American Journal of Plant Sciences
Vol.3 No.6(2012), Article ID:20049,5 pages DOI:10.4236/ajps.2012.36097
Comparative Morphology and Anatomy of Non-Rheophytic and Rheophytic Types of Adenophora triphylla var. japonica (Campanulaceae)
![]()
1Faculty of Agriculture, Kochi University, Kochi, Japan; 2Faculty of Science, Yamagata University, Yamagata, Japan.
Email: tfukuda@kochi-u.ac.jp
Received April 10th, 2012; revised April 30th, 2012; accepted May 18th, 2012
Keywords: Rheophyte; Leaf; Ecotype; Adenophora triphylla var. japonica
ABSTRACT
The morphology and anatomy of leaves of rheophytic and non-rheophytic types of Adenophora triphylla (Thunb.) ADC var. japonica (Regel) H. Hara were compared in order to clarify how leaf characteristics differ. Our results revealed that the leaf of the rheophytic type of A. triphylla var. japonica was narrower than the leaf of the non-rheophytic type because of fewer cells that were also smaller. Moreover, surprisingly, the rheophytic ecotype of A. triphylla var. japonica was thinner than that of the non-rheophytic type, although the general tendency is that the rheophytic leaf is thicker than the closely related non-rheophytic species, suggesting that the rheophytic type of A. triphylla var. japonica adapts differently, as compared to other rheophytic plants, to solar radiation and evaporation.
1. Introduction
Morphological diversity is a special feature of angiosperms, and a key challenge in biology is to understand how this extraordinary morphological diversity is generated. Leaves are the main photosynthetic organs of vascular plants, and they show a tremendous degree of natural variation in shape, making them an attractive system for studying the evolution of form. It is important to understand how morphological diversity in leaf forms was generated and whether it arose in response to natural selection. Rheophytic plants, which grow along rivers and streams, are subjected to flash floods after heavy raining, and these act as strong selective pressures. These plants have narrow lanceolate or cuneate leaves as adaptive modifications [1,2]. Moreover, in rheophytes, which grow in sunny riverbeds in forest gaps or margins, the thick cuticles on the leaf surface have been implicated as adaptations to avoiding or resisting thermal damage in open environments [3]. In addition, [4] reported that morphological and anatomical differences in leaf shape between rheophytes and closely related species appear to be correlated with differences in ecological setting. In fact, some studies using rheophytes and closely related species have been conducted as model cases for understanding leaf modification from fern to angiosperm (Osmunda lancea Thunb. (Osmundaceae): [5]; Aster microcephalus (Miq.) Franch. et Sav. var. ripensis Makino (Asteraceae): [6]; Dendranthema yoshinaganthum Makino ex Kitam. (Asteraceae): [7]; Farfugium japonicum (L.) Kitam. var. luchuense (Masam.) Kitam. (Asteraceae): [8]; Rhododendron indicum (L.) Sweet f. otakumi T. Yamaz. (Ericaceae): [9]).
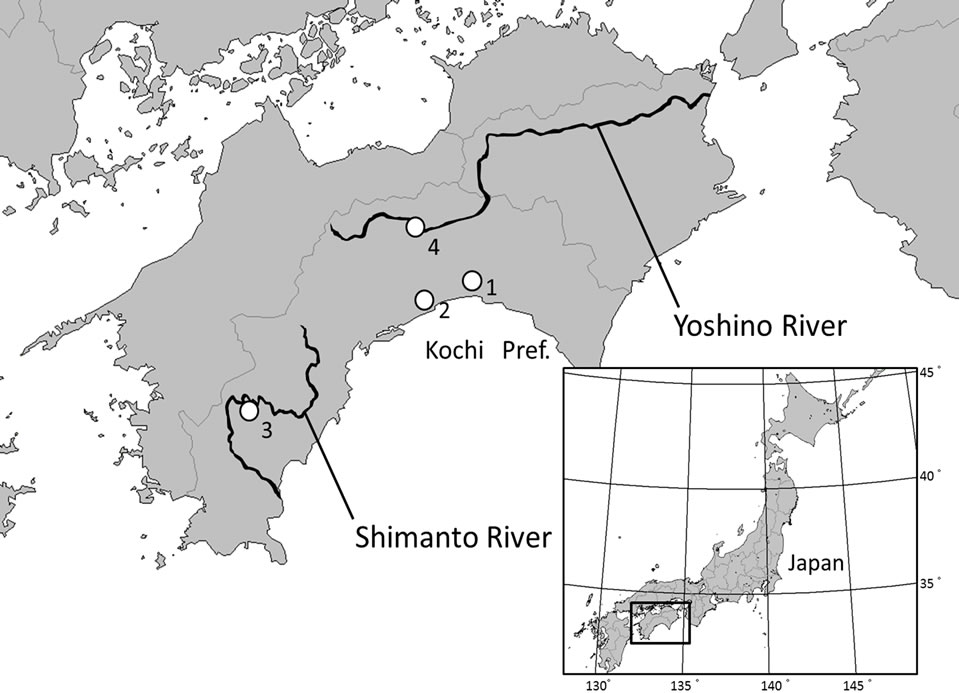
Adenophora triphylla (Thunb.) ADC var. japonica (Regel) H. Hara belongs to Campanulaceae and is distributed in Japan, Korea, and Sahalin [10]. [11] reported that the stenophyllization of A. triphylla var. japonica, which is a putative rheophytic ecotype, occurs in some areas of Kochi Prefecture in Japan (Figure 1). Although comparative morphological and anatomical analyses using the narrow leaves of A. triphylla var. japonica have not been made so far, the processes involved in the stenophyllization of A. triphylla var. japonica such as decreases in cell number and/or cell size remain unclear. In general, one of the indicators that is widely used to examine stenophyllization in putative rheophytes is the leaf index, which is the ratio of the length and width of the leaf [12], and some studies indicated that there are significant differences between rheophytic and non-rheophytic taxa in this regard (e.g., [6,7,13]). Therefore, to investigate the morphological and anatomical differentiation of the
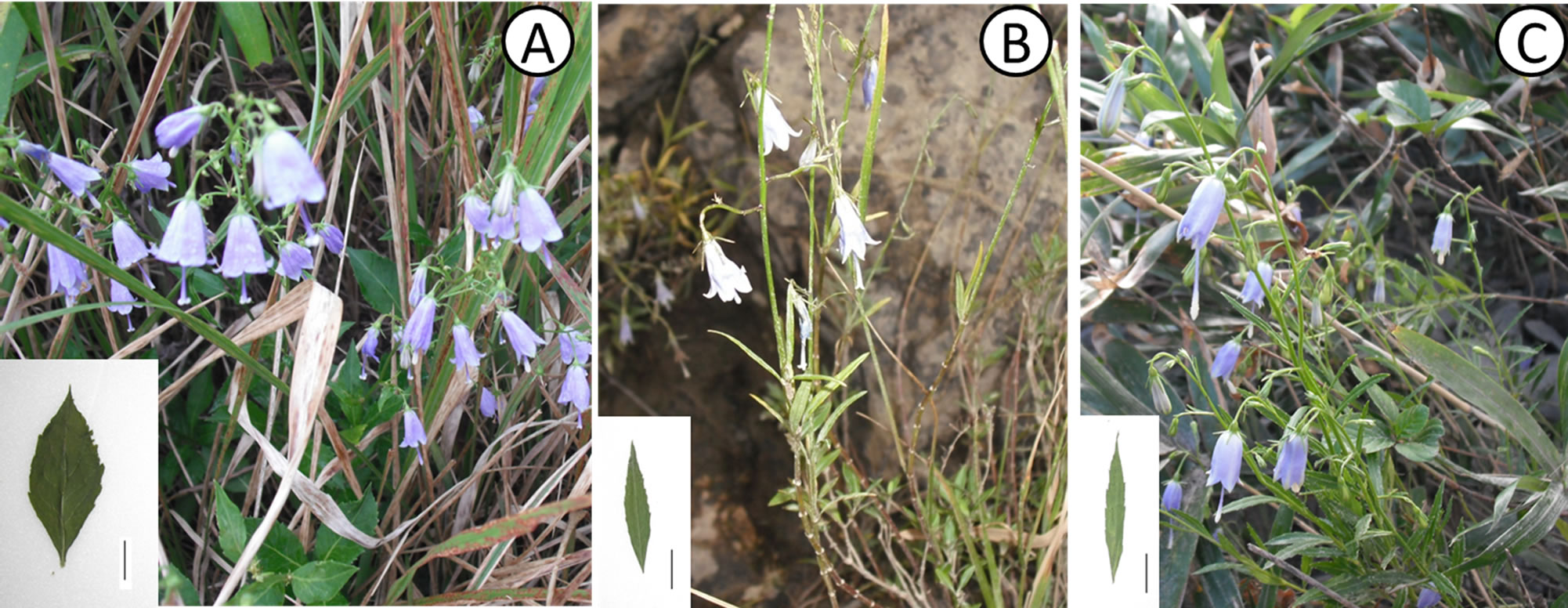
Figure 1. Plants of Adenophora triphylla var. japonica. (A) Non-rheophytic type in Higashisako; (B) Rheophytic type in Shimanto River; (C) Rheophytic type in Yoshino River. Bar = 1 cm.
rheophytic ecotype of A. triphylla var. japonica, we conducted comparative morphological and anatomical analyses, using rheophytic and non-rheophytic types.
2. Materials and Methods
All specimens of non-rheophytic and rheophytic types of Adenophora triphylla var. japonica examined in this study were collected from the fields. A total of 65 individuals of the non-rheophytic type of A. triphylla var. japonica (Hitsuzan: 35; Higashisako: 30) were analyzed. A total of 61 individuals of the non-rheophytic type of A. triphylla var. japonica, representing 2 populations of the Shimanto (31 individuals) and the Yoshino rivers (30 individuals), were analyzed. Collecting localities are indicated in Figure 2 and Table 1.
For morphological analysis, individual specimens were measured for the following continuous macromorphological variables of leaves: length and width of the leaf blade and angle of the leaf base. Measurements were made using a pair of digital calipers. Three fully expanded leaves per specimen were measured. We calculated the mean leaf length, width, and thickness of each specimen. For anatomical analysis, the fully expanded leaves from each specimen were collected. The leaves were fixed overnight in a solution of formaldehyde, ethanol, and acetic acid (FAA). To count the number of cells on the blade, the surface of the fixed leaves were peeled off using the Suzuki's Universal Micro-Printing (SUMP) method. The middle part of the blade along the midrib was examined to determine the number and size of the epidermal cells. Replicas of each leaf (1 mm2) were made to measure the density of the stomata. These copied SUMP images were examined twice for each leaf by using a light microscope.
3. Results
We analyzed the leaf morphology of non-rheophytic and rheophytic types of Adenophora triphylla var. japonica. Table 2 presents a summary of these measurements. Leaf lengths of non-rheophytic (2 populations from Higashisako and Hitsuzan) and rheophytic types (from Shimanto and Yoshino Rivers) of A. triphylla var. japonica were 50.6 ± 9.9, 39.2 ± 13.2, 33.6 ± 10.7, and 25.8 ± 8.2 mm, respectively; their widths were 17.9 ± 2.8, 13.1 ± 5.1, 6.7 ± 2.2, and 6.5 ± 2.6 mm, respectively; leaf thicknesses were 211.0 ± 19.0, 199.6 ± 23.3, 163.0 ± 16.9, and 173.8 ± 12.3 µm, respectively; and the average angles of the leaf base were 46.1 ± 10.6, 46.1 ± 17.5, 23.8 ± 5.7, and 27.0 ± 7.3 degrees, respectively. Each trait differed significantly between the non-rheophytic and rheophytic types, except leaf length. Moreover, we calculated the mean leaf size for the specimens. The leaf sizes of the
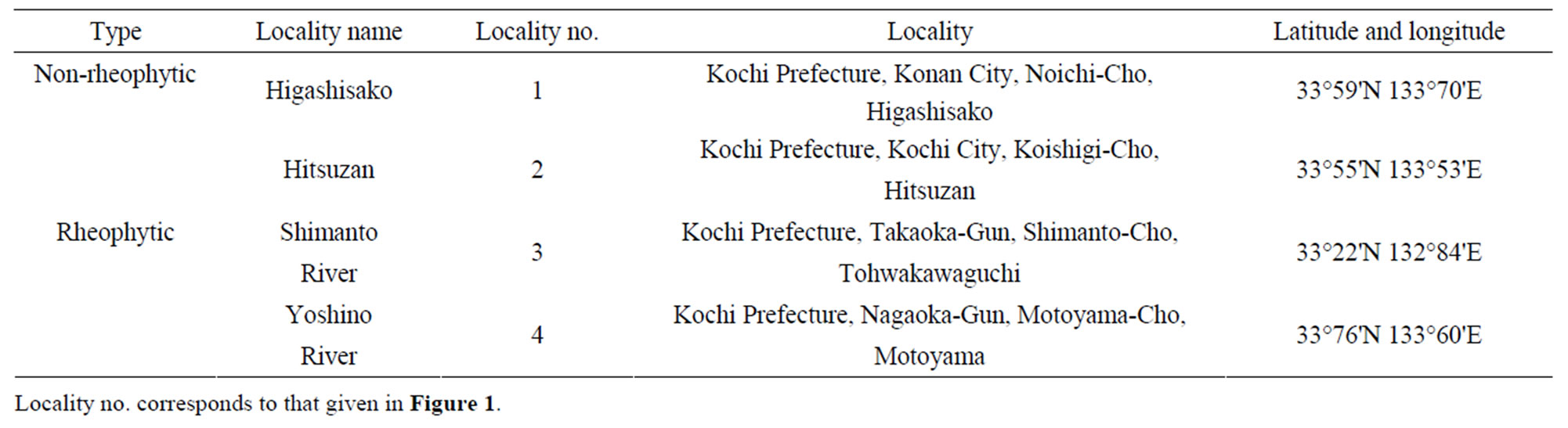
Table 1. Sampling localities used in this study.
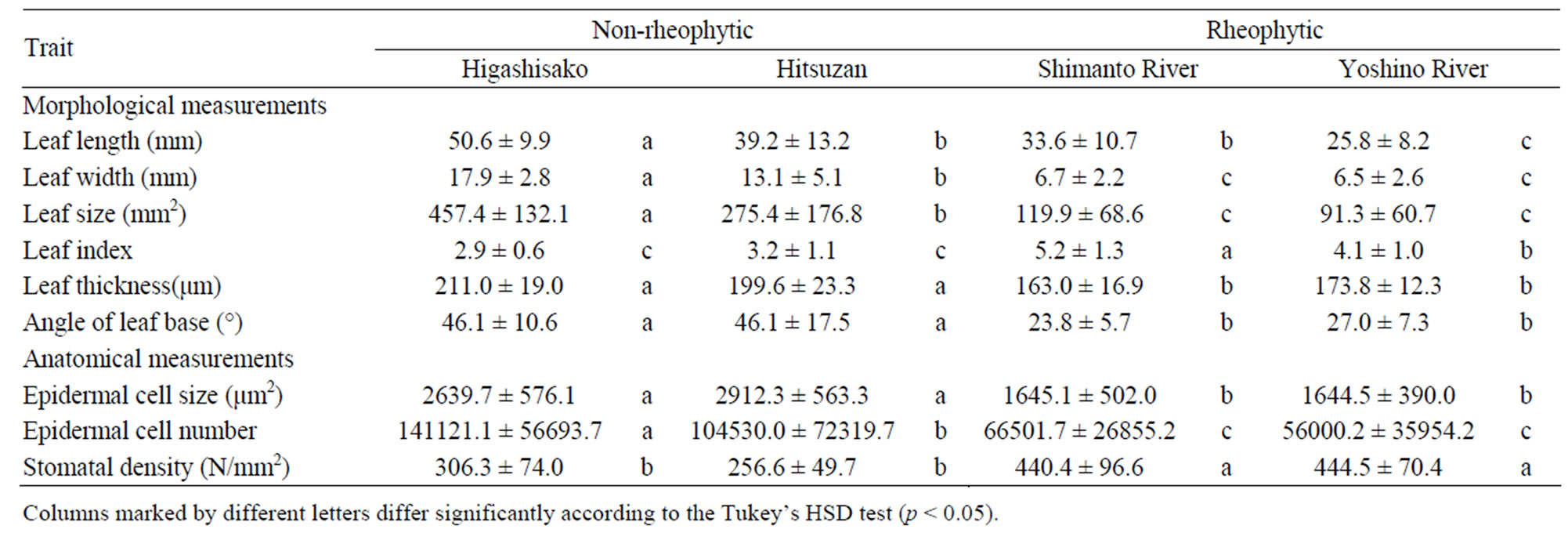
Table 2. Morphological and anatomical measurements (average ± standard deviation) of Adenophora triphylla var. japonica.
non-rheophytic (2 populations from Higashisako and Hitsuzan) and rheophytic (from Shimanto and Yoshino Rivers) types of A. triphylla var. japonica were 457.4 ± 132.1, 275.4 ± 176.8, 119.9 ± 68.6, and 91.3 ± 60.7 mm2, respectively, and the non-rheophytic and rheophytic types differed significantly in this regard.In addition, the leaf index values were calculated as the ratio of leaf length to leaf width similar to the method used by [12]. The non-rheophytic and rheophytic types of A. triphylla var. japonica also differed significantly in terms of leaf index (p < 0.05; non-rheophytic type: 2.9 ± 0.6 (Higashisako), 3.2 ± 1.1 (Hitsuzan) and rheophytic type: 5.2 ± 1.3 (Shimanto River), 4.1 ± 1.0 (Yoshino River)).
We calculated the mean epidermal cell size using the length and width of a cell measured from SUMP samples of non-rheophytic and rheophytic types of Adenophora triphylla var. japonica from all localities we examined. The cell sizes were 2639.7 ± 576.1 (Higashisako), 2912.3 ± 563.3 (Hitsuzan), 1645.1 ± 502.0 (Shimanto River), and 1644.5 ± 390.0 (Yoshino River) µm2, and the nonrheophytic and rheophytic types differed significantly in this regard (p < 0.05). Based on the leaf size and cell size, we calculated the cell number of a leaf. Total cell numbers of leaves were estimated to be 141,121.1 ± 56,693.7 (Higashisako), 104,530.0 ± 72,319.7 (Hitsuzan)66,501.7 ± 26,855.2 (Shimanto River), and 56,000.2 ± 35,954.2, (Yoshino River), and the non-rheophytic and rheophytic types differed significantly in this regard (p < 0.05). In addition, the stomatal density (440.4 ± 96.6 and 444.5 ± 70.4) of the rheophytic type was significantly higher than that of the non-rheophytic ecotype of A. triphylla var. japonica (306.7 ± 74.0 and 256.6 ± 49.7).
4. Discussion
[4] reported that rheophytic species are included in more than 60 families, from bryophytes to angiosperms, and some comparative anatomical studies have indicated that stenophyllization occurs in the leaves of these species.
In fern, for example, a comparative anatomical study of a rheophyte (Osmunda lancea: Osmundaceae) and its closely related dry land species (O. japonica Thunb.) indicated that there is a strong correlation between gross morphology and leaf anatomy [1]. Thus, stenophyllization in O. lancea appears to be caused by the decrease in cell size along the leaf-width direction [1]. Regarding angiosperms, [9] reported that the speciation from Rhododendron indicum f. indicum to rheophytic R. indicum f. otakumi (Ericaceae) is involved in decreasing the number of leaf cells. A similar process of stenophyllization was indicated in the rheophyte Farfugium japonicum (L. fil.) Kitam. var. luchuense (Masam.) Kitam. (Asteraceae) [8]. In other rheophytic species of Asteraceae, in contrast, the variation in leaf-width involved both the size and number of leaf cells as in case of the rheophyte Dendranthema yoshinaganthum [7] and Aster microcephalus var. ripensis [6]. Therefore, the developmental processes of narrow leaves in the rheophytes of angiosperms may have evolved from involving only the number of leaf cells into involving both the size and number of leaf cells in Asteraceae. We found that the rheophytic ecotype of Adenophora triphylla var. japonica has a significantly narrower leaf than the non-rheophytic type, and moreover, our result indicated that the decrease in both the number and size of leaf cells contributed to stenophyllization in a rheophytic ecotype of A. triphylla var. japonica. According to the overall framework of angiosperm phylogeny [14-18], Campanulaceae species, including A. triphylla var. japonica, are located at the ancestral position of Asteraceae, suggesting that rheophytic adaptation involves the same process of stenophyllization, and it evolved independently in each lineage of Campanulaceae and Asteraceae.
Comparative analyses of various rheophytic plants of angiosperms can potentially provide a general adaptation scenario for morphological characteristics. Previous studies of rheophytes reported comparative results of various morphological characteristics. In fact, recent studies of rheophytes of angiosperms has revealed some aspects of plant adaptation, with the interaction of environmental factors along streams and rivers being understood best. For example, the stomatal number was higher in the rheophytic F. japonicum var. luchuense than in inland specimens [19]. Moreover, the angle of the leaf base strongly correlated with both leaf length and width in As. microcephalus var. ripensis, and the decreasing angle of the leaf base led to the formation of lanceolate leaves [6]. Our results regarding the stomatal number and angle of the leaf base also indicated similar differentiation between rheophytic and non-rheophytic types of A. triphylla var. japonica, suggesting that with regard to these characteristics, the general tendency in the leaf morphology of rheophytes is to adapt to the environment along streams and rivers. However, surprisingly, the rheophytic ecotype of A. triphylla var. japonica had thinner leaves than the non-rheophytic ecotype, and it is very interesting the decreased leaf thickness in rheophytic plants. In general, the leaf thickness and stomatal number correlate positively with the mean solar radiation during leaf expansion [20,21], supporting the reports of rheophytic adaptation in some species (e.g., [1,21]). The rheophytic ecotype of A. triphylla var. japonica grows mainly in riverside habitats; on sunny, moist rocks; and on riverbanks along with other rheophytic plants such as O. lancea, R. ripense, and As. microcephalus var. ripensis in the Yoshino and Shimanto rivers. Therefore, although the high irradiance in riverside habitats of the rheophytic ecotype of A. triphylla var. japonica is the same as that received by other rheophytic plants, increased stomatal number and decreased leaf thickness is observed in this taxa (A. triphylla var. japonica). This finding suggests that the rheophytic ecotype of A. triphylla var. japonica shows different adaptation as compared with other rheophytic plants to solar radiation and evaporation. Therefore, further anatomical, ecological, and physiological studies using the rheophytic ecotype of A. triphylla var. japonica are needed.
5. Acknowledgements
We wish to thank Matsuyama K., Yoshimi Y., Yokoyama N., Isomoto S., Miyata H., Tsuchiya Y., Takei S., Sunami T., Kakimoto N. and Kumekawa Y. for their help. This study was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan and the River Fund in charge of the Foundation of River and Watershed Enviroment Management (FOREM), Japan (to TF).
REFERENCES
- R. Imaichi and M. Kato, “Comparative Leaf Development of Osmunda lancea and O. japonica (Osmundaceae): Heterochronic Origin of Rheophytic Stenophylly,” The Botanical Magazine, Vol. 105, No. 2, 1992, pp. 199-213. doi:10.1007/BF02489415
- M. Kato and R. Imaichi, “Leaf Anatomy of Tropical Fern Rheophytes with Its Evolutionary and Ecological Implications,” Canadian Journal of Botany, Vol. 70, No. 1, 1992, pp. 165-174. doi:10.1139/b92-022
- R. Imaichi and M. Kato, “Speciation and Morphological Evolution in Rheophytes,” In: K. Iwatsuki and P. H. Raven, Eds., Evolution and Diversification of Landplants, Springer-Verlag, Tokyo, 1997, pp. 309-318.
- C. G. G. J. van Steenis, “Rheophyte of the World,” Sijthoff and Noordhoff, Alpen aan den Rijn, 1981.
- R. Imaichi and M. Kato, “Comparative Leaf Morphology of Young Sporophytes of Rheophytic Osmunda lancea and Dryland O. japonica,” Journal of Plant Research, Vol. 106, No. 1, 1993, pp. 37-45. doi:10.1007/BF02344371
- Y. Yamada, H. Hayakawa, Y. Minamiya, K. Ito, Z. Shibayama, R. Arakawa and T. Fukuda, “Comparative Morphology and Anatomy of Rheophytic Aster microcephalus (Miq.) Franch. et Sav. var. ripensis Makino (Asteraceae),” Journal of Phytogeography and Taxonomy, Vol. 59, No. 1, 2011, pp. 35-42.
- H. Tsukaya, “Leaf anatomy of a rheophyte, Dendranthema yoshinaganthum (Asteraceae), and of Hybrids between D. yoshinaganthum and a Closely Related NonRheophyte, D. indicum,” Journal of Plant Research, Vol. 115, No. 5, 2002, pp. 329-333. doi:10.1007/s10265-002-0041-y
- M. Usukura, R. Imaichi and M. Kato, “Leaf Morphology of a Facultative Rheophyte, Farfugium japonicum var. luchuense (Compositae),” Journal of Plant Research, Vol. 107, No. 3, 1994, pp. 263-267. doi:10.1007/BF02344253
- H. Setoguchi and G. Kajimura, “Leaf Morphology of the Rheophyte, Rhododendron indicum f. otakumi (Ericaceae),” Acta Phytotaxomica et Geobotanica, Vol. 55, No. 1, 2004, pp. 45-54.
- J. Okazaki, “Adenophora Fisch,” In: K. Iwatsuki, T. Yamazaki, E. B. David and H. Ohba, Eds., Flora of Japan, Angiospermae Dictyedoneae Sympetalae (a), Kodansha, Tokyo, 1993, pp. 406-410.
- T. Yamanaka and K. Takezaki, “Distribution and Ecology of Rhododendron ripense Makino, with Reference to the Vegetation and Flora on Rocky River-Bank,” Journal of Japanese Botany, Vol. 34, 1959, pp. 215-224.
- H. Tsukaya, “Interpretation of Mutants in Leaf Morphology: Genetic Evidence for a Compensatory System in Leaf Morphogenesis That Provides a New Link between Cell and Organismal Theory,” International Review of Cytology, Vol. 217, 2002, pp. 1-39. doi:10.1016/S0074-7696(02)17011-2
- M. Kato, “Adaptation and Evolution of Rheophytes,” In: M. Kato, Ed., Evolutionary Morphology of Plants, University of Tokyo Press, Tokyo, 1999, pp. 131-183.
- S. Mathews and D. J. Donoghue, “The Root of Angiosperm Phylogeny Inferred from Duplicate Phytochrome Genes,” Science, Vol. 286, No. 5441, 1999, pp. 947-950. doi:10.1126/science.286.5441.947
- C. L. Parkinson, K. L. Adams and J. D. Palmer, “Maltigene Analyses Identify the Three Earliest Lineages of Extant Flowering Plants,” Current Biology, Vol. 9, 1999, pp. 1485-1488. doi:10.1016/S0960-9822(00)80119-0
- Y. L. Qiu, J. Lee, F. Bernasconi-Quadroni, D. E. Soltis, P. S. Soltis, M. Zain, E. A. Zimmer, Z. Chen, V. Savolainen and M. W. Chase, “The Earliest Angiosperms: Evidence from Mitochondrial, Plastid and Nuclear Genomes,” Nature, Vol. 402, No. 6760, 1999, pp. 404-407. doi:10.1038/46536
- P. S. Soltis, D. E. Soltis and M. W. Chase, “Angiosperm Phylogeny Inferred from Multiple Genes as a Tool for Comparative Biology,” Nature, Vol. 402, No. 6760, 1999, pp. 402-404. doi:10.1038/46528
- D. E. Soltis, P. S. Soltis, M. W. Chase, M. E. Mort, D. C. Albach, M. Zanis, V. Savolainen, W. H. Hahn, S. B. Hoot, M. F. Fay, M. Axtell, S. M. Swenson, M. A. Prince, W. J. Kress, K. C. Nixon and J. S. Farris, “Angiosperm Phylogeny Inferred from a Combined Data Set of 18S rDNA, rbcL and atpB Sequences,” Botanical Journal of the Linnean Society, Vol. 133, No. 4, 2000, pp. 381-461. doi:10.1006/bojl.2000.0380
- N. Nomura, H. Setoguchi and T. Takaso, “Functional Consequences of Stenophylly for Leaf Productivity: Comparison of the Anatomy and Physiology of a Rheophyte, Farfugium japonicum var. luchuence, and a Related NonRheophyte, F. japonicum (Asteraceae),” Journal of Plant Research, Vol. 119, No. 6, 2006, pp. 645-656. doi:10.1007/s10265-006-0024-5
- Ü. Niinemets, “Components of Leaf Dry Mass per Area— Thickness and Density—Alter Leaf Photosynthetic Capacity in Reverse Directions in Woody Plants,” New Phytolgist, Vol. 144, No. 1, 1999, pp. 35-47. doi:10.1046/j.1469-8137.1999.00466.x
- Ü. Niinemets, “Global-Scale Climatic Controls of Leaf Dry Mass per Area, Density and Thickness in Trees and Shrubs,” Ecology, Vol. 82, No. 2, 2001, pp. 453-469. doi:10.1890/0012-9658(2001)082[0453:GSCCOL]2.0.CO;2
NOTES
*Corresponding author.