American Journal of Plant Sciences
Vol.3 No.10(2012), Article ID:24151,4 pages DOI:10.4236/ajps.2012.310175
The Ecological Characteristics of Seed Germination and Seedling Establishment of Manglietia patungensis: Implication for Species Conservation*
![]()
Engineering Research Center of the Ministry of Education for the Three Gorges Reservoir Region’s Eco-Environment, The China Three Gorges University, Yichang, China.
Email: fqchen@ctgu.edu.cn
Received August 13th, 2012; revised September 12th, 2012; accepted September 24th, 2012
Keywords: Soil Type; Shade Degree; Seed Germination; Seedling Survival; Manglietia patungensis
ABSTRACT
Manglietia patungensis is an endangered species distributed aggregately in evergreen broad-leaved forest communities in Southwest China. A controlling experiment was employed to test the effects of soil type and shade degree on seed germination and seedling establishment by using four soil types and three shade treatments with five replicates. Results showed that the germination rate of M. patungensis seeds was relatively low ranging from 19% to 31%. Shade degree and soil type had no significant effect on seed germination rate, but influenced germination dynamics. Both shade degree and soil type treatments significantly affected seedling survival and seedling growth. Seedling survival rate increased with increasing light density. Increased light also promoted biomass accumulation and root development of seedlings. The biomass of the seedlings under full light condition increased 72% comparing with the seedlings under 80% shade degree. Root depth, root area and cross number increased with the increase of light density. Seedlings on farmland soil survived better than that on other three kinds of soil. The seedling survival rate in the farmland soil reached 91.4%, but was reduced to 80.3%, 78.0% and 52.8% in old-field soil, sandy soil and forest soil respectively. Total biomass, aboveground biomass and root biomass of seedlings in forest soil was the highest, followed by seedlings in sandy and old-field soils respectively, and seedlings in farmland soil ranked the least. Some suggestions were finally put forward for the conservation of M. patungensis based on the research results.
1. Introduction
Natural population regeneration comprises seed production, dispersal, germination and successful seedling establishment. Seed germination and seedling establishment are critical stages in life cycle, which determine plant population dynamics and ultimately community development, structure and sustainability in the recruitment of plant populations [1,2]. On the other hand, seed germination and seedling establishment are the most vulnerable stages to environmental stress and are characterized by extremely high mortality rate and most intense natural selection of the entire life-cycle [3,4]. Seedlings are therefore often regarded as the bottleneck in the life histories of species [4]. A large of proportion of plant species are becoming rare and endangered in the world because the processes of development, dispersal, and germination of diaspore are hampered or limited [5-7].
Pattern of seedling recruitment depends on a number of factors, particularly the availability of seed production, heritability, viability and adaptability as well as germination conditions [8,9]. Emergence, survival and final establishment of seedlings are greatly affected by habitat characteristics [10-12]. Of the habitat characteristics studied, ground cover, elevation, soil depth, slope and canopy openness are the main parameters that classified seedling habitat with respect to their emergence [13,14]. Establishment limitation may be caused by germination failure, seedling mortality, or juvenile death, all of which may be caused by adverse environmental conditions [15-17]. Each seed-bearing species has its own characteristic set of requirements for germination that may be regarded as adaptations for maximizing survival in a patchy and unpredictable environment [18].
Small-sized populations are particularly susceptible to extinction because of reduced fitness resulting from inbreeding depression and to random demographic and environmental effects [8,19]. Currently, for many threatened plant taxa, the relative importance of the contemporary ecological factors which constrain population growth are poorly understood and little is known about the management actions required to maintain stable populations of plants. Consequently, there is little scientific guidance for conservation managers to determine which threats/ actions have the highest priority. For conservation purposes, it is important to characterize traits of seed germination and seedling establishment which limit endangered species from expanding its geographic range, increasing in abundance, or persisting even at low abundances [20].
Manglietia patungensis, one of the Tertiary relict evergreen tree species, is distributed aggregately in evergreen broad-leaved forest communities in Southwest China including Hubei and Hunan province and Chongqing municipality, in a narrow area from north latitude 28˚47'10" to 30˚51'53", longitude from 107˚9' to 110˚38'9", height from 374 - 1029 m above sea level [21,22]. M. patungensis is 15 - 25 m in height. Its branches are light brown and glabrous. The species usually sets its white flowers from May to June and fruit in October. Its seeds have the after-ripening characteristics maturing in autumn and germinating in the spring of the following year. M. patungensis has lost most habitats under human disturbance and is listed as an endangered species under the Chinese State protection (Category II). The surviving populations are distributed in a fragmented landscape in very small sizes with tens or even several adult plants, and few young trees and seedlings appear in populations [23]. The genetic and developmental characteristics have been studied to uncover its endangered causes [24-27]. But the traits of seed germination and seedling establishment are still not clear in relation to population regeneration, stability, migration and distribution.
Soil type and shade degree are the critical environmental factors influencing population regeneration and distribution pattern in forest communities [28,29]. It is believed that soil type affects indirectly seed germination and seedling establishment by directly affecting soil moisture, nutrients and aeration [28,30], and that shade degree induces and breaks seed dormancy, influences seedling growth and biomass allocation [29]. Consideration of the germination requirements is essential to an understanding of ecological aspects of reproduction by seed and the endangered causes. In this study, we examine the ecological impacts of soil type and shade degree on seed germination and seedling establishment of M. patungensis by addressing three specific questions: 1) How does soil type and shade degree affect seed germination and seedling establishment? 2) There are few seedlings and young trees appearing in populations, and are there any limitation for seed germination or seedling establishment which contributes to this? 3) What ways to conserve the species during the stage of seed germination and seedling establishment?
2. Materials and Methods
2.1. Plant Materials
Fruits (polysecus) of M. patungensis were collected randomly from 5 plants in a field population at Badong county, Hubei province in October. Seeds were stripped from fruits and stored in moist sand at 4˚C in dark to break dormancy. Matured seeds were selected for the experiment of seed germination and seedling establishment in April next year.
2.2. Experiment Design
A controlling experiment was employed to test the effects of soil type and shade degree on seed germination and seedling establishment by using four soil types and three shade treatments with five replicates. Soils included sandy soil, forest soil, old-field soil and farmland soil that M. patungensis may spread and establish in field in its distribution area. Three shade levels were 0%, 40% and 80% of full sunlight to simulate the light regimes in the clearing, gap and understory respectively. The experimental unit consisted of 60 mature seeds placed on a plastic pots (25.5 cm tall, 17.5 cm diameter) filled with one kind of soil substrate and was arranged in a randomized complete block design with the three blocks based on the different shades. Within each block, seedlings in all treatments were rotated randomly every two days to minimize positional effects. Room humidity was controlled at a range from 70% to 75%. Soil water content was managed every two days, and germinated seeds were counted on a daily basis. Germination observation was ended when no further germination appeared at any treatment in three days. The experiment was continued to test the effects of soil type and shade degree on seedling establishment. Ten seedlings of each pot were randomly harvested and carefully washed in nylon web bags and sorted into belowground and aboveground parts when the experiment of seedling establishment was over. The leaf number, height and root system of each seedling were measured. Plant material then was dried to a constant mass at 60˚C and weighed.
The experiment was conducted in greenhouse of Three Gorges University. Experiment began on 19 April and ended on 19 June.
2.3. Data Analysis
Pots were considered the unit of replication and measurements for individual plants were averaged within pots.
The highest seedling number of each pot was used to count its germination rate, so were the seedling number of each pot at the end of experiment to count its survival rate. Biomass and biomass allocation, and root growth of seedlings were calculated for all tests. An univariate analysis was conducted with the dependent variable “seed germination”, “seedling survive”, “seedling biomass” and some index about seedling growth, and fixed factors “soil type” and “shade degree” to investigate the effects of soil type, shade degree and their interaction on seed germination and seedling establishment. All analyses were conducted using SPSS software (13.0).
3. Results
3.1. Seed Germination
Seeds of M. patungensis began to germinate after seeding 10 days and finished germination within 35 days (Figure 1). Soil types had no effect on germination dynamic meanwhile shade degree influenced the germination progress. Seeds both under 0% and 40% shade treatments germinated quickly after seeding 15 days, however, seeds under 80% shade treatment entered the fast germination period till seeded 20 days.
The final germination rate of M. patungensis seeds was relatively low. Univariate tests showed that soil type, shade degree and their interaction did not significantly influence seed germination rate (Table 1). Seeds germinated 25.1%, 28.2%, 21.5% and 29.6% in sandy soil, forest soil, old-field soil and farmland soil respectively and 27.4%, 24.0% and 26.9% in full light, 40% shade and 80% shade treatments, respectively (Figure 2).
3.2. Seedling Survival
Shade degree significantly influenced seedling survival (p < 0.01). Survival rate of seedlings decreased with increasing shade degree. Seedling survival rate under full
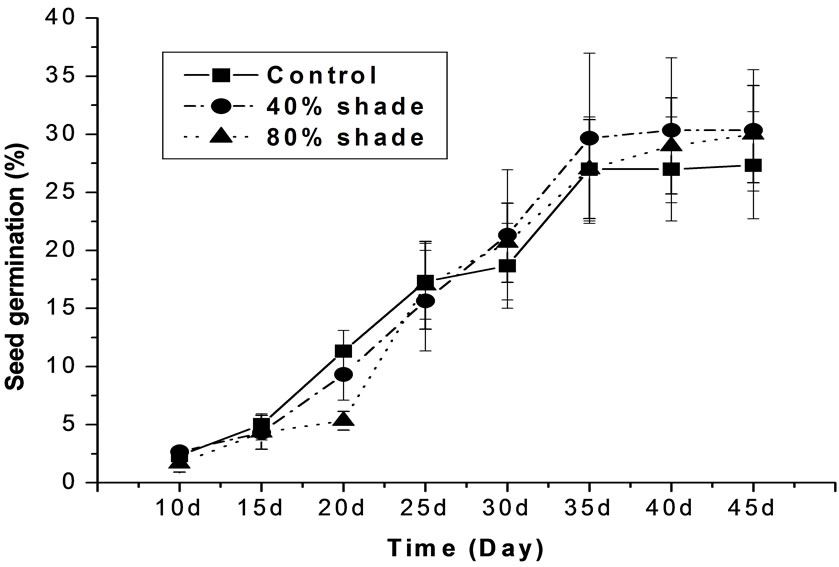
Figure 1. The germination dynamics of M. patungensis seeds under different shade (mean ± SE).
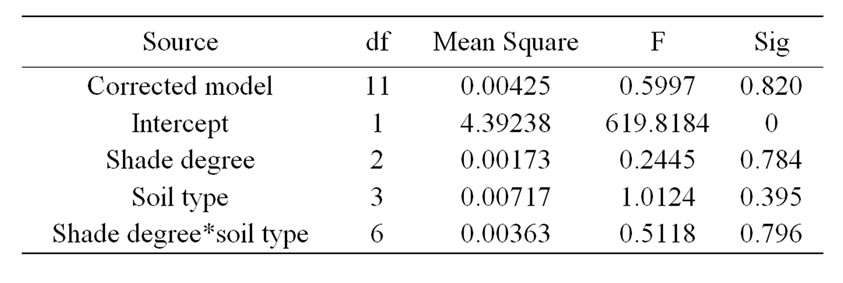
Table 1. Univariate analysis of the effects of shade and soil on seed germination.
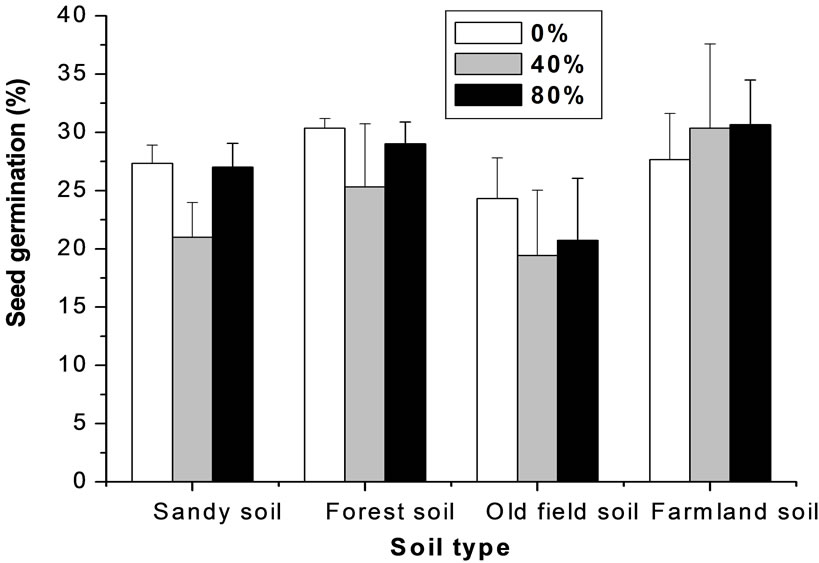
Figure 2. The effect of soil type and shade on the germination of M. patungensis seeds.
exposure reached 87.2%, but reduced to 73.2% and 62.8% under 40% and 80% shade treatments respectively. Soil type also markedly affected seedling survival (P < 0.001). The average seedling survival rate in the farmland soil is 91.4%, but reduced to 73.0% and 80.3% on sandy soil and old-field soil, respectively and seedlings in forest soil only survived 52.8% (Figure 3).
3.3. Seedling Growth
Seedling growth was influenced by both soil type and shade degree treatments. Biomass accumulation and biomass allocation differed significantly among treatments. Total biomass, aboveground biomass and root biomass of seedlings in forest soil was the highest, followed by seedlings in sandy and old-field soils respectively, and seedlings in farmland soil ranked the least. Therefore, the best soil for seedling growth was the forest soil. The effect of shade degree on seedling growth was also significant. Biomass of seedling decreased with increased shade degree. The biomass of seedlings under full light increased 72% comparing with the seedlings under 80% shade treatment. Seedlings under full light had the highest biomass and best biomass allocation. In addition, shoot biomass, underground biomass and their ratio also showed that forest soil and full sunlight conditions promoted seedling growth (Table 2).
Shade degree also influenced the development of seedling root systems. All root growing indexes, including
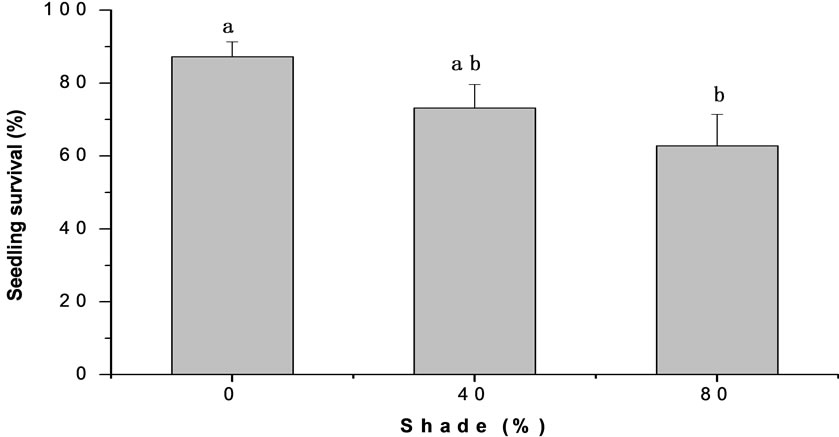
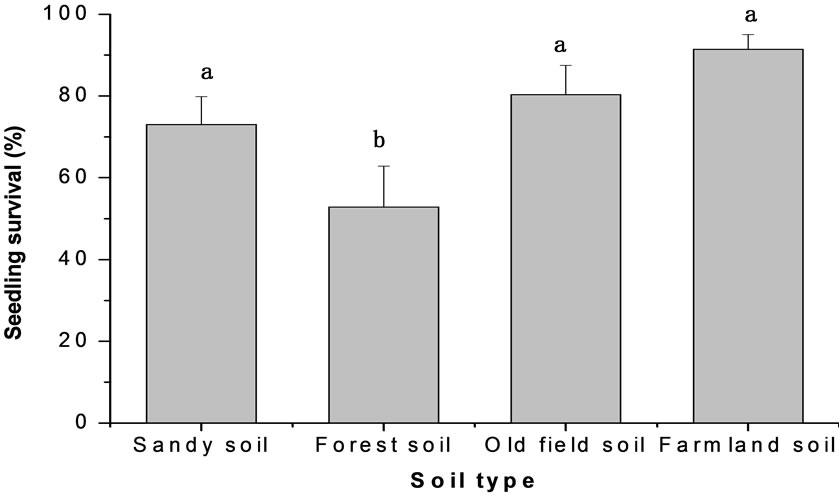
Figure 3. The effects of soil type and shade on seedling survival of M. patungensis.
root depth, root surface area and root cross number of seedlings under full sunlight treatment were bigger than that of seedling under 40% and 80% shade treatments (Table 3). It suggested full sunlight was appropriate for the development of root systems. Soil type only affected significantly root depth and root cross number (P < 0.01), but not the root surface area (P > 0.05). The root system of seedlings grown on forest soil, sandy soil and slop soil developed better than on farmland soil.
4. Discussion
Seed germination and seedling establishment determines the long-term persistence of populations and species. The obstacle to produce seeds and seedlings makes a species endangered [31]. Successful seed germination and seedling establishment firstly depends on the vitality which is closely related to seed development. Seed viability will decrease or cease when development ability degrades or environmental condition is inappropriate [32]. The germination rate of M. patungensis seeds was relative low, ranging from 19% to 31% in our experiment. Chen et al. suggested that the pistil and stamen of M. patungensis flower within the same individual matured at different times making the seed development difficult and most seeds developed incompletely [25]. Artificial insemination could resolve the problem of low maturing rate and obtain large number of seeds and seedlings [24]. In fact, asynchronous mature of pistil and stamen happens to most species to promote cross-pollination and keep seeds viable. The gene flow and seed development would be limited when population were fragmented [33]. The size of surviving M. patungensis populations ranging from several to tens of individuals are distributed away from each other in a fragmented landscape. Therefore, the key reason making M. patungensis endangered is its population size is too small to cross-pollinate within population, which further affects seed development and viability.
Many tree seeds are dormant at the time of dispersal, requiring a period of after-ripening before seeds germinate [34,35]. During that time various environmental stresses, animal feeding and pathogenic infection may make seeds lose viability [36,37]. Seeds of M. patungensis fall into the soil in autumn, and germinate in the spring next year. It takes 5 - 6 months for seeds to finish the morphological and physiological ripening process which only are completed under the conditions of low temperature and good moisture [25]. Seeds in field soil banks experience stresses of drought and frost, pathogen infection and animal feeding which makes the species lost its seed resources further.
Successful recruitment of plants also depends on the conditions of germination and establishment. Many environmental factors, including light, moisture, temperature, canopy density and habitat disturbance etc., may influence seed germination and seedling establishment. Every kind of species has its appropriate conditions for seed germination. Germination progress is stopped when environmental conditions are unsuitable. Light intensity appeared to be a primary factor controlling successful establishment and growth of seedlings. Light intensity has a positive effect on germination to some species [32]. Low light intensity significantly reduced height growth, leaf production and biomass gain of seedlings. The presence of herbaceous and woody plants may decrease light intensity and hence the ability of species to establish and grow, thus restricting recruitment of individuals into the field [32]. Kollmanna et al. found that natural regeneration of Abies guatemalensis was negatively with upper tree canopy cover and leaf area index (LAI) [38]. Light did not affect the germination rate of M. patungensis seeds. However, the establishment of its seedling was influenced significantly by shade. The survival rate decreased with the increase of shade degree. This suggested that seedlings of M. patungensis were likely to grow under sunny condition, which was demonstrated further by the experimental data about seedling growth. The increase of light promotes the accumulation of biomass and development of root system of M. patungensis.
Soil type is another important factor influencing seedling establishment. Seedlings of M. patungensis grown on forest soil had the largest biomass because the high

Table 2. Effects of soil type and shade on the growth of M. patungensis seedlings.
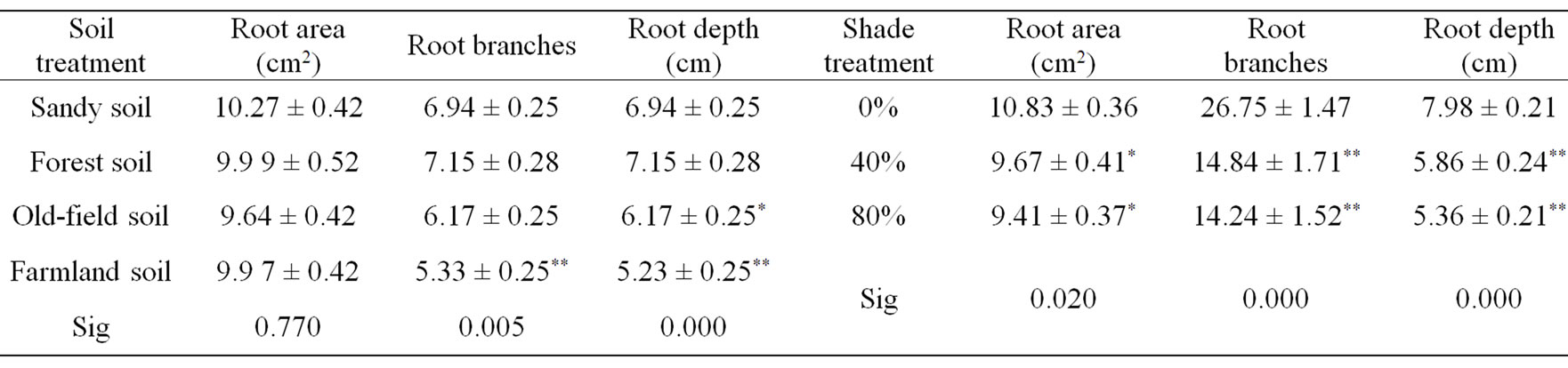
Table 3. The effects of soil type and shade on root system development of M. patungensis seedlings.
soil fertility promoted seedling growth. However, the survival rate of seedling grown on forest soil was lowest (52.8%). Most of seedlings died from the decay of root collar. A reasonable explanation is that pathogen infection from forest soil inducing seedling dying. Grime thought that the death of seedlings in the understory was almost always inevitable infringement of other plants and related fungi [39]. Forest soil is rich in pathogens and its harmful consequences increased with the increase of shade degree. Seedlings growing under canopy gaps are prone to pathogen infection. In an experiment about the factors affecting seedling survival, Carol found that shade conditions affected early survival, and the death of seedlings of six species in nine species contributed to pathogens infection [40].
To ensure that rare and threatened flora extinctions be prevented or at least minimized, numerous attempts have been made to create new populations of endangered plants by translocation [41,42]. A successful translocation can be defined by addressing four goals of abundance, extent, resilience and persistence [43]. The crucial issue addressed in reintroduction and restoration projects is how to promote seed germination and seedling establishment, especially in natural population. However, it is difficult for seeds to find a “safe site” to germinate and establish under the disturbances imposed to ecosystem by humans. Creating artificially appropriate environmental conditions for seed germination and seedling establishment is an effective means to improve species conservation. The establishment of M. patungensis seedlings is a critical stage to successful population regeneration. Seedlings of M. patungensis prefer sunny conditions. The dilemma is that forest soil is better for seedling growth but not for seedling survival because of pathogen infection. According to the results of this study, providing artificial seedbed with fertile soil under sunny condition can promote seed germination and seedling establishment. For the natural population, thinning canopy, removing litter and clearing weed shrubs to increase light density and reduce pathogen infection can promote population regeneration.
5. Acknowledgements
We thank Three Gorges University for providing field experimental plots. Jennifer Tvergyak provides helpful comments on this manuscript.
REFERENCES
- L. Baeten, H. Jacquemyn, H. van Calster, E. van Beek, R. Devlaeminck, K. Verheyen and M. Hermy, “Low Recruitment across Life Stages Partly Accounts for the Slow Colonization of Forest Herbs,” Journal of Ecology, Vol. 97, No. 1, 2009, pp. 109-117. doi:10.1111/j.1365-2745.2008.01455.x
- F. Q. Chen and Z. Q. Xie, “Reproductive Allocation, Seed Dispersal and Germination of Myricaria laxiflora, an Endangered Species in the Three Gorges Reservoir Area,” Plant Ecology, Vol. 191, No. 1, 2007, pp. 67-75. doi:10.1007/s11258-006-9214-4
- M. A. Leck, V. T. Parker and R. L. Simpson, “Seedling Ecology and Evolution,” Cambridge University Press, Cambridge, 2008. doi:10.1017/CBO9780511815133
- A. Kolb and K. Barsch, “Environmental Factors and Seed Abundance Influence Seedling Emergence of a Perennial Forest Herb,” Acta Oecologica, Vol. 36, No. 5, 2010, pp. 507-513. doi:10.1016/j.actao.2010.07.003
- J. Y. Colin and M. B. Linda, “Assessing Limitations on Population Growth in Two Critically Endangered Acacia Taxa,” Biological Conservation, Vol. 108, No. 1, 2002, pp. 13-26. doi:10.1016/S0006-3207(02)00084-8
- J. Gulias, A. Traveset, N. Riera and M. Mus, “Critical Stages in the Recruitment Process of Rhamnus alaternus L.,” Annals Botany, Vol. 93, No. 6, 2004, pp. 723-731. doi:10.1093/aob/mch100
- J. Manfred, P. Lesley and S. Birgitte, “Habitat Specificity, Seed Germination and Experimental Translocation of the Endangered Herb Brachycome mueller (Asteraceae),” Biological Conservation, Vol. 116, No. 2, 2004, pp. 251-267. doi:10.1016/S0006-3207(03)00196-4
- A. Quilichinia and M. Debusschea, “Seed Dispersal and Germination Patterns in a Rare Mediterranean Island Endemic (Anchusa crispa Viv., Boraginaceae),” Acta Oecologica, Vol. 21, No. 6, 2000, pp. 303-313. doi:10.1016/S1146-609X(00)01089-4
- B. R. Frey, M. S. Ashton, J. J. McKenna, D. Ellum and A. Finkral, “Topographic and Temporal Patterns in Tree Seedling Establishment, Growth, and Survival among Masting Species of Southern New England Mixed-Deciduous Forests,” Forest Ecology and Management, Vol. 245, No. 1-3, 2007, pp. 54-63. doi:10.1016/j.foreco.2007.03.069
- C. S. Lee, J. H. Kimm, H. Yi and Y. H. You, “Seedling Establishment and Regeneration of Korean Red Pine (Pinus densiflora S. et Z.) Forests in Korea in Relation to Soil Moisture,” Forest Ecology and Management, Vol. 199, No. 2-3, 2004, pp. 423-432. doi:10.1016/j.foreco.2004.05.053
- L. Gomez-Aparicio, F. Valladares, R. Zamora and J. L. Quero, “Response of Tree Seedlings to the Abiotic Heterogeneity Generated by Nurse Shrubs: An Experimental Approach at Different Scales,” Ecography, Vol. 8, No. 6, 2005, pp. 757-768. doi:10.1111/j.2005.0906-7590.04337.x
- M. Jusaitisa, L. Polomkab and B. Sorensenc, “Habitat Specificity, Seed Germination and Experimental Translocation of the Endangered Herb Brachycome muelleri (Asteraceae),” Biological Conservation, Vol. 116, No. 2, 2004, pp. 251-266. doi:10.1016/S0006-3207(03)00196-4
- P. I. Politi, M. Arianoutsou and G. P. Stamou, “Patterns of Abies cephalonica Seedling Recruitment in Mount Aenos National Park, Cephalonia, Greece,” Forest Ecology and Management, Vol. 258, No. 7, 2009, pp. 1129-1136. doi:10.1016/j.foreco.2009.05.038
- F. Q. Chen and Z. Q. Xie, “Survival and Growth Responses of Myricaria laxiflora Seedlings to Summer Flooding,” Aquatic Botany, Vol. 90, No. 4, 2009, pp. 333-338. doi:10.1016/j.aquabot.2008.12.006
- Z. Münzbergová and T. Herben, “Seed, Dispersal, Microsite, Habitat and Recruitment Limitation: Identification of Terms and Concepts in Studies of Limitations,” Oecologia, Vol. 145, No. 1, 2005, pp. 1-8. doi:10.1007/s00442-005-0052-1
- R. Nathan and H. C. Muller-Landau, “Spatial Patterns of Seed Dispersal, Their Determinants and Consequences for Recruitment,” Trends in Ecological Evolution, Vol. 15, No. 7, 2000, pp. 278-285. doi:10.1016/S0169-5347(00)01874-7
- L. Turnbull, M. Crawley and M. Rees, “Are Plant Populations Seed-Limited? A Review of Seed Sowing Experiments,” Oikos, Vol. 88, No. 2, 2000, pp. 225-238. doi:10.1034/j.1600-0706.2000.880201.x
- M. Fenner, “Seed Ecology,” Chapman and Hall, London, 1985. doi:10.1007/978-94-009-4844-0
- I. Hensen and K. Wesche, “Relationships between Population Size, Genetic Diversity and Fitness Components in the Rare Plant Dictamnus albus in Central Germany,” Biodiversity and Conservation, Vol. 15, No. 7, 2006, pp. 2249-2261. doi:10.1007/s10531-004-7183-2
- C. J. Yates and L. M. Broadhurst, “Assessing Limitations on Population Growth in Two Critically Endangered Acacia Taxa,” Biological Conservation, Vol. 108, No. 1, 2002, pp. 13-26. doi:10.1016/S0006-3207(02)00084-8
- L. G. Fu, “The Red Data Book of Chinese Plants: Rare and Endangered Plants (I),” Science Press, Beijing, 1992, pp. 438-439.
- X. D. Li, H. W. Huang, Z. Z. Li, J. S. He, X. W. Li and J. Q. Li, “Distribution and Conservation Strategy of Endangered Manglietia patungensis Hu,” Journal of Wuhan Botanical Research, Vol. 22, No. 5, 2004, pp. 421-427.
- F. J. Chen, H. W. Liang, X. Wang, Z. Q. He and F. L. Li, “Seed Dormancy and Germination Characteristics of Manglietia patungensis, an Endangered Plant Endemic to China,” Biodiversity Science, Vol. 15, No. 5, 2007, pp. 492-499. doi:10.1360/biodiv.060238
- X. D. Li, H. W. Huang, J. Q. Li and Y. Y. Zan, “Community Structure of Manglietia patungensis in Xiaoxi Natural Reserve, Hunan Province,” Journal of Wuhan Botanical Research, Vol. 24, No. 1, 2006, pp. 31-37.
- F. J. Chen, F. L. Li, H. W. Liang, L. Yao and Z. Q. He, “Megasporogenesis, Microsporogenesis and Development of Gametophytes in the Rare Endangered Plant Manglietia patungensis Hu,” Bull Botanical Research, Vol. 28, No. 6, 2008, pp. 657-662.
- J. He, Z. Li and H. Huang, “Allozymic Genetic Diversity in Manglietia patungensis, an Endangered Species, and Its Conservation Strategies,” Biodiversity Science, Vol. 13, No. 1, 2005, pp. 27-35. doi:10.1360/biodiv.040104
- J. S. He and H. W. Huang, “The Allozyme Diversity in Mnaglietia patungensis Hu,” Journal of Wuhan Botanical Research, Vol. 21, 2003, pp. 544-546.
- T. M. Langhans, C. Storm and A. Schwabe, “Biological Soil Crusts and Their Microenvironment: Impact on Emergence, Survival and Establishment of Seedlings,” Flora, Vol. 204, No. 2, 2009, pp. 157-168. doi:10.1016/j.flora.2008.01.001
- K. P. McLaren1 and M. A. McDonald, “The Effects of Moisture and Shade on Seed Germination and Seedling Survival in a Tropical Dry Forest in Jamaica,” Forest Ecology and Management, Vol. 183, No. 1-3, 2003, pp. 61-75. doi:10.1016/S0378-1127(03)00100-2
- B. J. Graae, T. Hansen and P. B. Sunde, “The Importance of Recruitment Limitation in Forest Plant Species Colonization: A Seed Sowing Experiment,” Flora, Vol. 199, No. 3, 2004, pp. 263-270. doi:10.1078/0367-2530-00154
- W. Schütz, P. Milberg and B. B. Lamont, “Germination Requirements and Seedling Responses to Water Availability and Soil Type in Four Eucalypt Species,” Acta Oecologica, Vol. 23, No. 1, 2002, pp. 23-30. doi:10.1016/S1146-609X(01)01130-4
- M. Burkarta, K. Alslebena, S. Lachmuthb, J. Schumachera, R. Hofmanna, F. Jeltsch and F. M. Schurrb, “Recruitment Requirements of the Rare and Threatened Juncus atratus,” Flora, Vol. 205, No. 9, 2010, pp. 583-589. doi:10.1016/j.flora.2009.08.003
- A. Kolb, “Habitat Fragmentation Reduces Plant Fitness by Disturbing Pollination and Modifying Response to Herbivory,” Biological Conservation, Vol. 141, No. 2, 2008, pp. 2540-2549. doi:10.1016/j.biocon.2008.07.015
- D. T. Bell, “The Process of Germination in Australian Species,” Australian Journal of Botany, Vol. 47, No. 4, 1999, pp. 475-517. doi:10.1071/BT98007
- Z. Peishi, J. A. Plummer, D. W. Turner, D. Choengsaat and D. T. Bell, “Lowand High-Temperature Storage Effects on Viability and Germinability of Seeds of Three Australian Asteraceae,” Australian Journal of Botany, Vol. 47, No. 2, 1999, pp. 265-275. doi:10.1071/BT97105
- C. J. Clark, J. R. Poulsen, D. J. Levey and C. W. Osenberg “Are Plant Populations Seed Limited? A Critique and Meta-Analysis of Seed Addition Experiments,” The American Naturalist, Vol. 170, No. 1, 2007, pp. 128-142. doi:10.1086/518565
- M. B. Falknera, R. D. Lavena and G. H. Aplet, “Experiments on Germination and Early Growth of Three Rare and Endemic Species of Hawaiian Tetramolopium (Asteraceae),” Biological Conservation, Vol. 80, No. 1, 1997, pp. 39-47. doi:10.1016/S0006-3207(96)00070-5
- J. Kollmanna, J. P. P. Co’rdova and R. M. Andersena, “Factors Limiting Regeneration of an Endangered Conifer in the Highlands of Guatemala,” Journal for Nature Conservation, Vol. 16, No. 3, 2008, pp. 146-156. doi:10.1016/j.jnc.2008.06.002
- J. P. Grime, “Plant Strategies and Vegetation Processes,” Wiley, Chichester, 1979.
- K. A. Carol, “Seedling Survival of Tropical Tree Species Interactions of Dispersal Distance, Light Gaps and Pathogens,” Ecology, Vol. 65, No. 6, 1984, pp. 1705-1712. doi:10.2307/1937766
- S. G. Mueck, “Translocation of the Plains Rice-Flower (Pimelea spinescens ssp. spinescens), Laverton, Victoria,” Ecological Management and Restoration, Vol. 1, No. 2, 2000, pp. 111-116. doi:10.1046/j.1442-8903.2000.00032.x
- J. Rowland and M. A. Maun, “Restoration Ecology of an Endangered Plant Species: Establishment of New Populations of Cirsium pitcheri,” Restoration Ecology, Vol. 9, No. 1, 2001, pp. 60-70. doi:10.1046/j.1526-100x.2001.009001060.x
- D. W. Schemske, B. C. Husband, M. H. Ruckelshaus, C. Goodwillie, I. M. Parker and J. G. Bishop, “Evaluating Approaches to the Conservation of Rare and Endangered Plants,” Ecology, Vol. 75, No. 3, 1994, pp. 584-606. doi:10.2307/1941718.
NOTES
*This work is supported by the National Natural Science Foundation of China (51079076).

