Food and Nutrition Sciences
Vol. 2 No. 8 (2011) , Article ID: 7893 , 6 pages DOI:10.4236/fns.2011.28122
Influence of Processing on Dietary Fiber, Tannin and in Vitro Protein Digestibility of Pearl Millet
![]()
Department of Studies in Food Science and Nutrition, University of Mysore, Manasagangotri, India.
Email: *asnaurooj@foodsci.uni-mysore.ac.in
Received July 14th, 2011; revised September 6th, 2011; accepted September 13th, 2011.
Keywords: Pearl Millet, Commercial Varieties, Dietary Fibre, Tannins, In-Vitro Protein Digestibility
ABSTRACT
From the nutritional point of view, data on dietary fiber content, tannin and in vitro protein digestibility of processed millet is of importance, because millets are never eaten raw. Effects of commonly used traditional methods on dietary fiber, tannin content and %IVPD of two locally available pearl millet varieties (Kalukombu and Maharashtra Rabi Bajra) were investigated. The millet was subjected to various processing methods like milling (whole flour, semi refined flour and bran rich fraction) roasting, boiling, pressure cooking & germination respectively. Processing had little effect on the total dietary fiber (TDF) content in both varieties; however the bran rich fraction showed highest TDF content of around 29%. Tannins effectively lowered upon boiling and pressure cooking respectively, but significantly increased (P ≥ 0.05) upon germination. Although the % IVPD of the millet (45.5 - 49.3 g/100g) was low, it significantly increased upon milling (bran rich fraction), roasting and germination respectively.
1. Introduction
Pearl millet, a lesser known and underutilized crop can be grown at low maintenance cost, is relatively a cheaper source of nutrients and staple for population below poverty line for economic reasons. It has the distinct advantage of being a drought-resistant crop and hence acts as a principle source of energy, protein, fat and minerals for poor people living in these regions. However, it has some limitations, due to the presence of antinutritional factors such as phytate, tannins and dietary fibre. These compounds are known to interfere with mineral bioavailability, carbohydrate and protein digestibility [1-3].
Protein digestibility is essentially a measure of the susceptibility of protein to proteolysis. A protein with high digestibility is potentially of better nutritional value than one with low digestibility because it provides more amino acids for absorption on proteolysis. Exogenous (interaction of proteins with non-protein components like polyphenols, non-starch polysaccharides, starch, tannins, dietary fibre, phytates and lipids.) and endogenous factors (changes within the proteins themselves) contribute to poor digestibility of proteins. During the process of milling and cooking, proteins may interact with non-protein components and the proteins themselves thereby affecting their digestibility [4]. Studies have indicated that both dietary fiber and tannins contribute to lower nutritional value of dietary proteins with soluble dietary fiber playing a major role in reducing its in vitro digestibility. In beans, soluble dietary fiber seems to play a more important role than insoluble dietary fiber in reducing protein digestibility [5].
Fermentation is one of the processes that decreases the level of antinutrients and enhance protein digestibility thereby improving protein quality and availability in pearl millet and sorghum varieties [6,7]. Irradiation has also been shown to be a useful processing method for reducing the antinutritional compounds and therefore, improving protein digestibility of sorghum grain. This improvement occurred due to modification in protein structure; as a result, more peptide bonds were exposed to hydrolysis [8,9].
The protein digestibility of pearl millet is low and hence there is a growing importance on the enhancement of protein digestibility which can be achieved by the means of traditional processing methods. Food uses of pearl millet are usually traditional and the methods of processing may involve boiling, pressure cooking, roasting or can be served raw after sprouting in the form of salads. Information on the effect of these simple processing methods on dietary fiber, tannins and protein digestibility in pearl millet appears to be lacking. Also in view of the fact that both dietary fiber and tannins contributed to lower protein digestibility because of its heat-stable nature are believed to participate in lowering the nutritional value of bean proteins [5] an attempt was made to find out if dietary fiber and tannins present in the millet are interfering with protein digestibility.
Hence the present study was carried out to analyze the effect of these cost effective processing treatment on the levels of dietary fiber, tannins and their impact on in vitro protein digestibility of the two commercial pearl millet varieties.
2. Material and Methods
Two commercially available Pearl millet varieties namely ‘Kalukombu’ (K) and Maharashtra Rabi Bajra (MRB) were procured from the local market of Mysore, India for the study.
‘Kalukombu’ (K) is a native variety traditionally grown by farmers in India (Karnataka, Tamilnadu and Maharashtra). This variety is not improved by the modern plant breeding system. It is considered nutritionally very superior by the local people and is used as food crop to make roti, dumpling and chapattis. The seeds of Kalukombu are small and elongated with persisting glumes/husk.
‘Maharashtra Rabi Bajra’ (MRB) is a commercially grown hybrid developed by the modern improved plant breeding technique by a commercial seed company. It is basically a winter crop. The seeds are grey/slate coloured, bold and round shaped without a persisting glumes/husk [10].
2.1. Processing of Pearl Millet
2.1.1. Milling
The cleaned grains were pulverized using a plate mill to obtain whole flour (WF). A part of the whole flour was further sieved through a 44 mesh sieve (BSS). The ‘+’ fraction was termed as the bran rich fraction (BRF) and the ‘–’ fraction was termed as semi-refined flour (SRF).
2.1.2. Wet and Dry Heat Treatment
Each batch of the two commercially available pearl millet varieties was pressure cooked for 10mins (9.8 × 104 Pa) and boiled for 30 minutes respectively. The processed grains were dried in an oven at 50˚C and milled into flour. Each of the millet varieties was roasted in an open pan for 10 - 15 minutes at 200˚C and milled into flour.
2.1.3. Germination
Pearl millet varieties were soaked in water overnight. The water was drained and the grains were tied in a moist muslin cloth and left to sprout at room temperature for 72 hr. After sprouting the grains were dried in an oven at 50˚C and milled into whole flour.
These processed millet flours were kept in air tight polythene bags and stored in a cool and dry place until use.
2.2. Total Dietary Fiber
Total dietary fibre (TDF) was measured as the sum soluble and insoluble dietary fiber as described by Asp et al. [11].
2.3. Tannin Content
Tannin content in the samples was measured using the method described by AOAC [12].
2.4. In-Vitro Protein Digestibility
In-vitro protein digestibility was estimated by enzymatic method of Akeson and Stahmann [13]. Samples were homogenized and suspended in 15 ml of 0.1 N HCl containing 1.5 mg of pepsin and incubated at 37˚C for 3 hr. The suspension was than neutralized with 0.5 N NaOH and treated with 4 mg of pancreatin in 7.5 ml of phosphate buffer (pH 8.0) containing 0.005 M sodium azide. The mixture was gently shaken and incubated at 37˚C for 24 h. After incubation, the sample was treated with 10 ml of 10% TCA and centrifuged at 3000 rpm for 20 min. Protein in the supernatant was estimated by Lowry’s method [14]. %IVPD was calculated using the following formula:
 .
.
2.5. Statistical Analysis
The data was subjected to analysis of variance (ANOVA) test and the differences between the means were compared for their significance (P ≤ 0.05) using SPSS software v.17. Pearson correlation coefficient was used to evaluate the relationship of in-vitro protein digestibility values with tannin content and dietary fiber content.
3. Results and Discussion
3.1. Effect of Processing on Dietary Fiber Content Equations
The influence of common household processing methods on the dietary fiber composition of pearl millet is depicted in Table 1. The total dietary fiber content of the whole flour was higher in K variety (13.3%) than MRB variety seeds (11.91%). The partial removal of bran by sieving to obtain semi refined flour retained significant amount of dietary fiber (10.6%-MRB & 9.2%-K). Dehusking has been reported to decrease dietary fiber content in pulses [15]. The bran rich fraction, a byproduct of flour milling contained around 29% of total dietary fiber of which around 1.5% was soluble and 27% was insoluble fraction. By virtue of its high fiber content, the bran rich fraction can be used as a novel source of dietary fiber. Semi refined flour of pearl millet can also be used in bakery products as it will contribute to both the texture and fiber content of the products.
From the nutritional point of view, data on dietary fiber content of processed millet is of importance, because millets are never eaten raw. In this study, wet and dry heat treatment of the millets did not considerably change the insoluble and soluble dietary fiber content. Although, boiling,

Table 1. Effect of processing on the total dietary fibre and its fractions (g/100g) of pearl millet.
pressure cooking and roasting increased the SDF of MRB variety, the difference was not statistically significant. Changes in dietary fiber composition of processed cereal and pulses have been reported where increase in TDF content could be due to formation of resistant starch [15].
Germination is an inexpensive technique for improveing the nutritional quality of millet seeds. The total dietary fiber content of germinated millet was 9.68% for MRB and 13.4% for K variety seeds. The results indicate that germination did not bring about any significant change in the TDF and its fractions. Studies have indicated that germination has a significant impact on the dietary fiber content. In legumes, germination brought about a significant increase in the dietary fiber content. While another study reported to decreased dietary fiber content due to germination, which could be partly attributed to the conversion of complex carbohydrates into simpler molecules by the action of hydrolyzing enzymes [16,17].
Tannins are polyphenolic compounds which bind to proteins, carbohydrates and minerals thereby reducing digestibility of these nutrients [18]. To reduce these harmful effects, traditional processing methods like milling, boiling, pressure cooking, roasting and germination were employed. In this study, pearl millet contained a good amount of tannins which differed between varieties (Table 2). Tannin levels in MRB variety seeds was 0.21% while that for K variety seeds was 0.23% tannic acid equivalents. These values were within the range reported for millets [19,20].
All millets contain phenolic acids, which are located in the pericarp, testa, and aleurone layer [21]. Therefore it was of interest to study the effect of semi refining of pearl millet flour on tannin levels. Semi refining of the millet flour significantly (P ≤ 0.05) lowered the tannin levels for K variety seeds. The bran rich fraction of both varieties retained most of the tannin content (about 0.32%). This increase can be attributed to concentration of tannins in the seed coat of the grain [22].
Tannin content significantly (P ≤ 0.05) reduced upon wet and dry heat treatments but results varied between the two varieties. A reduction in tannin levels due to boiling and pressure cooking was significant (P ≤ 0.05) in K variety grains. Several possible reasons have been suggested for reduction of tannins during cooking. Losses may result from leaching into cooking water, as tannins readily dissolve in water and alcohol to form colloidal solutions [23]. During processing, tannin binds to molecules like proteins, carbohydrates or minerals making them difficult to extract [18]. Roasting did not influence the tannin levels in both varieties.
Germination has been reported to reduce the tannin content and improve in vitro digestibility of proteins in legumes [24]. In contrast, germination of pearl millet significantly (P ≤ 0.05) increased the tannin content in both varieties (from 0.21% to 0.28% for MRB and 0.23% to 0.36% for K). A study on the effect of germination on tannin content of sorghum revealed that tannin content increased when germinated for different periods (1 - 5 days). During germination, a part of the tannins may enter into the endosperm along with the imbibed water and are likely to form complexes with reserve seed protein and hydrolytic enzymes thus inactivating them [20,25].
3.2. Effect of Processing on in-Vitro Protein Digestibility (IVPD)
Data in Table 3 indicated that protein digestibility of pearl millet was low (45.5% for K and 49.3% for MRB variety). The relatively low protein digestibility may be attributed to the influence of antinutrients such as enzyme

Table 2. Effect of processing on tannin content of pearl millet.
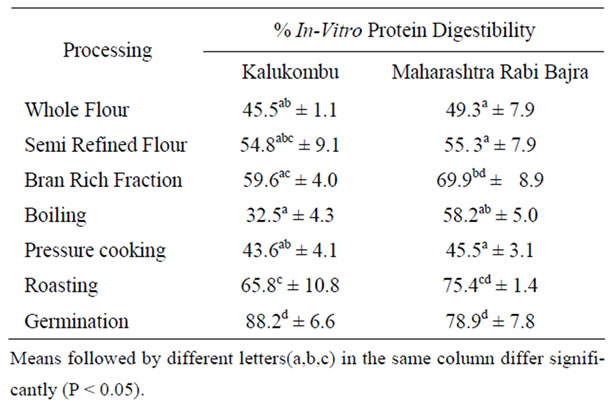
Table 3. Effect of processing on % in-vitro protein digestibility (IVPD) of pearl millet.
inhibitors, lectins, phytates, tannins and dietary fiber which inhibits protein digestion and also due to presence of protein structures that resist digestion.
Studies have demonstrated that high-tannin sorghum varieties formed indigestible protein-tannin complexes which are a major limiting factor in protein utilization [26]. Even though semi refining effectively reduced the tannin content, it did not alter protein digestibility of the millet. However this was not evident in case of the bran rich fraction of MRB variety. Regardless of the presence of high tannin levels (0.32 g/100g), IVPD was significantly (P ≤ 0.05) higher (about 70%) than that of the whole flour which contained comparatively lower tannin levels (0.21 g/100g). The IVPD of the bran rich fraction of pearl millet was comparable to that of wheat bran (69%) [27].
Results on the effect of heat treatments like boiling, pressure cooking and roasting on IVPD significantly (P ≤ 0.05) varied between the two varieties. Wet heat treatments (boiling & pressure cooking) did not improve the protein digestibility of the millet. These results are in agreement with earlier reports on some cereals and legumes. Protein cross-linking mainly through disulphide bonding and reduced protein extractability in cooked samples appears to be the most important factor affecting protein digestibility in cooked cereals [28-30]. In contrast, cooking improved IVPD of foxtail, finger and common millet [31]. Nevertheless, roasting markedly improved IVPD of pearl millet from 45.5% to 65.8% for K variety and 49.3% to 75.4% for MRB variety suggesting that dry heat treatment is more effective in improving protein digestibility than wet heat treatment. The improvement caused by heat treatment may be due to protein denaturation and/or decreasing resistance of protein to enzyme attack [32].
Germination significantly (P ≤ 0.05) increased the protein digestibility of both varieties compared to the un-germinated millet (from 45.5% to 88.2% for K variety and 49.3% to 78.9% for MRB variety). These findings are in agreement with an earlier study on pearl millet [33]. In this study, the increased tannin content due to germination did not negatively affect IVPD. The increase in IVPD can be attributed to an increase in soluble proteins, due to partial hydrolysis of storage proteins by endogenous proteases produced during the germination process. Such partially hydrolyzed storage proteins may be more easily available for pepsin digestion [34].
Tannins and dietary fiber are well known for their ability to bind and precipitate protein. A correlation study was carried out between tannins, TDF, IDF and SDF with %IVPD to ascertain whether IVPD was influenced by these factors (Table 4). A positive correlation, was found between % IVPD and tannin content (r = 0.605, p < 0.01). For IDF, SDF and TDF the correlations were positive although not signifycant. In this study, protein digestibility showed a strong association with tannin levels. The low protein digestibility of pearl millet was not due to tannins or dietary fiber content. It could be due to other factors like interaction of proteins with non-protein components and the proteins themselves thereby affecting their digestibility [4].
4. Conclusions
Considering the various processing methods employed in this study, it was apparent from the results that roasting

Table 4. Association of %IVPD with tannin and dietary fiber content of pearl millet.
and germination improved protein digestibility in pearl millet. In this study the full effect of tannins in reducing protein digestibility was not seen suggesting that tannins were not responsible for low protein digestibility. In conclusion, it is suggested that roasting and germination techniques may be employed to improve the nutritional quality of pearl millet.
5. Acknowledgements
The first author expresses sincere thanks to CSIRNew Delhi for the award of Senior Research Fellowship.
REFERENCES
- F. O. Sade, “Proximate, Antinutritional Factors and Functional Properties of Processed Pearl Millet (Pennisetum glaucum),” Journal of Food Technology, Vol. 7, No. 3, 2009, pp. 92-97.
- S. A. Sehgal and A. Kwatra, “Nutritional Evaluation of Pearl Millet Based Sponge Cake,” Journal of Food Science and Technology, Vol. 43, No. 3, 2006, pp. 312-313.
- M. Malik, U. Singh and S. Dahiya, “Nutrient Composition of Pearl Millet as Influenced by Genotypes and Cooking Methods,” Journal of Food Science and Technology, Vol. 39, No. 5, 2002, pp. 463-468. doi:10.1016/S0733-5210(03)00016-X
- K. G. Duodu, J. R. N. Taylora, P. S. Beltonb and B. R. Hamaker, “Factors Affecting Sorghum Protein Digestibility,” Journal of Cereal Science, Vol. 38, No. 2, 2003, pp. 117-131.
- J. S. Hughes, E. Acevedo, R. Bressani and G. B. Swanson, “Effects of Dietary Fiber and Tannins on Protein Utilization in Dry Beans (Phaseolus Vulgaris),” Food Research International, Vol. 29, No. 3-4, 1996, pp. 331-338. doi:10.1016/0963-9969(96)00027-0
- A. M. Maha Ali, A. H. El Tinay and A. H. Abdalla, “Effect of Fermentation on the in Vitro Protein Digestibility of Pearl Millet,” Food Chemistry, Vol. 80, No. 1, 2003, pp. 51-54. doi:10.1016/S0308-8146(02)00234-0
- A. G. H. Ilham and A. H. El Tinay, “Effect of Fermentation on Tannin Content and in-Vitro Protein and Starch Digestibility of Two Sorghum Cultivars,” Food Chemistry, Vol. 53, No. 2, 1995, pp. 149-151. doi:10.1016/0308-8146(95)90780-B
- P. Shawrang, A. A. Sadeghi, M. Behgar, H. Zareshahi and G. Shahhoseini, “Study of Chemical Compositions, Anti-Nutritional Contents and Digestibility of Electron Beam Irradiated Sorghum Grains,” Food Chemistry, Vol. 125, No. 2, 2011, pp. 376-379. doi:10.1016/j.foodchem.2010.09.010
- E. N. Fombang, J. R. N. Taylor, C. M. F. Mbofung and A. Minnaar, “Use of γ-Irradiation to Alleviate the Poor Protein Digestibility of Sorghum Porridge,” Food Chemistry, Vol. 91, No. 4, 2005, pp. 695-703. doi:10.1016/j.foodchem.2004.06.042
- Anon, All India Coordinated Pearl Millet Improvement Project, Annual Report 2009-2010, Mandor, 2010.
- G. Asp, C. G. Jonson, H. Hollment and M. Slijestrom, “Rapid Enzymatic Assay of Soluble and Insoluble Dietary Fiber,” Journal of Agricultural Food Chemistry, Vol. 33, No. 3, 1983, pp. 476-82. doi:10.1021/jf00117a003
- Association of Official Analytical Chemists (AOAC), “Official Methods of Analysis,” 10th Edition, Washington D.C., 1970, pp. 154-170.
- W. R. Akeson and M. A. Stahmann, “A Pepsin-Pancreatin Digest Index of Protein Quality,” Journal of Nutrition, Vol. 83, No. 2, 1964, pp. 257-261.
- N. Raghuramulu, M. Nair and S. Kalyansundaram, “A Manual for Laboratory Techniques,” National Institute of Nutrition, Indian Council for Medical Research, Jami-Osmania, Hyderabad, 1983.
- P. Ramulu and P. Udayasekhara-Roa, “Effect of Processing on Dietary Fiber Content of Cereals and Pulses,” Plants for Human Nutrition, Vol. 50, No. 3, 1997, pp. 249-257. doi:10.1007/BF02436061
- S. Mahadevamma and R. N. Tharanathan, “Processing of Legumes: Resistant Starch and Dietary Fiber Content,” Journal of Food Quality, Vol. 27, No. 4, 2003, pp. 289- 303. doi:10.1111/j.1745-4557.2004.00620.x
- R. H. Mathers, “Fermented Foods, Legumes: Chemistry, Technology and Human Nutrition,” Marcel Dekker, New York, 1989, pp. 161-163.
- L. Dykes and L. W. Rooney, “Sorghum and Millet Phenols and Antioxidants,” Journal of Cereal Science, Vol. 44, No. 3, 2006, pp. 236-251. doi:10.1016/j.jcs.2006.06.007
- V. D. Pawar and G. S. Parlikar, “Reducing the Polyphenols and Phytate and Improving the Protein Quality of Pearl Millet by Dehulling and Soaking,” Journal of Food Science and Technology, Vol. 27, No. 3, 1990, pp. 140- 143.
- S. B. Ahmed, S. A. Mahgoub and B. E. Babiker, “Changes in Tannin and Cyanide Contents and Diastic Activity during Germination and the Effect of Traditional Processing on Cyanide Content of Sorghum Cultivars,” Food Chemistry, Vol. 56, No. 2, 1996, pp. 159-162. doi:10.1016/0308-8146(95)00157-3
- C. M. McDonough and L. W. Rooney, “The Millets,” In: K. Kulp, Ed., Handbook of Cereal Science and Technology, Culinary and Hospitality Industry Publications, New York, 2000, pp. 177-200.
- S. Chopra and A. Sankhala, “Effect of Soaking and Sprouting on Tannin Phytate and in Vitro Iron in Underutilized Legumes—Hosed Gram (Dolichos Biflorus) and Moth Bean (Phaseolus Aconitifolius),” Journal of Food Science and Technology, Vol. 41, No. 5, 2004, pp. 547-550.
- E. Haslam, “Chemistry of Vegetable Tannins,” Academic press, New York, 1966.
- E. E. Maeda, C. N. Sule and M. J. Samuel, “Effect of Sprouting on Bean Nutritional Quality,” In: S. Sefa-Dedeh, Ed., Proceedings of Seminar on Development of High ProteinEnergy Foods from Grain Legumes, University of Ghana, Legon, 1991, pp. 62-74.
- M. L. Price, S. V. Socoyoc and L. G. Butler, “A Critical Evaluation of the Vanillin Reaction as an Assay for Tannin in Sorghum Grain,” Journal of Agricultural and Food Chemistry, Vol. 26, No. 5, 1978, pp. 1214-1218. doi:10.1021/jf60219a031
- B. A. K. Chibber, E. T. Mertz and J. D. Axtell, “In Vitro Digestibility of High-Tannin Sorghum at Different Stages of Dehulling,” Journal of Agricultural and Food Chemistry, Vol. 28, No. 1, 1980, pp. 160-161. doi:10.1021/jf60227a035
- R. M. Saunders, M. A. Connor, R. H. Edwards and G. O. Kohler, “Enzymatic Processing of Wheat Bran: Effects on Nutrient Availability,” American Association of Cereal Chemists, Vol. 49, 1972, pp. 438-442.
- S. M. Aisha Fageer, E. E. Babiker and A. H. El Tinay, “Effect of Malt Pre-Treatment and/or Cooking on Phytate and Essential Amino Acid Content and in Vitro Protein Digestibility of Corn Flour,” Food Chemistry, Vol. 88, No. 2, 2004, pp. 261-265. doi:10.1016/j.foodchem.2004.01.040
- K. Vijayakumari, M. Pugalenthi and V. Vadivel, “Effect of Soaking and Hydrothermal Processing Methods on the Levels of Antinutrients and in Vitro Protein Digestibility of Bauhinia Purpurea L. Seeds,” Food Chemistry, Vol. 103, No. 3, 2007, pp. 968-975. doi:10.1016/j.foodchem.2006.07.071
- U. Singh and R. Jambunathan, “Studies on Desi and Kabuli Chichpea (Cicer arietinum L) Cultivars: Levels of Protease Inhibitors, Levels of Polyphenolic Compounds and in Vitro Digestibility,” Journal of Food Science, Vol. 46, No. 5, 1981, pp. 1364-1367. doi:10.1111/j.1365-2621.1981.tb04176.x
- G. Ravindran, “Seed Protein of Millets; Amino Acid Composition, Proteinase and in-Vitro Protein Digestibility,” Food Chemistry, Vol. 44, No. 1, 1992, pp. 13-17. doi:10.1016/0308-8146(92)90251-V
- S. K.Sathe, V. Iyer and D. K. Salunkhe, “Functional Properties of the Great Nothern Bean (Phaseolus Vulgaris, L.) Proteins. Amino Acid Composition, in Vitro Digestibility and Application to Cookies,” Journal of Food Science, Vol. 47, No. 1, 1982, pp. 8-11. doi:10.1111/j.1365-2621.1982.tb11014.x
- A. Shaturvedi and G. Sarojini, “Malting of PEARL MILLET (Pennisetum Typhoideum)—Its Effect on Starch and Protein Digestibility,” Journal of Food Science and Technology, Vol. 33, No. 4, 1996, pp. 342-344.
- V. J. Bhise, J. K. Chavan and S. S. Kadam, “Effects of Malting on Proximate Composition and in Vitro Protein and Starch Digestibilities of Grain Sorghum,” Association of Food Scientists and Technologists, Vol. 25, No. 6, 1988, pp. 327-329.

