal and M. K. Hellerstein, “Calorie Restriction Increases Fatty Acid Synthesis and Whole Body Fat Oxidation Rates,” American Journal Physiology Endocrinology Metabolism, Vol. 298, No. 1, 2010, pp. E108-E116. http://dx.doi.org/10.1152/ajpendo.00524.2009
- W. Stünkel and R. M. Campbell, “Sirtuin 1 (SIRT1): The Misunderstood HDAC,” Journal of Biomolecular Screening, Vol. 16, No. 10, 2011, pp. 1153-1169. http://dx.doi.org/10.1177/1087057111422103
- K. Xu, X. L. Dai, H. C. Huang and Z. F. Jiang, “Targeting HDACs: A Promising Therapy for Alzheimer’s Disease,” Oxidative Medicine and Cellular Longevity, Vol. 2011, 2011, pp. 1-5. http://dx.doi.org/10.1155/2011/143269
- H. Funato, S. Oda, J. Yokofujita, H. Igarashi and M. Kuroda, “Fasting and High-Fat Diet Alter Histone Deacetylase Expression in the Medial Hypothalamus,” PLoS One, Vol. 6, No. 4, 2011, Article ID: e18950. http://dx.doi.org/10.1371/journal.pone.0018950
- M. M. Mihaylova, D. S. Vasquez, K. Ravnskjaer, P. D. Denechaud, R. T. Yu, J. G. Alvarez, M. Downes, R. M. Evans, M. Montminy and R. J. Shaw, “Class IIa Histone Deacetylases Are Hormone-Activated Regulators of FOXO and Mammalian Glucose Homeostasis,” Cell, Vol. 145, No. 4, 2011, pp. 607-621. http://dx.doi.org/10.1016/j.cell.2011.03.043
- I. Milagre, M. J. Nunes, M. Moutinho, I. Rivera, A. Fuso, S. Scarpa, M. J. Gama and E. Rodrigues, “ChromatinModifying Agents Increase Transcription of CYP46A1, a Key Player in Brain Cholesterol Elimination,” Journal of Alzheimer’s Disease, Vol. 22, No. 4, 2010, pp. 1209- 1221.
- S. V. Chittur, N. Sangster-Guity and P. J. McCormick, “Histone Deacetylase Inhibitors: A New Mode for Inhibition of Cholesterol Metabolism,” BMC Genomics, Vol. 9, No. 507, 2008, pp. 1-14.
- A. B. Crujeiras, E. Goyenechea, I. Abete, M. Lage, M. C. Carreira, J. A. Martínez and F. F. Casanueva, “Weight Regain after a Diet-Induced Loss Is Predicted by Higher Baseline Leptin and Lower Ghrelin Plasma Levels,” Journal of Clinical Endocrinology and Metabolism, Vol. 95, No. 11, 2010, pp. 5037-5044. http://dx.doi.org/10.1210/jc.2009-2566
- K. A. Boyd, D. G. O’Donovan, S. Doran, J. Wishart, I. M. Chapman, M. Horowitz and C. Feinle, “High-Fat Diet Effects on Gut Motility, Hormone, and Appetite Responses to Duodenal Lipid in Healthy Men,” American Journal of Physiology and Gastrointestinal Liver Physiology, Vol. 284, No. 2, 2003, pp. G188-G196.
- T. J. Little and C. Feinle-Bisset, “Effects of Dietary Fat on Appetite and Energy Intake in Health and Obesity— Oral and Gastrointestinal Sensory Contributions,” Physiology & Behavior, Vol. 104, No. 4, 2011, pp. 613-620. http://dx.doi.org/10.1016/j.physbeh.2011.04.038
- C. Brøns, C. B. Jensen, H. Storgaard, N. J. Hiscock, A. White and J. S. Appel, “Impact of Short-Term High-Fat Feeding on Glucose and Insulin Metabolism in Young Healthy Men,” Journal Physiology, Vol. 587, No. 10, 2009, pp. 2387-2397. http://dx.doi.org/10.1113/jphysiol.2009.169078
- C. Pil-Byung, Y. Shin-Hwan, K. Il-Gyu, H. Gwang-Suk, Y. Jae-Hyun and L. Han-Joon, “Effects of Exercise Program on Appetite-Regulating Hormones, Inflammatory Mediators, Lipid Profiles, and Body Composition in Healthy Men,” Journal Sports Medical Physical Fitness, Vol. 51, No. 4, 2011, pp. 654-663.
- K. Porikos and S. Hagamen, “Is Fiber Satiating? Effects of a High Fiber Preload on Subsequent Food Intake of Normal-Weight and Obese Young Men,” Appetite, Vol. 7, No. 2, 1986, pp. 153-162. http://www.sciencedirect.com/science/article/pii/S0195666386800150 http://dx.doi.org/10.1016/S0195-6663(86)80015-0
- R. A. Samra and G. H. Anderson, “Insoluble Cereal Fiber Reduces Appetite and Short-Term Food Intake and Glycemic Response to Food Consumed 75 Min Later by Healthy Men,” American Journal of Clinical Nutrition, Vol. 86, No. 4, 2007, pp. 972-979.
- S. Ibrugger, M. Kristensen, M. S. Mikkelsen and A. Astrup, “Flaxseed Dietary Supplements for Suppression of Appeptite and Food Intake,” Appetite, Vol. 58, No. 2, 2012, pp. 490-495. http://dx.doi.org/10.1016/j.appet.2011.12.024
- J. Darzi, G. S. Frost and M. D. Robertson, “Do SCFA Have a Role in Appetite Regulation?” Proceedings of the Nutrition Society, Vol. 70, No. 1, 2011, pp. 119-128. http://dx.doi.org/10.1017/S0029665110004039
- H. V. Lin, A. Frassetto, E. J. Kowalik, A. R. Nawrocki and M. M. Lu, “Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms,” PLoS One, Vol. 7, No. 4, 2012, Article ID: e35240. http://dx.doi.org/10.1371/journal.pone.0035240
- B. W. Smith and L. A. Adams, “Non-Alcoholic Fatty Liver Disease,” Critical Review Clinical Laboratory Science, Vol. 48, No. 3, 2011, pp. 97-113. http://dx.doi.org/10.3109/10408363.2011.596521
- H.-S. Lai, W.-H. Lin, P.-R. Chen, H.-C. Wu, P.-H. Lee and W.-J. Chen, “Effects of a High-Fiber Diet on Hepatocyte Apoptosis and Liver Regeneration after Partial Hepatectomy in Rats with Fatty Liver,” Journal of Parenteral & Enteral Nutrition, Vol. 29, No. 6, 2005, pp. 401-407. http://dx.doi.org/10.1177/0148607105029006401
- M. Sleeth, A. Psichas and G. Frost, “Weight Gain and Insulin Sensitivity: A Role for the Glycaemic Index and Dietary Fibre?” British Journal of Nutrition, Vol. 109, No. 9, 2012, pp. 1539-1541. http://dx.doi.org/10.1017/S0007114512005016
- N. Rafiq and Z. N. Younossi, “Effects of Weight Loss on NonalcoholicFatty Liver,” Seminars in Liver Disease, Vol. 28, No. 4, 2008, pp. 427-433. http://dx.doi.org/10.1055/s-0028-1091986
- M. S. Jansen, S. C. Nagel, J. P. Miranda, E. K. Lobenhofer, C. A. Afshari and D. P. McDonnell, “Short-Chain Fatty Acids Enhance Nuclear Receptor Activity through Mitogen-Activated Protein Kinase Activation and Histone Deacetylase Inhibition,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 101, No. 18, 2004, pp. 7199-7204. http://dx.doi.org/10.1073/pnas.0402014101
- M. V. Liberato, A. S. Nascimento, S. D. Ayers, J. Z. Lin, A. Cvoro and R. L. Silveira, “Medium Chain Fatty Acids Are Selective Peroxisome Proliferator Activated Receptor γ Activators and Pan-PPAR Partial Agonists,” PLoS One, Vol. 7, No. 5, 2012, Article ID: e36297. http://dx.doi.org/10.1371/journal.pone.0036297
- F. Schroeder, A. D. Petrescu, H. Huang, B. P. Atshaves, A. L. McIntosh and G. G. Martin, “Role of Fatty Acid Binding Proteins and Long Chain Fatty Acids in Modulating Nuclear Receptors and Gene Transcription,” Lipids, Vol. 43, No. 1, 2008, pp. 1-17. http://dx.doi.org/10.1007/s11745-007-3111-z
- D. Bosco, A. Fava, M. Plastino, T. Montalcini and A. Pujia, “Possible Implications of Insulin Resistance and Glucose Metabolism in Alzheimer’s Disease Pathogenesis,” Journal of Cell and Molecular Medicine, Vol. 15, No. 9, 2011, pp. 1807-1821. http://dx.doi.org/10.1111/j.1582-4934.2011.01318.x
- C. L. Masters, G. Simms, N. A. Weinman, G. Multhaup and K. Beyreuther, “Amyloid Plaque Core Protein in Alzheimer’s Disease and Down’s Syndrome,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 82, No. 12, 1985, pp. 4245-4249. http://dx.doi.org/10.1073/pnas.82.12.4245
- S. Bodovitz and W. L. Klein, “Cholesterol Modulates α- Secretase Cleavage of Amyloid Precursor Protein,” Journal of Biological Chemistry, Vol. 271, No. 8, 1996, pp. 4436-4440. http://dx.doi.org/10.1074/jbc.271.8.4436
- R. Vassar, B. D. Bennett, S. Babu-Khan, S. Kahn, E. A. Mendiaz, P. Denis, et al., “Beta-Secretase Cleavage of Alzheimer’s Amyloid Precursor Protein by the Transmembrane Aspartic Protease BACE,” Science, Vol. 286, No. 5540, 1999, pp. 735-741. http://dx.doi.org/10.1126/science.286.5440.735
- F. S. Esch, P. S. Keim, E. C. Beattie, R. W. Blacher, A. R. Culwell, T. Oltersdorf, D. McClure and P. J. Ward, “Cleavage of Amyloid Beta Peptide during Constitutive Processing of Its Precursor,” Science, Vol. 248, No. 4959, 1990, pp. 1122-1124. http://dx.doi.org/10.1126/science.2111583
- S. Soriano, A. S. Chyung, X. Chen, G. B. Stokin, V. M. Lee and E. H. Koo, “Expression of β-Amyloid Precursor Protein-CD3γ Chimeras to Demonstrate the Selective Generation of Amyloid β1-40 and Amyloid β1-42 Peptides within Secretory and Endocytic Compartments,” Journal of Biological Chemistry, Vol. 274, No. 45, 1999, pp. 32295-32300. http://dx.doi.org/10.1074/jbc.274.45.32295
- H. Hayashi, N. Kimura, H. Yamagauchi, K. Hasegawa, T. Yokoseki, M. Shibata, N. Yamamoto, M. Michikawa, Y. Yoshikawa, K. Terao, K. Matsuzaki, C. A. Lemere, D. J. Selkoe, H. Naiki and K. Yanagisawa, “A Seed for Alzheimer Amyloid in the Brain,” Journal of Neuroscience, Vol. 24, No. 20, 2004, pp. 4894-4902. http://dx.doi.org/10.1523/JNEUROSCI.0861-04. 2004
- J. Bieschke, Q. Zhang, E. T. Powers, R. A. Lerner and J. W. Kelly, “Oxidative Metabolites Accelerate Alzheimer’s Amyloidogenesis by a Two Step Mechanism, Eliminating the Requirement for Nucleation,” Biochemistry, Vol. 44, No. 13, 2005, pp. 4977-4983. http://dx.doi.org/10.1021/bi0501030
- A. E. Roher, Y. M. Kuo, K. M. Kokjohn, M. R. Emmerling and S. Gracon, “Amyloid and Lipids in the Pathology of Alzheimer’s Disease,” Amyloid, Vol. 6, No. 2, 1998, pp. 136-145. http://dx.doi.org/10.3109/13506129909007315
- Y. M. Kuo, M. R. Emmerling, C. L. Bisgaier, A. D. Essenburg, H. C. Lampert, D. Drumm and A. E. Roher, “Elevated Low Density Lipoprotein in Alzheimer’s Disease Correlates with Brain Aβ 1-42 Levels,” Biochemical and Biophysical Research Communications, Vol. 252, No. 3, 1998, pp. 711-715. http://dx.doi.org/10.1006/bbrc.1998.9652
- A. Merched, Y. Xia, S. Visvikis, J. M. Serrot and G. Siest, “Decreased High Density Lipoprotein Cholesterol and Serum Apolipoprotein AI Concentrations Are Highly Correlated with the Severity of Alzheimer’s Disease,” Neurobiology of Aging, Vol. 21, No. 1, 2000, pp. 27-30. http://dx.doi.org/10.1016/S0197-4580(99)00103-7
- M. Dean, Y. Hamon and G. Chimini, “The Human ATPBinding Cassette (ABC) Transporter Superfamily,” Journal of Lipid Research, Vol. 42, No. 7, 2001, pp. 1007- 1017.
- D. H. Mauch, K. Nagler, S. Shumacher, C. Goritz, E. C. Muller, A. Otto and F. W. Pfreiger, “CNS Synaptogenesis Promoted by Glia-Derived Cholesterol,” Science, Vol. 294, No. 5545, 2001, pp. 1354-1357. http://dx.doi.org/10.1126/science.294.5545.1354
- R. P. Koldamova, I. M. Lefterov, M. D. Ikonomovic, J. Skoko, P. I. Lefterov, B. A. Isanski, S. T. DeKosky and J. S. Lazo, “22R-Hydroxy Cholesterol and 9-cis-Retinoic Acid Induce ATP Binding Cassette Transporter A1 Expression and Cholesterol Efflux in Brain Cells and Decrease Amyloid β Secretion,” Journal of Biological Chemistry, Vol. 278, No. 15, 2003, pp. 13244-13256. http://dx.doi.org/10.1074/jbc.M300044200
- S. E. Wahrle, H. Jiang, M. Parasadanian, J. Legleiter, X. Han, J. D. Fryer, T. Kowalewski and D. M. Holtzman, “ABCA1 Is Required for Normal Central Nervous System ApoE Levels and for Lipidation of Astrocyte-Secreted ApoE,” Journal of Biological Chemistry, Vol. 279, No. 39, 2004, pp. 40987-40993. http://dx.doi.org/10.1074/jbc.M407963200
- A. Sundqvist and J. Ericsson, “Transcription Dependent Degradation Controls the Stability of the SREBP Family of Transcription Factors,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 100, No. 24, 2003, pp. 13833-13838. http://dx.doi.org/10.1073/pnas.2335135100
- R. Ehehalt, P. Keller, C. Haass, C. Thiele and K. Simons, “Amyloidogenic Processing of the Alzheimer’s β-Amyloid Precursor Protein Depends on Lipid Rafts,” Journal of Cell Biology, Vol. 160, No. 1, 2003, pp. 113-123. http://dx.doi.org/10.1083/jcb.200207113
- K. S. Vetrivel, H. Cheng, W. Lin, T. Sakurai, T. Li, N. Nakina, P. C. Wong, H. Xu and G. Thinakaran, “Association of γ-Secretase with Lipid Rafts in Post Golgi and Endosome Membranes,” Journal of Biological Chemistry, Vol. 279, No. 43, 2004, pp. 44945-44954. http://dx.doi.org/10.1074/jbc.M407986200
- M. Simons, P. Keller, B. DeStrooper, K. Beyreuther, C. G. Dotti and K. Simons, “Cholesterol Depletion Inhibits the Generation of βamyloid in Hippocampal Neurons,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 95, No. 11, 1998, pp. 6460-6464. http://dx.doi.org/10.1073/pnas.95.11.6460
- E. Kojro, G. Gimpl, S. Lammich, W. März and F. Fahrenholz, “Low Cholesterol Stimulates the Nonamyloidogenic Pathway by Its Effect on the α-Secretase ADAM 10,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 98, No. 10, 2001, pp. 5815-5820. http://dx.doi.org/10.1073/pnas.081612998
- M. O. W. Grimm, H. S. Grim, H. J. Patzold, E. G. Zinser, R. Halomen, M. Durering, J. A. Tschäpe, B. De Strooper, U. Müller, J. Shen and T. Hartmann, “Regulation of Cholesterol and Sphingomyelin Metabolism by Amyloid-β and Presenilin,” Nature Cell Biology, Vol. 7, No. 11, 2005, pp. 1118-1123. http://dx.doi.org/10.1038/ncb1313
- A. Miyazaki, M. Sakai, Y. Sakamoto and S. Horiuchi, “Acyl-Coenzyme A: Cholesterol Acyl Transferase Inhibitors for Controlling Hypercholesterolemia and Atherosclerosis,” Current Opinion in Investigational Drugs, Vol. 4, No. 9, 2003, pp. 1095-1099.
- L. Puglielli, B. C. Ellis, L. A. Ingano and D. M. Kovacs, “Role of Acyl-Coenzyme A: Cholesterol Acyl Transferase Activity in the Processing of Amyloid Precursor Protein,” Journal of Molecular Neuroscience, Vol. 24, No. 1, 2004, pp. 93-96. http://dx.doi.org/10.1385/JMN:24:1:093
- B. Hutter-Paier, H. J. Huttunen, L. Puglielli, C. B. Eckman, D. Y. Kim, A. Hofmeister, R. D. Moir, S. B. Domnitz, M. P. Frosch, M. Windisch and D. M. Kovacs, “The ACAT Inhibitor CP-113, 818 Markedly Reduces Amyloid Pathology in a Mouse Model of Alzheimer’s Disease,” Neuron, Vol. 44, No. 2, 2004, pp. 227-238. http://dx.doi.org/10.1016/j.neuron.2004.08.043
- D. Cao, K. Fukuchi, H. Q. Wan, H. Kim and L. Li, “Lack of the LDL Receptor Aggravates Learning Deficits and Amyloid Deposits in Alzheimer’s Disease Transgenic Mice,” Neurobiology of Aging, Vol. 27, No. 11, 2006, pp. 1632-1643. http://dx.doi.org/10.1016/j.neurobiolaging.2005.09.011
- W. Retz, J. Thorne, N. Durany, A. Harsanyi, P. Retz-Junginger, J. Kornhuber, P. Riederer and M. Rosler, “Potential Genetic Markers of Sporadic Alzheimer’s Dementia,” Psychiatric Genetics, Vol. 11, No. 3, 2001, pp. 115-122. http://dx.doi.org/10.1097/00041444-200109000-00002
- C. V. Zerbinatti, S. E. Wahrle, H. Kim, J. A. Cam, K. Bates, S. M. Paul, D. M. Holtzman and G. Bu, “Apolipoprotein E and Low Density Lipoprotein Receptor Related Protein Facilitate Intraneuronal Aβ42 Accumulation in Amyloid Model Mice,” Journal of Biological Chemistry, Vol. 281, No. 47, 2006, pp. 36180-36186. http://dx.doi.org/10.1074/jbc.M604436200
- R. Deane, R. Bell, A. Sagare and B. V. Zlokovic, “Clearance of Amyloid-Beta Peptide Across the Blood-Brain Barrier: Implication for Therapies in Alzheimer’s Disease,” Central Nervous System & Neurology Disorder-Drug Targets, Vol. 8, No. 1, 2009, pp. 16-30. http://dx.doi.org/10.2174/187152709787601867
- S. Jaeger and C. J. Pietrzik, “Functional Role of Lipoprotein Receptors in Alzheimer’s Disease,” Current Alzheimer’s Research, Vol. 5, No. 1, 2008, pp. 15-25. http://dx.doi.org/10.2174/156720508783884675
- M. P. Marzolo and G. J. Bu, “Lipoprotein Receptors and Cholesterol in APP Trafficking and Proteolytic Processing, Implications for Alzheimer’s Disease,” Seminars in Cell & Developmental Biology, Vol. 20, No. 2, 2009, pp. 191- 200. http://dx.doi.org/10.1016/j. semcdb.2008.10.005
- E. Waldron, S. Jaeger and C. U. Pietrzik, “Functional Role of the Low Density Lipoprotein Receptor Related Protein in Alzheimer’s Disease,” Neurodegeneration Disease, Vol. 3, No. 4, 2006, pp. 233-238. http://dx.doi.org/10.1159/000095261
- I. Bjorkhem and S. Meaney, “Brain Cholesterol: Long Secret Life behind a Barrier,” Arteriosclerosis, Thrombosis and Vascular Biology, Vol. 24, No. 5, 2004, pp. 806-815. http://dx.doi.org/10.1161/01.ATV.0000120374.59826.1b
- I. Bjorkhem, U. Diczfalusy and D. Lutjohann, “Removal of Cholesterol from Extrahepatic Sources by Oxidative Mechanisms,” Current Opinion in Lipidology, Vol. 10, No. 2, 1999, pp. 161-165. http://dx.doi.org/10.1097/00041433-199904000-00010
- J. M. Dietschy and S. D. Turley, “Cholesterol Metabolism in the Brain,” Current Opinion in Lipidology, Vol. 12, No. 2, 2001, pp. 105-112. http://dx.doi.org/10.1097/00041433-200104000-00003
- J. E. Vance, H. Hayashi and B. Karten, “Cholesterol Homeostasis in Neurons and Glial Cells,” Seminar in Cell & Developmental Biology, Vol. 16, No. 2, 2005, pp. 193-212. http://dx.doi.org/10.1016/j.semcdb.2005.01.005
- U. Funfschilling, G. Saher, L. Xiao, W. Mobius and K. A. Nave, “Survival of Adult Neurons Lacking Cholesterol Synthesis in Vivo,” BMC Neuroscience, Vol. 8, No. 1, 2007, pp. 1-9. http://dx.doi.org/10.1186/1471-2202-8-1
- G. Saher, B. Brugger, C. Lappe-Sietke, W. Mobius, R. Tozawa, M. C. Wehr, F. Wieland, F. Ishibashi and K. Nave, “High Cholesterol Level Is Essential for Myelin Membrane Growth,” Nature Neuroscience, Vol. 8, No. 4, 2005, pp. 468-475.
- F. W. Pfreiger, “Cholesterol Homeostasis and Function in Neurons of the Central Nervous System,” Cellular and Molecular Life Sciences, Vol. 60, No. 6, 2003, pp. 1158- 1171.
- E. G. Lund, J. M. Guileyard and D. W. Russell, “cDNA Cloning of Cholesterol 24-Hydroxylase, a Mediator of Cholesterol Homeostasis in the Brain,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 96, No. 13, 1999, pp. 7238-7243. http://dx.doi.org/10.1073/pnas.96.13. 7238
- D. Lutjohann, O. Breuner, G. Ahlberg, I. Nemesno, A. Siden, U. Diczfalusy and I. Bjorkhem, “Cholesterol Homeostasis in Human Brain: Evidence for an Age Dependent Flux of 24S-Hydroxycholesterol from the Brain into the Liver,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 93, No. 18, 1996, pp. 9799-9804. http://dx.doi.org/10.1073/pnas.93.18.9799
- D. Lutjohann, A. Papassotiropoulos, I. Björkhem, S. Locatelli, M. Bagli and R. D. Oehring, “Plasma 24S-Hydroxycholesterol Is Increased in Alzheimer’s and Vascular Demented Persons,” Journal of Lipid Research, Vol. 41, No. 2, 2000, pp. 195-198.
- A. Papassotiropoulos, D. Lutjohann, M. Bagli, M. Locatelli, F. Jessen, M. L. Rao, W. Maier, I. Bjürkhem, K. von Bergmann and R. Heun, “Plasma 24S-Hydroxycholesterol: A Peripheral Indicator of Neuronal Degeneration and Potential State Marker for Alzheimer’s Disease,” Neuroreport, Vol. 11, No. 9, 2000, pp. 1959-1962. http://dx.doi.org/10.1097/00001756-200006260-00030
- A. Papassotiropoulos, D. Lütjohann, M. Bagli, S. Locatelli, F. Jessen, R. Buschfort, U. Ptok, I. Björkhem, K. von Bergmann and R. Heun, “24S-Hydroxycholesterol in Cerebrospinal Fluid Is Elevated in Early Stages of Dementia,” Journal of Psychiatric Research, Vol. 36, No. 1, 2002, pp. 27-32. http://dx.doi.org/10.1016/S0022-3956(01)00050-4
- H. Jick, G. I. Zornberg, S. S. Jick, S. Seshadri and D. A. Drachman, “Statins and the Risk of Dementia,” The Lancet, Vol. 356, No. 9242, 2000, pp. 1627-1631. http://dx.doi.org/10.1016/S0140-6736(00)03155-X
- B. Wolozin, W. Kellman, P. Rousseau, C. G. Celessia and G. Siegel, “Decreased Prevalence of Alzheimer’s Disease Associated with 3-Hydroxy-3methyl-Glutaryl Coenzyme: A Reductase Inhibitors,” JAMA Neurology, Vol. 57, No. 10, 2000, pp. 1439-1143. http://dx.doi.org/10.1001/archneur.57.10.1439
- N. B. Chauhan, G. J. Siegel and D. L. Feinstein, “Effects of Lovastatin and Pravastatin on Amyloid Processing and Inflammatory Response in TgCRND8 Brain,” Neurochemical Research, Vol. 29, No. 10, 2004, pp. 1887-1911. http://dx.doi.org/10.1023/B:NERE.0000042217.90204.8d
- S. S. Petanceska, S. DeRosa, V. Olm, N. Diaz, A. Sharma, T. Thomas-Bryant, K. Duff, M. Pappolla and L. M. Refolo, “Statin Therapy for Alzheimer’s Disease, Will It Work?” Journal of Molecular Neuroscience, Vol. 19, No. 1-2, 2002, pp. 155-161. http://dx.doi.org/10.1007/s12031-002-0026-2
- L. M. Refolo, B. Malester, J. LaFrancois, T. Bryant-Thomas, R. Wang, G. S. Tint, K. Sambamurti, K. Duff and M. A. Pappolla, “Hypercholesterolemia Accelerates the Alzheimer’s Amyloid Pathology in a Transgenic Mouse Model,” Neurobiology of Disease, Vol. 7, No. 4, 2000, pp. 321-331. http://dx.doi.org/10.1006/nbdi.2000.0304
- L. M. Refollo, M. A. Papolla, J. LaFrancois, B. Malester, S. D. Schmidt, T. Thomas-Bryant, G. S. Tint, R. Wang, M. Mercken, S. S. Petanceska and K. E. Duff, “A Cholesterol Lowering Drug Reduces β-Amyloid Pathology in a Transgenic Mouse Model of Alzheimer’s Disease,” Neurobiology of Disease, Vol. 8, No. 5, 2001, pp. 890-899. http://dx.doi.org/10.1006/nbdi.2001.0422
- K. Fassbender, M. Simons, C. Bergmann, M. Stroick, D. Lutjohann, P. Keller, H. Runz, S. Kuhl, T. Bertsch, K. von Bergmann, M. Hennerici, K. Beyreuther and T. Hartmann, “Simvastatin Strongly Reduces Levels of Alzheimer’s Disease β-Amyloid Peptides Aβ 42 and Aβ 40 in Vitro and in Vivo,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 98, No. 10, 2001, pp. 5856-5861. http://dx.doi.org/10.1073/pnas.081620098
- I. J. Martins, E. Hone, J. K. Foster, S. I. Sunram-Lea, A. Gnjec, S. J. Fuller, D. Nolan, S. E. Gandy and R. N. Martins, “Apolipoprotein E, Cholesterol Metabolism, Diabetes and the Convergence of Risk Factors for Alzheimer’s Disease and Cardiovascular Disease,” Molecular Psychiatry, Vol. 11, No. 8, 2006, pp. 721-736. http://dx.doi.org/10.1038/sj. mp.4001854
- I. J. Martins, T. Berger, M. J. Sharman, G. Verdile, S. J. Fuller and R. N. Martins, “Cholesterol Metabolism and Transport in the Pathogenesis of Alzheimer’s Disease,” Journal of Neurochemistry, Vol. 111, No. 6, 2009, pp. 1275-1308. http://dx.doi.org/10.1111/j.1471-4159.2009.06408.x
- H. Chen, S. M. Zhang, M. A. Hernan, W. C. Willet and A. Ascherio, “Diet and Parkinson’s Disease: A Potential Role of Dairy Products in Men,” Annals of Neurology, Vol. 52, No. 6, 2002, pp. 793-801.
- H. Chen, E. O’Reilly, M. L. McCullough, C. Rodriguez, M. A. Schwarzschild, E. E Calle, M. J. Thun and A. Ascherio, “Consumtion of Dairy Products and Risks of Parkinson’s Disease,” American Journal of Epidemiology, Vol. 165, No. 9, 2007, pp. 998-1006. http://dx.doi.org/10.1093/aje/kwk089
- M. Park, G. W. Ross, H. Petrovitch, L. R. White, K. H. Masaki, J. S. Nelson, C. M. Tanner , J. D. Curb, P. L. Blanchette and R. D. Abbott, “Consumption of Milk and Calcium in Midlife and the Future Risk of Parkinson’s Disease,” Neurology, Vol. 64, No. 6, 2005, pp. 1047-1051. http://dx.doi.org/10.1212/01.WNL.0000154532.98495. BF
- G. Logroscino, K. Marder, L. Cote, M. X. Tang, S. Shea and R. Mayeux, “Dietary Lipids and Antioxidants in Parkinson’s Disease: A Population-Based, Case-Control Study,” Annals of Neurology, Vol. 39, No. 1, 1996, pp. 89-94. http://dx.doi.org/10.1002/ana.410390113
- M. Barichella, E. Cereda and G. Pezzoli, “Major Nutritional Issues in the Management of Parkinson’s Disease,” Movement Disorders, Vol. 24, No. 13, 2009, pp. 1881-1892. http://dx.doi.org/10.1002/mds.22705
- R. A. Alcalay, Y. Gu, H. Mejia-Santana, L. Cote, K. S. Marder and N. Scarmeas, “Mediterranean Diet Adherence and Parkinson’s Disease,” Movement Disorders, Vol. 27, No. 6, 2012, pp. 771-774. http://dx.doi.org/10.1002/mds.24918
- K. Marder, Y. Gu, S. Eberly, C. M. Tanner, N. Scarmeas, D. Oakes and I. Shoulson, “Relationship of Mediterranean Diet and Caloric Intake to Phenoconversion in Huntington Disease,” JAMA Neurology, Vol. 70, No. 11, 2013, pp. 1382-1388.
- A. Trejo, R. M. Tarrats, M. E. Alonso, M. C. Boll, A. Ochoa and L. Velásquez, “Assessment of the Nutrition Status of Patients with Huntington’s Disease,” Nutrition, Vol. 20, No. 2, 2004, pp. 192-196.
- M. J. Dowie, E. L. Scotter, E. Molinari and M. Glass, “The Therapeutic Potential of G-Protein Coupled Receptors in Huntington’s Disease,” Pharmacology & Therapeutics, Vol. 128, No. 2, 2010, pp. 305-323. http://dx.doi.org/10.1016/j. pharmthera.2010.07.008
- L. Wang, B. Martins, R. Brenneman, L. M. Lutrell and S. Maudsley, “Allosteric Modulators of G Protein-Coupled Receptors: Future Therapeutics for Complex Physiological Disorders,” Journal of Pharmacology and Experimental Therapeutics, Vol. 331, No. 2, 2009, pp. 340-348. http://dx.doi.org/10.1124/jpet.109.156380
- E. J. Goetzl, “Diverse Pathways for Nuclear Signaling by G Protein-Coupled Receptors and Their Ligands,” FASEB Journal, Vol. 21, No. 3, 2007, pp. 638-642. http://dx.doi.org/10.1096/fj.06-6624hyp
- R. Alemany, J. S. Perona, J. M. Sánchez-Dominguez, E. Montero, J. Cañizares, R. Bressani, P. V. Escribá and V. Ruiz-Gutierrez, “G Protein-Coupled Receptor Systems and Their Lipid Environment in Health Disorders during Aging,” Biochimica et Biophysica Acta, Vol. 1768, No. 4, 2007, pp. 964-975. http://dx.doi.org/10.1016/j. bbamem.2006.09.024
- F. Gobeil, A. Fortier, T. Zhu, M. Bossolasco, M. Leduc, M. Grandbois, N. Heveker, G. Bkaily, S. Chemtob and D. Barbaz, “G-Protein-Coupled Receptors Signalling at the Cell Nucleus: An Emerging Paradigm,” Canadian Journal of Physiology and Pharmacology, Vol. 84, No. 3-4, 2006, pp. 287-297.
- Y. D. Paila and A. Chattopadhyay, “Membrane Cholesterol in the Function and Organization of G-Protein Coupled Receptors,” Subcellular Biochemistry, Vol. 51, 2010, pp. 439-466. http://dx.doi.org/10.1007/978-90-481-8622-8_16
- A. K. Gupta, C. G. Savopoulos, J. Ahuja and A. I. Hatzitolios, “Role of Phytosterols in Lipid Lowering: Current Perspectives,” Quarterly Journal of Medicine, Vol. 104, No. 4, 2011, pp. 301-308. http://dx.doi.org/10.1093/qjmed/hcr007
- F. Gomez-Pinilla, “Brain Foods: The Effects of Nutrients on Brain Function,” Nature Reviews Neuroscience, Vol. 9, No. 7, 2008, pp. 568-578. http://dx. doi.org/10.1038/nrn2421
- S. B. Patel, “Plant Sterols and Stanols: Their Role in Health and Disease,” Journal of Clinical Lipidology, Vol. 2, No. 2, 2008, pp. S11-S19. http://dx.doi.org/10.1016/j.jacl.2008.01.007
- R. E. Ostlund, “Phytosterols in Human Nutrition,” Annual Review in Nutrition, Vol. 22, 2002, pp. 533-549. http://dx.doi.org/10.1146/annurev.nutr.22.020702.075220
- S. P. Choudhary and L. S. Tran, “Phytosterols: Perspectives in Human Nutrition and Clinical Therapy,” Current Medicinal Chemistry, Vol. 18, No. 29, 2011, pp. 4557-4567. http://dx.doi.org/10.2174/092986711797287593
- T. Vanmierlo, J. Popp, H. Kolsch, S. Friedrichs, F. Jessen, B. Stoffel-Wagner, T. Bertsch , T. Hartmann, W. Maier, K. von Bergmann, H. Steinbusch, M. Mulder and D. Lutjohann, “The Plant Sterol Brassicasterol as Additional CSF Biomarker in Alzheimer’s Disease,” Acta Psychiatrica Scandinavica, Vol. 124, No. 3, 2011, pp. 184-192. http://dx.doi.org/10.1111/j.1600-0447.2011.01713.x
- I. Demonty, R. T. Ras, H. C. M. van der Knaap, S. M. Guus, J. E. Duchateau, L. Meijer, P. L. Zock, J. M. Geleijnse and E. A. Trautwein, “Continuous Dose-Response Relationship of the LDL-Cholesterol-Lowering Effect of Phytosterol Intake,” Journal of Nutrition, Vol. 139, No. 2, 2009, pp. 271-284. http://dx.doi.org/10.3945/jn.108.095125
- M. K. Gul and S. Amar, “Sterols and the Phytosterol Content in Oilseed Rape (Brassica napus L.),” Journal of Cell and Molecular Biology, Vol. 5, No. 2, 2006, pp. 71-79.
- P. J. Jansen, D. Lütjohann, K. Abildayeva, T. Vanmierlo, T. Plösch, J. Plat, K. von Bergmannb, A. K. Groene, F. C. S. Ramaekersa, F. Kuipersc and M. Mulder, “Dietary Plant Sterols Accumulate in the Brain,” Biochimica et Biophysica Acta, Vol. 1761, No. 4, 2006, pp. 445-453. http://dx.doi.org/10.1016/j.bbalip.2006.03.015
- T. Vanmierlo, O. Weingärtner, S. van der Pol, C. Husche, A. Kerksiek, S. Friedrichs, E. Sijbrands, H. Steinbusch, M. Grimm, T. Hartmann, U. Laufs, M. Böhm, H. E. de Vries, M. Mulder and D. Lütjohann, “Dietary Intake of Plant Sterols Stably Increases Plant Sterol Levels in Themurine Brain,” Journal of Lipid Research, Vol. 53, No. 4, 2012, pp. 726- 735. http://dx.doi.org/10.1194/jlr.M017244
- D. Lütjohann, A. Brzezinka, E. Barth, D. Abramowski, M. Staufenbiel, K. von Bergmann, K. Beyreuther, G. Multhaup and T. A. Bayer, “Profile of Cholesterol-Related Sterols in Agedamyloid Precursor Protein Transgenic Mouse Brain,” Journal of Lipid Research, Vol. 43, No. 7, 2002, pp. 1078- 1085.
- C. B. Fricke, M. Schrøder, M. Poulsen , K. von Bergmann, I. Wester, I. Knudsen, A. Mortensen and D. Lütjohann, “Increased Plant Sterol and Stanol Levels in Brain of Watanabe Rabbits Fed Rapeseed Oil Derived Plant Sterol or Stanol Esters,” British Journal of Nutrition, Vol. 98, No. 5, 2007, pp. 890-899.
- C. C. Smith, P. J. Hyatt, L. Stanyer and D. J. Betteridge, “Platelet Secretion of Beta-Amyloid Is Increased in Hypercholesterolaemia,” Brain Research, Vol. 896, No. 1-2, 2001, pp. 161-164. http://dx.doi.org/10.1016/S0006-8993(01)02080-7
- C. Shi, J. Liu, F. M. Wu, X. M. Zhu, D. T. Yew and J. Xu, “β-Sitosterol Inhibits High Cholesterol-Induced Platelet β-Amyloid Release,” Journal of Bioenergetics and Biomembranes, Vol. 43, No. 6, 2011, pp. 691-697. http://dx.doi.org/10.1007/s10863-011-9383-2
- M. Katzi, I. Bartov, P. Budowski and A. Bondi, “Inhibition of Cholesterol Deposition in Livers of Mice Fed Phytosterols in Short-Term Experiments,” Journal of Nutrtion, Vol. 100, No. 10, 1970, pp. 1141-1148.
- D. S. MacKay and P. Jones, “Limitations of Lathosterol to plant Sterol Ratios and Serum Plant Sterols as Surrogate Markers for Cholesterol Absorption during Plant Sterol Supplementation,” Nutrition, Metabolism and Cardiovascular Diseases, Vol. 22, No. 9, 2012, p. e21. http://dx.doi.org/10.1016/j.numecd.2011.11.007
- T. A. Miettinen, H. Gylling and M. J. Nissinen, “The Role of Serum Non-Cholesterol Sterols as Surrogate Markers of Absolute Cholesterol Synthesis and Absorption,” Nutrition, Metabolism and Cardiovascular Disease, Vol. 21, No. 10, 2011, pp. 765-769. http://dx.doi.org/10.1016/j.numecd.2011.05.005
- E. Misawa, M. Tanaka, K. Nomaguchi, K. Nabeshima, M. Yamada, T. Toida and K. Iwatsuki, “Oral Ingestion of Aloe Vera Phytosterols Alters Hepatic Gene Expression Profiles and Ameliorates Obesity-Associated Metabolic Disorders in Zucker Diabetic Fatty Rats,” Journal of Agricultural and Food Chemistry, Vol. 60, No. 11, 2012, pp. 2799-2806. http://dx.doi.org/10.1021/jf204465j
- T. M. Jeitner, I. Voloshyna and A. B. Reiss, “Oxysterol Derivatives of Cholesterol in Neurodegenerative Disorders,” Current Medicinal Chemistry, Vol. 18, No. 10, 2011, pp. 1515-1525. http://dx. doi. org/10. 2174/092986711795328445
- V. Leoni, “Oxysterols as Markers of Neurological Disease—A Review,” Scandinavian Journal of Clinical & Laboratory Investigation, Vol. 69, No. 1, 2009, pp. 22-25. http://dx.doi.org/10.1080/00365510802651858
- A. Otaegui-Arrazola, M. Menéndez-Carreño, D. Ansorena and I. Astiasarán, “Oxysterols: A World to Explore,” Food and Chemical Toxicology, Vol. 48, No. 12, 2010, pp. 3289- 3303. http://dx.doi.org/10.1016/j.fct.2010.09.023
- K. Yasutake, M. Kohjima, M. Nakashima, K. Kotoh, M. Nakamuta and M. Enjoji, “Nutrition Therapy for Liver Diseases Based on the Status of Nutritional Intake,” Gastroenterology Research and Practice, Vol. 2012, 2012, Article ID: 859697. http://dx.doi.org/10.1155/2012/859697
- R. Brauner, C. Johannes, F. Ploess, F. Bracher and R. L. Lorenz, “Phytosterols Reduce Cholesterol Absorption by Inhibition of 27-Hydroxycholesterol Generation, Liver X Receptor α Activation and Expression of the Basolateral Sterol Exporter ATP-Binding Cassette A1 in Caco-2 Enterocytes,” Journal of Nutrition, Vol. 142, No. 6, 2012, pp. 981-989. http://dx.doi.org/10.3945/jn.111.157198
- Q. Chen, H. Gruber, C. Pakenham, W. M. N. Ratnayake and K. A. Scoggan, “Dietary Phytosterols and Phytostanols Alter the Expression of Sterol-Regulatory Genes in SHRSP and WKY inbred Rats,” Annals of Nutrition & Metabolism, Vol. 55, No. 4, 2009, pp. 341-350. http://dx.doi.org/10.1159/000252350
- N. S. Sabeva, C. M. McPhaul, X. G. Li and T. J. Cory, D. J. Feola and G. A. Graf, “Phytosterols Differentially Influence ABC transporter Expression, Cholesterol Efflux and Inflammatory Cytokine Secretion in Macrophage Foam Cells,” Journal of Nutritional Biochemistry, Vol. 22, No. 8, 2011, pp. 777-783. http://dx.doi.org/10.1016/j.jnutbio.2010.07.002
- C. P. Chuu, “Modulation of Liver X Receptor Signaling as a Prevention and Therapy for Colon Cancer,” Medical Hypotheses, Vol. 76, No. 5, 2011, pp. 697-699. http://dx.doi.org/10.1016/j.mehy.2011.01.037
- E. Ikonen, “Mechanisms for Cellular Cholesterol Transport: Defects and Human Disease,” Physiological Reviews, Vol. 86, No. 4, 2006, pp. 1237-1261. http://dx.doi.org/10.1152/physrev.00022.2005
- I. J. Martins, A. C. Wilson, W. L. F. Lim, S. M. Laws, S. J. Fuller and R. N. Martins, “Sirtuin 1 Mediates the Obesity Induced Risk of Common Degenerative Diseases: Alzheimer’s Disease, Coronary Artery Disease and Type 2 Diabetes,” Health, Vol. 4, No. 12A, 2012, pp. 1-9.
- S. V. Chochina, N. A. Avdulov, U. Igbavboa, J. P. Cleary, E. O. O’Hare and W. G. Wood, “Amyloid Beta-Peptide1-40 Increases Neuronal Membrane Fluidity: Role of Cholesterol and Brain Region,” Journal of Lipid Research, Vol. 42, No. 8, 2001, pp. 1292-1297.
- G. P. Eckert, N. J. Cairns, A. Maras, W. F. Gattaf and W. E. Muller, “Cholesterol Modulates the Membrane-Disordering Effects of Beta-Amyloid Peptides in the Hippocampus: Specific Changes in Alzheimer’s Disease,” Dementia and Geriatric Cognitive Disorders, Vol. 11, 2000, pp. 181-186. http://dx.doi.org/10.1159/000017234
- N. V. Koudinov, K. Anatol, T. T. Berezov and A. R. Koudinov, “Amyloid Beta, Neural Lipids, Cholesterol and Alzheimer’s Disease,” Neurobiology of Lipids, Vol. 1, No. 6, 2003, pp. 28-33.
- K. Hąc-Wydro, A. Zając and P. Dynarowicz-Łątka, “The Influence of Plant Stanol on Phospholipids Monolayers— The Effect of Phospholipid Structure,” Journal of Colloid and Interface Science, Vol. 360, No. 2, 2011, pp. 681-689. http://dx.doi.org/10.1016/j.jcis.2011.04.089
- K. H. Wydro, P. Wydro, A. Jogoda and J. Kapusta, “The Study on the Interaction between Phytosterols and Phospholipids in Model Membranes,” Chemistry and Physics of Lipids, Vol. 150, No. 1, 2007, pp. 22-34. http://dx.doi.org/10.1016/j.chemphyslip.2007.06.211
- S. R. Ji, Y. Wu and S. F. Sui, “Study of Beta-Amyloid Peptide (Abeta40) Insertion into Phospholipid Membranes Using Monolayer Technique,” Biochemistry, Vol. 67, No. 11, 2002, pp. 1283-1288. http://dx.doi.org/10.1023/A:1021361607611
- K. Hac-Wydro, “The Replacement of Cholesterol by Phytosterols and the Increase of Total Sterol Content in Model Erythrocyte Membranes,” Chemistry and Physics of Lipids, Vol. 163, No. 7, 2010, pp. 689-697. http://dx.doi.org/10.1016/j.chemphyslip.2010.07.001
- A. K. Bhattacharyya and D. A. Eggen, “Effect of Dietary Cholesterol Level on Plasma Campesterol Concentration in Rhesus Monkeys,” Annals of Nutrition and Metabolism, Vol. 31, No. 5, 1987, pp. 276-281. http://dx.doi.org/10.1159/000177280
- E. Bartnikowska, “Biological Activities of Phytosterols with Particular Attention to Their Effects on Lipid Metabolism,” Polish Journal Food & Nutrition Science, Vol. 59, No. 2, 2009, pp. 105-112.
- T. S. Tillman and M. Cascio, “Effects of Membrane Lipids on Ion Channel Structure and Function,” Cell Biochemistry and Biophysics, Vol. 38, No. 2, 2003, pp. 161-190. http://dx.doi.org/10.1385/CBB:38:2:161
- R. W. Gross, C. M. Jenkins, J. Yang, D. J. Mancuso and X. Han, “Functional Lipidomics: The Roles of Specialized Lipids and Lipid-Protein Interactions in Modulating Neuronal Function,” Prostaglandins & Other Lipid Mediators, Vol. 77, No. 1-4, 2005, pp. 52-64. http://dx.doi.org/10.1016/j.prostaglandins.2004.09.005
- M. Cascio, “Connexins and Their Environment: Effects of Lipid Composition on Ion Channels,” Biochimica et Biophysica Acta, Vol. 1711, No. 2, 2005, pp. 142-143.
- N. G. Bazan, “Synaptic Signalling by Lipids in the Life and Death of Neurons,” Molecular Neurobiology, Vol. 31, No. 1-3, 2005, pp. 219-230. http://dx.doi.org/10.1385/MN:31:1-3:219
- P. L. Yeagle, “Lipid Regulation of Cell Membrane Structure and Function,” FASEB Journal, Vol. 3, No. 7, 1989, pp. 1833-1842.
- C. Dart, “Lipid Microdomains and the Regulation of Ion Channel Function,” Journal of Physiology, Vol. 588, No. 17, 2010, pp. 3169-3178. http://dx.doi.org/10.1113/jphysiol.2010.191585
- N. A. Shirwany, D. Payette, J. Xie and Q. Guo, “The Amyloid Beta Ion Channel Hypothesis of Alzheimer’s Disease,” Neuropsychiatric Disease and Treatment, Vol. 3, No. 5, 2007, pp. 597-612.
- H. Jang, J. Zheng and R. Nussinov, “Models of Beta-Amyloid Ion Channels in the Membrane Suggest that Channel Formation in the Bilayer Is a Dynamic Process,” Biophysical Journal, Vol. 93, No. 6, 2007, pp. 1938-1949. http://dx.doi.org/10.1529/biophysj.107.110148
- I. Levitan, Y. Fang, A. Rosenhouse-Dantsker and V. Romanenko, “Cholesterol Binding and Cholesterol Transport Proteins: Cholesterol and Ion Channels,” Subcellular Biochemistry, Vol. 51, 2010, pp. 509-549. http://dx.doi.org/10.1007/978-90-481-8622-8_19
- P. Prangkio, E. Yusko, D. Sept, J. Yang and M. Mayer, “Multivariate Analyses of Amyloid-Beta Oligomer Populations Indicate a Connection between Pore Formation and Cytotoxicity,” PLoS ONE, Vol. 7, No. 10, 2012, Article ID: e47261. http://dx.doi.org/10.1371/journal.pone.0047261
- R. Capone, F. Garcia Quiroz, P. Prangkio, I. Saluja, A. M. Sauer, M. R. Bautista, S. Raymond, J. Yang and M. Mayer, “Amyloid-b-Induced Ion Flux in Artificial Lipid Bilayers and Neuronal Cells: Resolving a Controversy,” Neurotoxicity Research, Vol. 16, No. 1, 2009, pp. 1-13. http://dx.doi.org/10.1007/s12640-009-9033-1
- A. B. Awad, R. Roy and C. S. Fink, “Beta-Sitosterol, a Plant Sterol Induces Apoptosis and Activates Key Caspases in MDA-MB-231 Human Breast Cancer Cells,” Oncology Reports, Vol. 10, No. 2, 2003, pp. 497-500.
- P. J. Bouic, “The Role of Phytosterols and Phytosterols in Immune Modulation: A Review of the Past 10 Years,” Current Opinion in Clinical Nutrition and Metabolic Care, Vol. 4, No. 6, 2001, pp. 471-475.
- M. H. Moghadasian, B. M. McManu, P. H. Pritchard and J. J. Frohlich, “Tall Oil—Derived Phytosterols Reduce Atherosclerosis in ApoE-Deficient Mice,” Arteriosclerosis, Thrombrosis and Vascular Biology, Vol. 17, No. 1, 1997, pp. 119-126. http://dx.doi.org/10.1161/01.ATV.17.1.119
- A. Wächtershäuser and J. Stein, “Rationale for the Luminal Provision of Butyrate in Intestinal Diseases,” European Journal of Nutrition, Vol. 39, No. 4, 2000, pp. 164-171. http://dx.doi.org/10.1007/s003940070020
- G. Jacobasch, D. Schmiedl, M. Kruschewski and K. Schmehl, “Dietary Resistant Starch and Chronic Inflammatory Bowel Diseases,” International Journal of Colorectal Disease, Vol. 14, No. 4-5, 1999, pp. 201-211. http://dx.doi.org/10.1007/s003840050212
- M. H. Moghadasian, B. M. McManus, D. V. Godin, B. Rodrigues and J. J. Frohlich, “Proatherogenic and Antiatherogenic Effects of Probucol and Phytosterols in Apolipoprotein E-Deficient Mice: Possible Mechanisms of Action,” Circulation, Vol. 99, No. 13, 1999, pp. 1733-1739. http://dx.doi.org/10.1161/01.CIR.99.13.1733
- H. M. Hamer, D. M. Jonkers, A. Bast, S. A. Vanhoutvin, M. A. Fischer, A. Kodde, F. J. Troost, K. Venema and R. J. Brummer, “Butyrate Modulates Oxidative Stress in the Colonic Mucosa of Healthy Humans,” Clinical Nutrition, Vol. 28, No. 1, 2009, pp. 88-93. http://dx.doi.org/10.1016/j.clnu.2008.11.002
- W. J. L. Chen, J. W. Anderson and D. Jennings, “Propionate May Mediate the Hypocholesterolemic Effects of Certain Soluble Plant Fibres in Cholesterol Fed Rats,” Experimental Biology and Medicine, Vol. 175, No. 2, 1984, pp. 215-218. http://dx.doi.org/10.3181/00379727-175-41791
- M. A. Levrat, M. L. Favier, C. Moundras, C. Rémésy, C. Demigné and C. Morand, “Role of Dietary Propionic Acid and Bile Acid Excretion in the Hypocholesterolemic Effects of Oligosaccharides in Rats,” Journal of Nutrition, Vol. 124, No. 4, 1994, pp. 531-538.
- M. J. Eporin, Z. M. Yuan, D. L. Sentz, K. Plaisance and J. L. Eiseman, “Plasma Pharmacokinetics of Butyrate after Intravenous Administration of Sodium Butyrate or Oral Administration of Tributyrin Or Sodium Butyrate to Mice and Rats,” Cancer Chemotherapy and Pharmacology, Vol. 43, No. 6, 1999, pp. 445-453. http://dx.doi.org/10.1007/s002800050922
- I. Demonty, Y. M. Chan, D. Pelled and P. J. Jones, “FishOil Esters of Plant Sterols Improve the Lipid Profile of Dyslipidemic Subjects More than Do Fish-Oil or Sunflower Oil Esters of Plant Sterols,” American Journal of Clinical Nutrition, Vol. 84, No. 6, 2006, pp. 1534-1542.
- B. H. Kim and C. C. Akoh, “Modeling and Optimization of Lipase-Catalyzed Synthesis of Phytosteryl Esters of Oleic Acid by Response Surface Methodology,” Food Chemistry, Vol. 102, No. 1, 2007, pp. 336-342. http://dx.doi.org/10.1016/j.foodchem.2006.05.025
- G. Torrelo, C. F. Torres and G. Reglero, “Enzymatic Strategies for Solvent-Free Production of Short and Medium Chain Phytosteryl Esters,” European Journal of Lipid Science and Technology, Vol. 114, No. 6, 2012, pp. 670-676. http://dx.doi.org/10.1002/ejlt.201100346
- V. E. Shashoua, J. N. Jacob, R. Ridge, A. Campbell and R. J. Baldessarini, “Gamma-Aminobutyric Acid Esters, Synthesis, Brain Uptake and Pharmacological Studies of Aliphatic and Steroid Esters of Gamma-Aminobutyric Acid,” Journal of Medicinal Chemistry, Vol. 27, No. 5, 1984, pp. 659-664. http://dx.doi.org/10.1021/jm00371a018
- R. Ayesh, J. A. Weststrate, P. N. Drewitt and P. A. Hepburn, “Safety Evaluation of Phytosterol Esters. Part 5. Faecal Short-Chain Fatty Acid and Microflora Content, Faecal Bacterial Enzyme Activity and Serum Female Sex Hormones in Healthy Normolipidaemic Volunteers Consuming a Controlled Diet Either with or without a Phytosterol Ester-Enriched Margarine,” Food and Chemical Toxicology, Vol. 37, No. 12, 1999, pp. 1127-1138. http://dx.doi.org/10.1016/S0278-6915(99)00109-X
- K. M. Y. Engel, K. Schröck, D. Teupser, L. M. Holdt, A. Tönjes, et al., “Reduced Food Intake and Body Weight in Mice Deficient for the G Protein-Coupled Receptor GPR82,” PLoS ONE, Vol. 6, No. 12, 2011, Article ID: e29400. http://dx.doi.org/10.1371/journal.pone.0029400
- Y. Xiong, N. Miyamoto, K. Shibata, M. A. Valasek, T. Motoike, R. M. Kedzierski and M. Yanagisawa, “ShortChain Fatty Acids Stimulate Leptin Production in Adipocytes through the G Protein Coupled Receptor GPR41,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 101, No. 4, 2004, pp. 1046- 1050. http://dx.doi.org/10.1073/pnas.2637002100
- M. R. Bomhof, “Gut Bugs, Energy Balance and Obesity,” Molecular Genetic and Clinical Research in Obesity, Vol. 3, 2012, pp. 70-71.
- A. Wchtershauser and J. Stein, “Rationale for the Luminal Provision of Butyrate in Intestinal Diseases,” European Journal of Nutrition, Vol. 39, No. 4, 2000, pp. 164-171. http://dx.doi.org/10.1007/s003940070020
- I. Yin, G. laevsky and C. Giardina, “Butyrate Suppression of Colonocyte NF-κB Activation and Cellular Proteosome Activity,” Journal of Biological Chemistry, Vol. 276, No. 48, pp. 44641-44646. http://dx.doi.org/10.1074/jbc.M105170200
- B. F. Hinebusch, S. Meng, J. T. Wu, S. Y. Archer and R. A. Hodin, “The Effects of Short-Chain Fatty Acids on Human Colon Cancer Cell Phenotype Are Associated with Histone Hyperacetylation,” Journal of Nutrition, Vol. 132, No. 5, 2002, pp. 1012-1017.
- X. Y. He, G. Merz, P. Mehta, H. Schulz and S. Y. Yang, “Human Brain Short Chain L-3 Hydroxyacyl Coenzyme A Dehydrogenase Is A Single-Domain Multifunctional Enzyme. Characterization of a Novel 17Beta-Hydroxysteroid Dehydrogenase,” Journal of Biological Chemistry, Vol. 274, No. 21, 1999, pp. 15014-15019. http://dx.doi.org/10.1074/jbc.274.21.15014
- J. M. Mariadason, G. A. Corner and I. H. Augenlicht, “Genetic Reprogramming in Pathways of Colonic Cell Maturation Induced by Short Chain Fatty Acids: Comparison with Trichostatin A, Sulindac and Curcumin and Implications for Chemoprevention of Colon Cancer,” Cancer Research, Vol. 60, No. 16, 2000, pp. 4561-4572.
- D. Chakravortty, Y. Kato, T. Sugiyama, M. M. Mu, T. Yoshida and T. Yokochi, “The Inhibitory Action of Butyrate on Lipopolysaccharide-Induced Nitric Oxide Production in RAW 264. 7 Murine Macrophage Cells,” Journal of Endotoxin Research, Vol. 6, No. 3, 2000, pp. 243- 247.
- U. Böcker, O. Yezerskyy, P. Feick, T. Manigold, A. Panja, U. Kalina, F. Herweck, S. Rossol and M. V. Singer, “Responsiveness of Intestinal Epithelial Cell Lines to Lipopolysaccharide Is Correlated with Toll-Like Receptor 4 but Not Toll-Like Receptor 2 or CD14 Expression,” International Journal of Colorectal Disease, Vol. 18, No. 1, 2003, pp. 25-32. http://dx.doi.org/10.1007/s00384-002-0415-6
- H. Luhrs, J. Schauber, G. Dusel, R. Melcher, W. Scheppach and T. Menzel, “Cytokine-Activated Degradation of Inhibitory κB Protein α Is Inhibited by the Short-Chain Fatty Acid Butyrate,” International Journal of Colorectal Disease, Vol. 16, No. 4, 2001, pp. 195-201. http://dx.doi.org/10.1007/s003840100295
NOTES
*Corresponding author.

