Food and Nutrition Sciences
Vol. 2 No. 7 (2011) , Article ID: 7241 , 5 pages DOI:10.4236/fns.2011.27107
Effects of Lignocellulosic in Wood Used as Substrate on the Quality and Yield of Mushrooms
![]()
Department of Chemistry, College of Science, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Email: *mbadu0@gmail.com
Received June 1st, 2011; revised July 29th, 2011; accepted August 6th, 2011.
Keywords: Lignin, Cellulose, Hemicellulose, Proximate Composition, Pleurotus Ostreatus
ABSTRACT
The objective of this study was to find out if the sawdust generated from some of the Ghanaian wood species can be used in the cultivation of pleurotus ostreatus (oyster mushroom) and their subsequent effect on the quality and yield of the mushrooms produced. Sawdust from three Ghanaian wood species (Triplochiton scleraxylon, Ceiba pentandra and Terminalia superba) were collected and their cellulose, hemicelluloses, lignin and nitrogen contents determined using standard methods. Triplochiton scleraxylon gave 46.76%, 15.69%, 27.55%, 0.01% w/w, Ceiba pentandra gave 44.79%, 15.32%, 34.08%, 0.02% w/w and Terminalia superba gave 46.64%, 16.29%, 31.17%, 0.02% w/w of the cellulose, hemicelluloses, lignin and nitrogen content respectively. Compost was then made from each of the wood and used as substrate for the cultivation of pleurotus ostreatus. The highest yield of mushroom was obtained from T. scleraxylon 334g followed by T. superba 277 g and C. pentandra gave the lowest yield of 193 g fresh weight after 3 flushes. The proximate composition of the mushrooms produced gave crude protein ranging 16.33 - 18.20, fat 1.67 - 2.07, carbohydrate 40.86 - 50.53, fibre 4.14 - 6.73 and ash content of 4.40% - 5.80%. The report has shown that the yield and nutritional content of the oyster mushroom on sawdust depends on the chemical constituents such as the cellulose content, the hemicellulose content, the lignin content, the nitrogen content of the particular substrate used. Triplochiton scleraxylon gave the best yield and nutritional content, considering that these substrates are freely available and regarded as “waste”, it can be used to cultivate edible mushrooms to supplement nutritional requirement and source of income to make life better for many people.
1. Introduction
The most abundant source of carbon is plant biomass, composed primarily of cellulose, hemicellulose, and lignin. Many microorganisms are capable of grading and utilizing cellulose and hemicellulose as carbon and energy source [1]. The potential of bioconversion of lingocellulosic waste into value-added products is emphasized in recent studies [2]. Although for the commercial production of Pleurotus mushrooms the principal substrate used is wheat straw, there are numerous past studies indicating the need for the examination of other agro-industrial waste (wood chips, sawdust, straw, sugarcane etc) [3].
The activities of the wood industries in the country have led to the generation of large amounts of sawdust and other wood residues in the system. These residues are left on the floors of the premises of some of these wood industries. Some are dumped in our landfill sites and others are incinerated. All these have associated environmental problems. The reprocessing of these residuals will help alleviate increasing pressures on landfill sites, whiles at same time converting materials that have been considered waste into a resource [4].
Mushrooms have a requirement for organic compounds as a source of energy and carbon which are used in normal cell metabolism. Organic and inorganic compounds are available to wood habiting fungi in several forms [5]. Oyster mushroom is edible basidiomycete which can grow naturally on rotten lignocellulosic materials. It has high nutritional properties, rich in mineral content and medicinal properties [6]. Mushrooms provide 29% of the recommended daily intake (RDI) for vitamin B2 (riboflavin) and 27% of the RDI for niacin and are one of the very few foods that provide a natural source of vitamin D. Biosynthesis of vitamin D levels from ergosterols in mushrooms is enhanced by exposure to sunlight during cultivation or to ultraviolet light during drying. [7]
Cultivation of Oyster mushroom has become attractive and commercially produced world wide. Many agricultural by-products, such as plant fibers [8], coconut palm leafstalk [9] and sugarcane residue [10,11] are being used as substrates for the mushroom cultivation. In Thailand, oyster mushroom is cultivated mainly on sawdust [12]. In view of this, the sawdust generated from some of the Ghanaian wood species are used in the cultivation of pleurotus ostreatus (oyster mushroom) and their subsequent effect on the quality and yield of the mushrooms produced are studied in this work.
2. Materials and Methods
2.1. Sample Preparation
Pure culture of pleurotus ostreatus was obtained from tissue culture made from mushrooms collected in the wild around dead decaying stumps of oak-wood in Kanyasi, a suburb of Kumasi in the Ashanti region of Ghana. The sawdust was collected from a local sawmill at Kumasi, Ghana. Portions of sawdust of the three wood species namely Triplochiton scleraxylon, ceiba petandra and terminalia superba were air dried for 72 hours, ground and kept in air tight plastic container before analysis.
2.2. Lignin Determination
To 1 g of the extractives free sawdust sample, 14 ml of cold 72% sulphuric acid was added and stirred. The mixture was left to stand for 2 hours. After the 2 hours, the mixture was then washed in a 1 L conical flask and diluted to 3% sulfuric acid. The mixture was then boiled for 4 hours under reflux. The insoluble material was allowed to settle and filtered. The residue was washed and dried in an oven at 105˚C after 2 hours this then cooled and weighed as the lignin content [13].
2.3. Holo Cellulose
To 2 g of the extractive free sample, 180 ml distilled water; 8.6 g sodium chloride, 6.0ml ethanoic acid and 6.6 g sodium chloride were added. The mixture was then digested in a 250 ml conical flask under reflux at 70˚C for 3 hours. It was then allowed to cool, filtered and the residue washed with five 20 ml portions of 100 ml distilled water, the residue was then dried at 105˚C for 24 hours to attain constant weight.
2.4. Cellulose
To 2 g of the extractive free sample was taken and put into a 250 ml beaker, 100 ml of 17.5% NaOH solution was added and stirred at 25˚C for 30 minutes. The content of the beaker was then filtered, washed with 25 ml of 9.5% NaOH solution and 20 ml portions of 100 ml distilled water. The residue was again washed with distilled water and 40 ml of 10% acetic acid and further with 1 L distilled water. The residue was then dried at 105˚C for 24 hours to constant weight.
2.5. Hemicellulose
On the bases of solubility in 17.5% NaOH solution, holoccellulose is sub divided into insoluble cellulose and the soluble hemecellulose. Therefore the hemecellullose is determined as the difference between the weight of holocellulose and weight of cellulose present.
2.6. Compost Preparations and Mushroom Cultivation
Portion of the sawdust collected was soaked in water and heaped for 7 days whiles turning over every 2 days to ferment. To 4 kg of the fermented substrates were mixed with 1% CaCO3 w/v and 1% w/v sugar on oven dry weight basis. Distilled water was added to the fermented substrates to adjust their moisture content to about 75%. After thoroughly mixing the supplements and substrates, 400 g of the compost was packed into each polyethylene bags of size 15 × 30 cm and the mouth tightened with a rubber band and plugged with cotton. Three replicate bags were prepared for each substrate. The bags were then autoclaved at 200˚C for 2 hours. The bags filled with the autoclaved compost were allowed to cool down to room temperature before they were inoculated at 5% spawning level (w/w). Inoculated bags were kept on a clean bench in the laboratory at 28˚C ± 2˚C and average relative humidity at 90%. After complete colonization of substrates by the mushroom mycelium, the bags were opened for fruiting. This was followed by periodic watering of bags every other day with 150 ml of distilled water per day to avoid dryness. Fresh mushroom yield were recorded a day after they had sprout out.
2.7. Proximate Composition
The nutritional content of the mushroom samples including fibre, protein, ash, fat and carbohydrate produced from the various wood species were analyzed.
2.7.1. Crude Fibre
The crude fibre was determined by acid and alkali digestion methods on the fat free mushroom sample according to the method describe Raghuramulu et al. (1983) [14].
2.7.2. Crude Protein
The crude protein content of the samples were estimated by the macro Kjeldhal method employed to find the total nitrogen content. The percent content of the total nitrogen was multiplied by a factor of 6.25 to find the crude protein of the mushrooms [15].
2.7.3. Determination of Ash
The ash content was determined by igniting the plant material in silica crucibles in a muffle furnace at 620˚C for 3 hours [15].
2.7.4. Crude Fat
Fat in the mushrooms was determined by extracting a known weight of powdered sample with petroleum ether using the soxhlet apparatus as described in the AOAC, (1990) [15].
2.7.5. Carbohydrate
After the fat extraction, the samples were heated for 3 hours with 100 ml water, 10 ml hydrochloric acid in a 600 ml conical flask and digested under reflux. After the digestion the sample was cooled, filtered and analysed using the UV/ Visible Spectrophotometer [16].
3. Results and Discussion
Digestion of cellulose produces glucose and cellobiose, while digestion of hemicellulose produces mostly xylose and other sugars, such as glucoronic acid and galacturonic acid as secondary products [17-19]. Since many sugars are released which are converted into sources of carbon when lignocellulosic substrates are digested, lignocellulosic substrates therefore are good substrate for pleurotus ostreatus cultivation [20].
Lignin plays a central role in carbon cycling on Earth. Its heterogeneous structure gives plants their structural rigidity and also serves to protect cellulose and hemicellulose from degradation [21]. Table 1 shows the lignin, cellulose and hemicellulose content of the various wood species that were used and their respective mushroom yields produced. Triplochiton scleraxylon gave the highest yield of 334 g, Terminalia superba gave 277 g whiles Ceiba pentandra gave the lowest of yield of 193 g. Comparing the lignocellulosic content with the yield of mushrooms produced it was observed that Triplochiton scleraxylon which had the highest cellulose content of 46.76%, hemicellulose 15.69% and lignin content 27.55%. Ceiba pentandra which had the highest lignin content and low cellulose content gave the lowest yield of 193 g. It was observed that Triplochiton scleraxylon which had the lowest lignin content showed the highest yield of 334 g followed by Terminalia superba and the Ceiba pentandra. This can be attributed to fact that lignin is a heterogeneous and irregular arrangement of phenylpropanol polymer that resists chemical or enzymatic degradation to protect the cellulose. The availability of the sugars to the fungi was limited by the lignin content. Table 2 shows the proximate composition of the mushrooms produced on the sawdust of the various wood species.
The Triplochiton scleraxylon mushroom was found to contain the highest carbohydrate content among the three wood species used. It showed a carbohydrate concentration of 50.53% ± 3.31%. Aletor, (1995) worked on the chemical composition and nutritive value of some edible American fungi and found that the carbohydrate content of pleurotus ostreatus was 81.8% dry weight of the mushroom [22]. This implies that the carbohydrate content of the pleurotus ostreatus can go as high as 81.8% depending on the substrate it is being cultivated on.
Generally cultivated mushrooms contain 45% - 65% dry weight of carbohydrate [23] but the range is below the value obtained for the mushrooms produced in this research. The crude protein content was found to be 18.20% and that of fat was found to be 1.72%. These values are comparable with what is given in the workdone by Diez and Alvarez, (2001) and Longvah and Deosthale (1999) [24,25]. Mushrooms grown on the Terminalia superba compost performed poorly on the nutriational content as compared with that of Triplochiton scleraxylon and Ceiba pentandra. It gave a carbohydrate content of 40.86%. This value is below the value given for carbohydrates in cultivated mushrooms in general [23].
Mushrooms are generally considered in nutrition guides as a vegetable. Cultivated mushrooms are a valuable source of several micronutrients and are a low kilojoule,
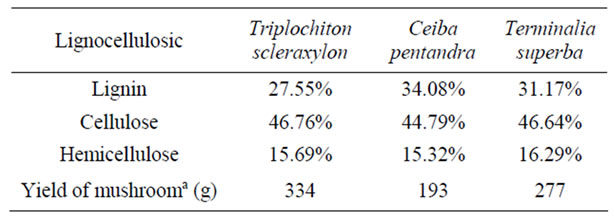
Table 1. Amount of the lignocellulosics in the various wood species and yield of mushrooms produced.
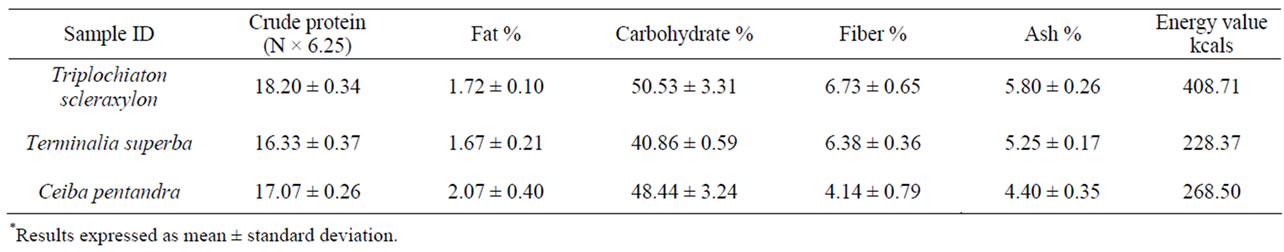
Table 2. Proximate composition of the mushrooms produced on the sawdust of the various wood species.
nutrient-dense food. The protein content, the fat content and fibre content were of low value but all were within the range in cultivated mushrooms the is 15% - 50%, 2% - 6%, 5% - 15% respectively [26] Mushrooms from Ceiba pentandra gave a carbohydrate content of 48.44%. The protein content, fat content and fibre content were found to be 17.07%, 2.07% and 4.14% respectively and with the exception of the fibre content which is a little below, the protein and fat contents are within the range reported by Oi-wah Lau (1982) [27].The high carbohydrate, protein and low fat is a similar characteristic of the edible wild mushrooms which have been studied widely [23,26, 28].
4. Conclusions
In this work it was found that the sawdust from all the three wood species have the ability to be used as compost in the cultivation of the Oyster mushroom. Triplochiton scleraxylon had the highest cellulose and the lowest lignin content amongs the three and it gave the highest mushroom yield and for the proximate composition it was found to be highest in carbohydrate content, protein content, fibre content and ash content. Ceiba pentandra had the lowest in cellulose and hemicel-luloses and the highest in lignin content. Ceiba pentandra produced the lowest yield of mushrooms and highest fat content. This shows that the yield and nutritional content of the pleurotus ostreatus cultivated on sawdust depends on the chemical constituents such as the cellulose content, the hemicellulose content, the lignin content, of the particular substrate used.
5. Acknowledgements
This work was funded by the Teaching and Learning innovation fund (TALIF) project. The authors appreciate Dr Kenneth J. Mensah and Mr. Kingsley Badu for proof reading the manuscript; we also do appreciate the assistance of the entire staff of the Chemistry Department of Kwame Nkrumah University of Science and technology, Kumasi Ghana.
REFERENCES
- D. Cullen and P. J. Kersten, “The Mgcota III, Biochemistry and Molecular Biology,” 2nd Edition, Springer-Verlag Heidelberg, Berlin, 2004, pp. 249-273.
- G. Zervakis and A. Philippoussis, “Management of AgroIndustrial Waste through the Cultivation of Edible MushRooms,” Proceedings of the Fourth European Waste Forum Innovation in Waste Management, Millan, 2000, pp. 87-90.
- A. Philippousis, G. Zervakis and P. Diamantopoulou, Bioconversion of Lignocellulosic Waste through the Cultivation of the Edible Mustrooms Agrocybe Aegeriha, Volvarialla Volvacea and Pleurotus sp,” World Journal of Microbiology and Biotechnology, Vol. 17, No. 20, 2001, pp. 191-200. doi:10.1023/A:1016685530312
- N. Morotroshi, “Chemical Characterisation of Wood and Its Components,” In: D. N.-S. Hon and N. Shiraishi, Eds., Wood Cellulose Chemistry, Marcel Dekker, New York, 1991
- T. K. Kirk and T. L. Highly, “Quantitative Changes in Structural Components of Conifer Woods during Decay by Whiteand Brown-Rot Fungi,” Phytopathology, Vol. 63, 1973, pp. 1338-1342
- Z. Bano and S. Rajarathnam, “Pleurotus Mushrooms. Part II, Nutritional Value, Post-Harvest Physiology, Preservation and Role as Human Food CRC Critical,” Reviews in Food Science and Nutrition, Vol. 27, No. 2, 1988, pp. 87-158.
- P. Roupas, M. Noakes, C. Margetts, J. Keogh and P. Taylor, “Mushrooms and Health National Research Flagships CSIRO,” Global Initiative on Mushrooms and Health Report, June 2010.
- Q. A. Mandeel, A. A. Al-Laith and S. A. Mohamed, “Cultivation of Oyster Mushrooms (Pluerotus sp.) on Various Lignocellulosics Wastes,” World Journal of Microbiology and Biotechnology, Vol. 21, No. 4, 2005, pp. 601-607
- G. V. Thomas, S. R. Prabhu, M. Z. Reeny and B. M Bopaiah, “Evaluation of Lignocellulosic Biomass from Coconut Palm as Substrate for Cultivation of Pleurotus Sajorcaju (Fr) Singer,” World Journal of Microbiology and Biotechnology, Vol. 14, No. 6, 1998, pp. 879-882. doi:10.1023/A:1008881124903
- A. K. Singh, “Cultivation of Oyster Mushroom (Pleurotus sp.) on Sugarcane Residues,” Journal of Mycology and Plant Pathology, Vol. 28, 1998, pp. 240-245.
- S. Vetayasuporn, “Bagasse as a Possible Substrate for Pleunotus ostreatus (Fr.) Kummer Cultivation for the Local Mushroom Farms I the Northest of Thailand,” Pakistan Journal of Biology Sciences, Vol. 9, No. 13, 2006, pp. 2512-2515.
- S. Vetayasuporn, “The Feasibility of Using Coconut Residue as a Substrate for Oyster Mushroom Cultivation,” Biotechnology, Vol. 6, No. 4, 2007, pp. 578-582.
- D. Templeton and T. Ehrman, “Determination of AcidInsoluble Lignin in Biomass,” Laboratory Analytical Procedure No. 003, National Renewable Energy Laboratory, Golden, CO. 1995.
- N. Raghuramulu, M. K. Nair and S. Kalayanasundaram, “A Manual of Laboratory Techniques,” National Institute of Nutrition, ICMR, Hyderabad, India, 1983.
- AOAC, “Official Methods of Analysis,” 15th Edition, Association of Official Analytical Chemists, Washington DC, USA, 1990.
- M. Dubois, K. A. Giles, J. K. Hamilton, P. A. Rebers and F. Smith, “Calorimetric Methods for Determination of Sugars and Related Substances,” Analytical Chemistry, Vol. 28, No. 3, 1956, pp. 350-356. doi:10.1021/ac60111a017
- T. Jefferies, “Biodegradation of Liqnin-Carbohydrate Complex,” Biodegradation, Vol. 1, No. 2-3, 1990, pp. 163-176.
- P. Albersheim, “The Primary Cell Wall ‘Plant Biochemistry’,” Academic Press, New York, 1976, pp. 225-274.
- A. J. Clarke, “Biodegradation of Cellulose: Enzymology and Biotechnology,” Technomic, Lancaster, PA, 1997.
- B. Keller, “Structural Cell Wall Proteins,” Plant Physiology, Vol. 101, No. 4, 1993, pp. 1127-1130.
- S. Vetayasuporn, “Oyster Mushroom Cultivation on Different Cellulose Substrates,” Research Journal of Agriculture and Biological Sciences, Vol. 2, No. 6, 2006, pp. 548-551.
- V. A. Aletor, “Compositional Studies On-Edible Tropical Species of Mushrooms,” Food Chemistry, Vol. 54, No. 3, 1995, pp. 265-268. doi:10.1016/0308-8146(95)00044-J
- A. Dundar, H. Acay and A. Yildiz, “Yield Performances and Nutritional Contents of Three Oyster Mushroom Species Cultivated on Wheat Stalk,” African Journal of Biotechnology, Vol. 7, No. 19, 2008, pp. 3497-3501.
- V. A. Diez and A. Alvarez, “Compositional and Nutritional Studies on Two Wild Mushrooms from Northwest Spain,” Food Chemistry, Vol. 75, No. 4, pp. 417-422. doi:10.1016/S0308-8146(01)00229-1
- T. Longvah and Y. G. Deosthale, “Composition and Nutritional Studies on Edible Wild Mushrooms from Northeast India,” Food Chemistry, Vol. 63, No. 3, 1999, pp. 331-334. doi:10.1016/S0308-8146(98)00026-0
- A. Yildiz, M. Karakaplan and F. Aydin, “Studies on Pleurotus ostreatus (Jacq. Ex Fr.) Kum. Var. salignus (Pers. Ex Fr.) Konr. et Maubl.: Cultivation, Proximate Composition, Organic and Mineral Composition of Carpophores,” Food Chemistry, Vol. 61, No. 1-2, 1998, pp. 127-130. doi:10.1016/S0308-8146(97)00066-6
- L. Oi-Wah, “Methods of Chemical Analysis of MushRoom,” Department of Chemistry, the Chinese University of Hong Kong Shatin, Hong Kong, 1982.
- A. Yildiz and M. Karakaplan, “Evaluation of Some Agricultural Wastes for the Cultivation of Edible Mushrooms (Pleurotus ostreatus var salignus),” Journal of Food Science and Technology, Vol. 40, 2003, pp. 290- 292.

