American Journal of Plant Sciences
Vol.3 No.4(2012), Article ID:18725,9 pages DOI:10.4236/ajps.2012.34064
Low Co-Cultivation Temperature at 20˚C Resulted in the Reproducible Maximum Increase in Both the Fresh Weight Yield and Stable Expression of GUS Activity after Agrobacterium tumefaciens-Mediated Transformation of Tobacco Leaf Disks
![]()
Department of Plant Pathology and Crop Physiology, Louisiana State University and LSU AgCenter, Baton Rouge, USA.
Email: nmurai@lsu.edu
Received December 24th, 2011; revised January 19th, 2012; accepted February 2nd, 2012
Keywords: Agrobacterium tumefaciens; Co-Cultivation Temperature; Fresh Weight Yield; Stable GUS Gene Expression; Tobacco Leaf Disks; Transformation
ABSTRACT
The importance of controlled temperature during the four-days co-cultivation period was evaluated under the most physiologically relevant conditions for Agrobacterium tumefaciens-mediated transformation of tobacco (Nicotiana tabacum L. cv. Xanthi (nn, Smith)) leaf disks. We compared the effect of temperatures ranging from 15˚C, 18˚C, 20˚C, 22˚C to 25˚C on the stable expression of β-glucuronidase (GUS) activity of 14 days old hygromycin-selected leaf disks, and on the increase in the fresh weight yield of 28 days old kanamycin-selected calli. The highest average of GUS activity was obtained at 20˚C among the five temperatures tested although the difference between the 18˚C and 20˚C treatment was not statistically significant. The GUS activity at 15˚C was statistically lower than those at 18˚C and 20˚C. The GUS activity in 22˚C treatment was an intermediate between the highest (18/20˚C) and second highest averages (15˚C), and was not statistically significantly different. The lowest average of GUS activity was observed at 25˚C. The highest increase in the plate average of fresh weight yield was obtained at 20˚C among the five temperature tested. The 20˚C treatment was statistically significantly better than the 15˚C and 18˚C treatments. The 20˚C co-cultivation treatment resulted in the higher FW yield than 22˚C and 25˚C even though the differences were not statistically significant. In conclusion, low co-cultivation temperature at 20˚C resulted in the reproducible maximum increase in both the fresh weight yield and stable expression of GUS activity after transformation of tobacco leaf disks.
1. Introduction
Agrobacterium tumefaciens is a Gram-negative soil bacterium and plant pathogen causing crown gall disease in angiosperms and gymnosperms [1]. Agrobacterium-plant interaction was one of the first model systems in which the molecular mechanism for plant pathogenicity has been elucidated in details [2,3]. About 20 kb segment of DNA (T-DNA) in a tumor-inducing plasmid (ca. 200 kb Ti plasmid) is transferred from the bacterium to the host plant genome by a molecular machinery closely resembling to a bacterial conjugal transfer [4-6]. The disease phenotype is a manifestation of expression of bacterial T-DNA genes in plant cells that is over-production of plant growth hormones cytokinin and auxin.
This natural DNA transfer system has been exploited to introduce genes of agronomic interest into plants resulted in the production of genetically modified crops by plant biotechnology industries. Initial approaches of gene transfer were to introduce a target gene into the T-DNA region of Ti plasmid after either a single- (co-integration) or double-homologous recombination between an intermediate vector (pRK290) and Ti plasmid [7,8]. A binary plant vector strategy was designed to separate the TDNA region in a small plasmid from the virulence genes in avirulent T-DNA-less Ti plasmid [9]. The small plant vectors with the T-DNA region have been simply now called binary Ti vectors [10,11].
A. tumefaciens-mediated transformation has been generally used for genetic transformation of higher plants since 1983. In a model plant Arabidopsis thaliana gene transfer became a routine using floral dip procedure [12]. However, floral dip procedure does not work well for most other plants, and there are significant technical barriers in transformation of major crops such as soybean, maize, sugarcane and wheat. We had learned hard lessons when we attempted to generate a large number of transgenic tobacco plants to study the effect of 5’-deletion mutations on the promoter activity of the bean seed storage protein phaseolin gene [13,14]. We intended to generate a minimum of ten independent transgenic plants for each of seven deletion constructs, and ended up repeating tobacco transformation experiments eight times since we found out only two experiments worked successfully to generate sufficient number of transgenic plants. At that moment we had no clue as to why we had to waste so much time and effort. Based on the results from our current experiments we now know why.
The importance of controlled temperature during the co-cultivation period was not taken seriously at all in the above-mentioned transformation experiments in 1989. The small culture room (160 cm wide × 142.4 cm deep × 215 cm high) housed one metal shelf rack with four shelves (45 cm wide × 120 cm deep) spaced 40 cm apart. Two fluorescent lamp fixtures each holding two 48 inch-long fluorescent lamps (Philip Westinghouse Lamps F40CW Cool White 40W) were hung from each shelve for constant light. The fluorescent lamps generated the heat in the small culture room resulting in the temperature gradient from the top to bottom shelves. While the room temperature of the building was maintained at around 24.5˚C, the temperature of the top shelve in the culture room could have been over 30˚C without a mechanical air-circulation. Accordingly, we observed that the transformation of tobacco leaf disks were most effective at the bottom shelf, and least effective at the top shelf.
Previous reports suggest that co-cultivation temperatures ranging from 19˚C to 22˚C may be an important environmental condition after A. tumefaciens-mediated transformation of Phaseolus acutifolius [15], cauliflower [16], cotton [17,18], garlic [19], and soybean [20]. However, the importance of controlled temperature during the co-cultivation period was not evaluated critically in rigorously quantitative manners under the most physiologically relevant conditions for plant transformation.
Here, in addition to providing the reproducibility and consistency of experimental results we found that low co-cultivation temperature at 20˚C was the most critical condition for the maximum increase in fresh weight yield and stable GUS gene expression after A. tumefaciensmediated transformation of tobacco leaf disks. We believe the controlled co-cultivation temperature at 20˚C under the most physiologically relevant conditions could be the most important determinant for efficient transformation of other higher plants.
2. Materials and Methods
2.1. Tobacco Plantlets Cultured in Vitro
Tobacco (Nicotiana tabacum L. cv. Xanthi (nn or Smith)) seeds were kindly provided by E. Ted Woodlief, Department of Crop Science, North Carolina State University, Raleigh, NC. Tobacco seeds were sterilized by 50% (v/v) Clorox and planted per petridish on MS-media [21] (per L 4.4 g Sigma MS salts, 0.1 g myo-inositol, 0.4 mg thiamine-HCl, 30 g sucrose, pH 5.6, 10 g agar for solid medium). Seeds were germinated and plantlets were grown in the growth room under constant light with 61 μE∙m–2∙s–1 at room temperature. Plantlets were subcultured at fourweek intervals.
2.2. Binary Ti Vectors
New binary Ti vectors pLSU-1 to 5 were constructed by Seokhyun Lee in this laboratory [22]. The pLSU-3 vector contained a plant-expressible kanamycin-resistance gene and β-glucuronidase gene from pCambia 1305.2 which was purchased from CAMBIA (Canberra, Australia). Plasmids pCambia 1305.1 and pLSU-3 were used for the GUS and growth analyses, respectively.
2.3. Agrobacterium tumefaciens
A. tumefaciens LBA4404 strain was purchased from Invitrogen (Carlsbad, CA). The bacteria was maintained at 28˚C in Agrobacterium media (A-media) (per L 5.0 g yeast extract, 2.0 g mannitol, 2.0 g (NH4)2SO4, 8.570 g K2HPO4, 4.192 g KH2PO4, 160 mg MgSO4.7H2O, 5.0 mg FeSO4·7H2O, 11.0 mg CaCl2·2H2O, and 2.0 mg MnCl2·4H2O).
2.4. Co-Cultivation of Tobacco Leaf Disks with A. tumefaciens
A. tumefaciens-mediated transformation of tobacco leaf disc was performed according to Burow et al. [13] as modified by Park [23] and Su [24]. A. tumefaciens LBA4404 strains were grown overnight in the liquid A medium at 28˚C, 250 rpm with appropriate antibiotics. Cells were harvested by centrifugation and resuspended in liquid MS media (per L 4.4 g MS salts, 30 g sucrose, pH 5.5) at the concentration of 3 × 107 cells/ml (0.3 A600 units/ml). Leaf disks were cut from mature leaf of four week-old tobacco plantlets using a cork borer with 1 cm inner diameter, mechanically wounded with multipleneedle devise, Kenzan from Stone Lantern (Passumpsic, VT) and soaked in 10 ml of A. tumefaciens inoculum containing 200 µM acetosyringone and 0.005% (v/v) Silwet L-77 for 60 minutes. Inoculated leaf disks were blotted thoroughly on sterilized brown paper towels. Ten leaf disks were plated per petridish on the co-cultivation media (per L 4.4 g MS basal salts, 1 mg nicotinic acid, 1 mg pyridoxine·HCl, 0.1 mg thiamine-HCl, 100 mg myoinositol, 1 mg N6-benzyl aminopurine, 0.1 mg α-naphtalene-3-acetic acid, 30 g sucrose, 10 g agar at pH 5.6) containing 200 μM acetosyringone without any antibiotics. Leaf disks were co-cultivated with A. tumefaciens at 20˚C or otherwise specified temperatures for four days.
2.5. Selection for Antibiotic-Resistant Calli after A. tumefaciens-Mediated Transformation
Co-cultivated leaf disks were transferred to the shoot selection medium (the same media composition as cocultivation media) containing 500 mg/L carbenicillin, and 300 mg/L kanamycin or 50 mg/L hygromycin. Leaf disks were incubated at 25˚C ± 0.5˚C under constant light for 14 days and then transferred to the fresh shoot selection media for another 14 days. At the end of 28 days selection the digital images of cultures were taken, the fresh weights were measured, and the calli and shoots were stored at –80˚C for later assay.
2.6. Quantitative β-Glucuronidase Assay
b-Glucuronidase activity was measured in tobacco leaf disks selected by hygromycin for 14 days by colorimetric assays [25,26] as modified by Park [23]. GUS activity was measured with Jasco FP-6300 Spectrofluorometer (Jasco Co., Great Dunmow, UK) in the laboratory of professor Marcia Newcomer, Department of Biological Sciences, LSU. The wavelength was set to 365 nm for excitation and 455 nm for emission. The spectrofluorometer was calibrated with GUS stop buffer using quarz cuvettes. A standard curve was prepared using 0.005, 0.05, 0.1, 0.2, 0.3, 0.4, and 0.5 mM 4-methylumbelliferone solution (MU). Relative intensity of MU light emission in sample solutions was read after the enzyme reaction for 1, 15, 30, and 60 min. The amount of the GUS was calculated based on the established standard curve.
Protein concentration was determined by colorimetric assay using the DC protein assay kit (Bio Rad). Absorbance was read by an ELISA reader at 630 nm after 15 min of reaction at room temperature.
2.7. Statistical Analysis
Each temperature treatment has five plates with 10 leaf disks per plate. The data were subjected to one-way ANOVA program of Statistical Analysis System (SAS) software, SAS Online Doc 9.1.3 from SAS Institute (Cary, NC). Means were compared using either Fisher’s Least Significant Difference (LSD) test (α = 0.05) (Figure 1 by SP) or Turkey’s Studentized Range (HSD) test (α = 0.05) (Figure 2 by GS).
3. Results
We were interested in evaluating the importance of controlled temperature during the co-cultivation period for A. tumefaciens-mediated transformation of tobacco leaf disks. The most physiologically relevant conditions for plant transformation were selected during the four days co-cultivation period. To reproduce the near-identical temperature and light conditions among different treatments we used three Tissue Culture Chambers of Model CU-36L5 of Percival Scientific. The temperatures during the four-day co-cultivation period were set from 15˚C, 18˚C, 20˚C, 22˚C to 25˚C with the range of 0.7˚C under constant light (56 mE∙m–2∙s–1). We compared the effect of co-cultivation temperatures on the stable expression of β-
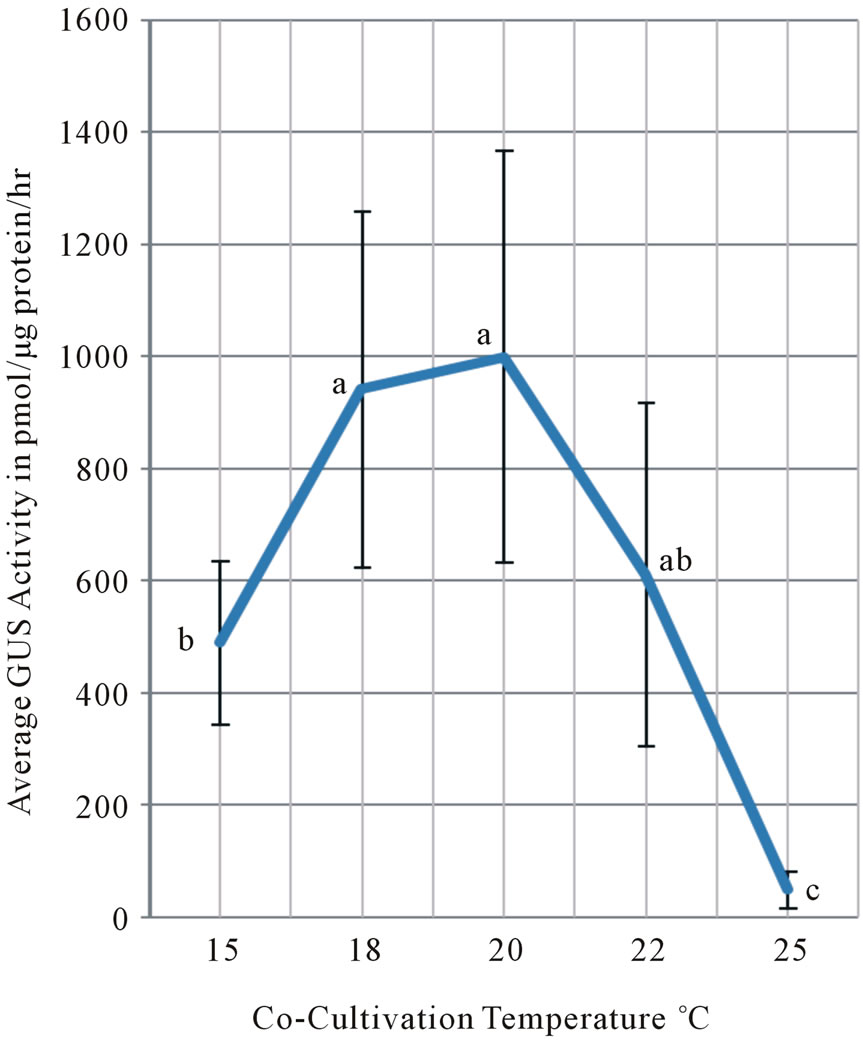
Figure 1. Co-cultivation temperature at 15˚C, 18˚C, 20˚C, 22˚C and 25˚C were compared in its effect on the stable expression of GUS activity measured after four-days cocultivation followed by 14-days hygromycin-selection at 25˚C. All values from four different experiments were pooled and averaged. Averages of GUS activity at each temperature were represented in pmol/μg protein/hour with standard deviations as vertical bars. Means followed by the same letter are not significantly different at a = 0.05 level using Fisher’s least significant difference (LSD) test. Experiment was conducted from 10/18 to 11/25/2005.
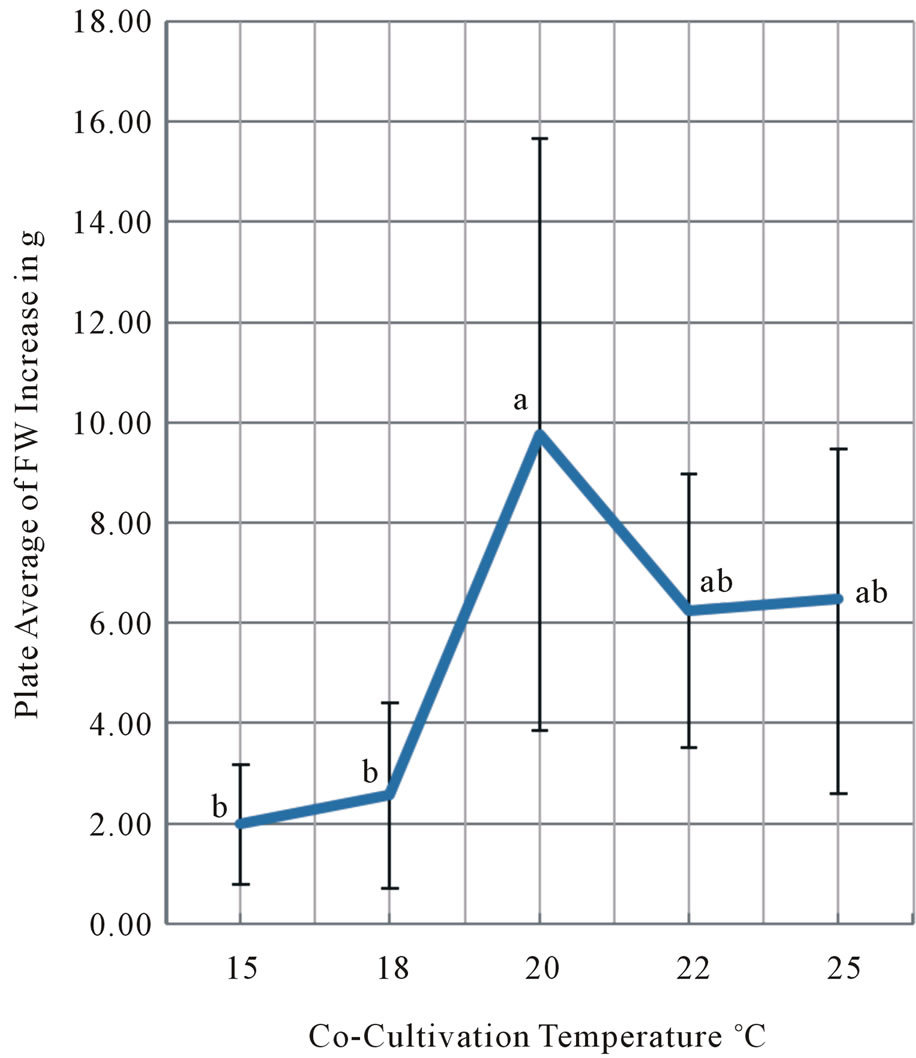
Figure 2. Co-cultivation temperature at 15˚C, 18˚C, 20˚C, 22˚C and 25˚C were compared in its effect on the final fresh weight yield increase of tobacco leaf disks after four days co-cultivation and 28 day kanamycin selection at 25˚C. Co-cultivation was performed from 11/9 to 11/13, the first selection from 11/13 to 11/27 and the second selection from 11/27 to 12/11/2009. Each temperature treatment has five plates with 10 leaf disks per plate. The same experiment was repeated at least three times. Vertical bars at the average FW yields indicate standard deviations. Means followed by the same letter are not significantly different at the α = 0.05 level using Tukey’s Studentized Range (HSD) test.
glucuronidase (GUS) activity of 14 days old hygromycin-selected calli (Figure 1), and on the increase in the fresh weight yield of 28 days old kanamycin-selected calli (Figures 2 and 3).
3.1. β-Glucuronidase Activity Assays on Co-Cultivation Temperature
Initially we chose to measure the stable expression of GUS activity to set up optimal experimental conditions during the co-cultivation period with A. tumefaciens [23]. A. tumefaciens-mediated transformation of tobacco leaf disks were conducted according to Burow et al. [13] as modified by Park [23] and Su [24]. Five important experimental parameters were compared singly or in combination among the range of concentrations or of time durations, and the most optimal conditions were selected and shown by bold [23]. Co-cultivation days ranged from 2, 4 to 6 days; bacteria concentrations from 3 × 105, 3 × 106, 3 × 107 to 108 cells/mL; surfactant Silwet L-77 con-

Figure 3. Effect of co-cultivation temperatures at 15˚C, 18˚C, 20˚C, 22˚C and 25˚C on the fresh weight yield increase and plantlet regeneration from tobacco leaf disks after four-days co-cultivation and 28-days kanamycin selection at 25˚C. Two representative plates out of five plates with 10 leaf disks per plate were shown for each temperature treatment with a vector-less control. The results from the same experiment were shown in Figures 2 and 3.
centrations from 0.001%, 0.005%, 0.05% to 0.1% (v/v); vacuum infiltration duration from 1.5, 4, 20 to 40 min; and acetosyringone concentrations from 100, 200 to 400 μM. The following standerdized conditions were used for subsequent experiments: A. tumefaciens strain LBA4404 containing pCAMBIA1305.1 was grown overnight at 28˚C in the liquid MS media and the concentration was adjusted to 3 × 107 cells/ml (0.3 A600 units/mL). Tobacco leaf disks were inoculated with bacteria under 50 mmHg vacuum infiltration for 20 min in the presence of the 0.005% (w/v) Silwet L-77. Leaf disks were co-cultivated without antibiotics in the presence of 200 μM of acetosyringone for four days under constant light at 20˚C or otherwise specified temperature. Co-cultured leaf disks were transferred to selection media containing 50 mg/L hygromycin and 500 mg/L carbenicillin, and grown for 14 days under constant light at 25˚C. The β-glucuronidase activity was measured at the end of growth period by quantitative GUS assay to compare the effect of experimental variables.
We compared the effect of five co-cultivation temperatures ranging from 15˚C, 18˚C, 20˚C, 22˚C to 25˚C on the GUS expression of 14 days old hygromycin-resistant calli. All GUS activity values were pooled from four separate experiments and averaged with a standard deviation at each temperature (Figure 1). The highest average of GUS activity was obtained at 20˚C among the five temperatures although the difference between the 18˚C and 20˚C treatment was not statistically significant. Statistically lower average of GUS activity was observed in the 15˚C than in the 18/20˚C treatments. The lowest average of GUS activity was observed in the 25˚C cocultivation treatment. The GUS activity in the 22˚C treatment was an intermediate between these highest averages at 18˚C and 20˚C and the lower average at 15˚C, and was not statistically significantly different. We concluded that co-cultivation at 20˚C is the most critical determinant for the stable expression of maximum GUS activity after transformation of tobacco leaf disks.
We observed during the vacuum infiltration period that significant portions of leaf disks were destroyed under the low vacuum and unable to produce calli resulting in undetectable GUS activity. We rectified this limitation by substituting the vacuum infiltration with mechanical wounding using a multiple-needles devise.
3.2. Fresh Weight Increase Growth Assays on Co-Cultivation Temperature
We decided to use growth comparison assay of rigorously selected calli by antibiotic selection agent to apply a qualitatively different measurement from the GUS activity for comparison. We chose the kanamycin concentration of 300 mg/L to provide a rigorous selection scheme that only transformed cells are selected for and survived [24].
To show the effectiveness of the kanamycin concentration at 300 mg/L for rigorous selection we selected kanamycin-resistant calli and regenerated over 100 transgenic tobacco plants [14]. The virtually universal expression among transgenic plants of transferred bean seed storage protein β-phaseolin gene was demonstrated by RNA dot blot assay and protein expression by EnzymeLinked Immunosorbent Assay [14].
The experimental conditions were essentially the same as GUS assay experiments except for the following conditions; leaf disks were mechanically wounded using a multiple-needle device. Wounded leaf disks were first co-cultured in liquid for 60 min with A. tumefaciens containing a new binary Ti vector pLSU-3. Co-cultured leaf disks were transferred to the shoot selection medium containing 500 mg/L carbenicillin and 300 mg/L kanamycin. Leaf disks were incubated at 25˚C ± 0.7˚C under constant light with 56 μE∙m–2∙s–1 for 14 days and then transferred to the fresh shoot selection media for additional 14 days. At the end of 28 days growth period digital images of leaf disk growth were taken, the fresh weight yields were measured in mg.
The temperatures during the four-day co-cultivation period were set from 15˚C, 18˚C, 20˚C, 22˚C to 25˚C with the range of 0.7˚C under constant light (56 μE∙m–2∙ s–1). The results from the temperature experiments were summarized in Figure 2. We found the four-days cocultivation at 20˚C resulted in the highest increase in the plate average of fresh weight yields. The 20˚C treatment was statistically significantly better than the 15˚C and 18˚C treatments. The 20˚C co-cultivation provided higher fresh weight (FW) yield than 22 and 25˚C even though the differences were not statistically significant. As evident in Figure 3, numerous tobacco plantlets were regenerated from leaf disks at the end of four weeks selection period. The plant regeneration frequency from tobacco leaf discs was reported by Burow et al. [13,14]. We concluded that co-cultivation at 20˚C is the most critical determinant for the maximum increase in FW yield after transformation of tobacco leaf disks.
4. Discussion
We found that low co-cultivation temperature at 20˚C is the most critical environmental determinant to achieve the reproducible and consistent results for the maximum increase in the fresh weight yield and stable GUS gene expression after A. tumefaciens-mediated transformation of tobacco leaf disks. Among the five temperatures from 15˚C, 18˚C, 20˚C, 22˚C to 25˚C tested, co-cultivation at 20˚C resulted in statistically greater growth than that at 15˚C and 18˚C. Statistically distinct and higher growth was observed after co-cultivation at 20˚C than at 22˚C and 25˚C. The highest average of stable GUS activity was also obtained after co-cultivation at 20˚C. Co-cultivation at 18˚C and 20˚C resulted in statistically higher GUS expression average than at 15˚C and 25˚C. Statistically distinct and intermediate average of GUS activity was observed at 22˚C. The results demonstrated that four-day co-cultivation with different temperature treatments determined the final outcomes of GUS expression and growth yield of transformed tissues after selection. Temperature profiles of fresh weight yield increase and GUS gene expression are in agreement with thermosensitivity profile of plasmid transfer during transconjugation and of stability of pili, a channel structure responseble for plasmid transfer. Although co-cultivation at 19˚C is the best condition for bacterial pili formation [27], it is not the most favorable condition for the growth of tobacco leaf disks, resulting in the higher fresh weight yield at 20˚C, 22˚C and 25˚C than at 15˚C and 18˚C. Tumor induction studies have been routinely performed in the range 25˚C to 28˚C because it has been reported that tumor are mostly formed over 25˚C in Nicotiana by Ricker et al. [28].
The temperature profile during the co-cultivation period in our experiments is different from those previously reported for GUS activity. Transient expression of quantitative GUS activity in Phaseolus acutifolius embryogenic callus had a peak at 22˚C in a range of co-cultivation temperature from 15˚C, 19˚C, 22˚C, 25˚C, 27˚C to 29˚C. Histochemical staining of GUS activity was also the highest at 22˚C for both Phaseolus calli and Nicotiana tobacum as reported by Dillen et al. [15]. For hypocotyls explants of cauliflower transformation, 22˚C co-cultivation resulted in the highest GUS expression, and no GUS expression was detected at 28˚C [16]. GUS expression in Phaseolus acutifolius transformation experiment also showed 22˚C co-cultivation is optimal condition to increase transformation efficiency [29]. The frequency of garlic calli with transient GUS expression is the highest at 22˚C followed by 20˚C and 24˚C [19]. The frequency of phosphinothricin-resistant soybean calli was higher at 22˚C than at 25˚C [20]. In an agreement with this study, all these previous experiments reported that GUS expression was reduced significantlly at co-cultivation temperatures over 25˚C.
The lower optimal co-cultivation temperature in this study may be partly accounted for by the differences between stable vs transient GUS expression, and also the difference in co-cultivation and selection conditions employed. We measured the increase in fresh weight yield and GUS expression of stable transformants, and have applied the most physiologically relevant conditions during the co-cultivation and selection period using identical tissue culture chambers of Model CU-36L5 of Percival Scientific. Leaf disks were placed during the four days co-cultivation on MS shoot media in a Petri dish under constant light (56 μE∙m–2∙s–1) with the temperature range of ± 0.7˚C. Selection for transformed cells were conducted for 14 or 28 days on MS shoot media containing hygromycin or kanamycin, respectively, in a Petri dish under constant light at 25˚C ± 0.7˚C. In comparison, Dillen et al. [15] had used less than physiologically appropriate conditions during the co-cultivation, no selection of tissues and shorter culture period before GUS assay. Leaves of Nicotiana tabacum cv SR1 Petit Havana were infiltrated with 0.8 A600 unit of A. tumefaciens under reduced pressure for 20 min, placed on a filter paper in a Petri dish which was sealed in a plastic bag, and submerged for three days under water in a water bath. Calli of Phaseolus acutifolius were placed in a glass jar submereged under water in a water bath. After two days co-cultivation, treated calli were cultured on non-selected medium for four days prior to GUS assay. Consequently, the reported GUS activity was significantly lower than in this study and had no standard deviation indicated. We assumed a minimum effect exerted by the difference in binary vectors, helper plasmids or strains of A. tumefaciens used in this and previous experiments.
Riker [30] made an initial observation of important role of temperature on the formation of crown gall tumors in tomato and other plants. The size of tumors was largest at the optimal temperature of 22˚C and was gradually decreased as the incubation temperature increased to below 28˚C. No tumor was formed at or above 30˚C. Braun [31] used this thermo-sensitivity to distinguish two phases of crown gall tumor formation between the temperature-sensitive inception phase and the temperature-insensitive development phase. The autonomous neoplastic growth of transformed cells was achieved only after plant cells reached to the second development phase. The inception phase was further divided to two processes, the thermo-sensitive induction process and the thermoinsensitive conditioning process. The conditioning of plant cells was initiated by wounding, took places either below or above 30˚C, and improved during the 48 hr period after wounding up to the time the first cell division was observed. Thus, Braun identified the thermosensitivity of the induction process in which Tumor Inducing Principal (TIP) transfers from the bacteria to plant cells. The nature of Braun’s TIP was later elucidated as T-strand of transfer DNA (T-DNA) of tumor-inducing plasmid (Ti-plasmid), and the components of T-DNA transfer machinery was further identified.
The thermo-sensitivity of conjugative transfer of Ti plasmid from the virulent to cured avirulent strains of A. tumefaciens was noted by Tempe et al. [32]. The frequency of plasmid transfer was 4.0 and 3.5 × 10–2 at 23˚C and 27˚C, respectively, reduced by four-fold to 0.9 × 10–2 at 30˚C, and went below 10–6 at 33˚C and 36˚C. IncQ group plasmid pML122 is a non-conjugative plasmid but can utilizes a transfer machinery of conjugative Ti plasmid of A. tumefaciens. Conjugal transfer of the IncQ plasmid mediated by A. tumefaciens was found thermosensitive as reported by Fullner and Nester [33]. The frequency of conjugal transfer was the highest 2.6 × 10–3 at the optimal temperature of 19˚C, gradually reduced to 1.1 × 10–3 at 22˚C, 5.3 × 10–4 at 25˚C, and 2.0 × 10–4 at 15˚C, and then drastically decreased by over 1000-fold at 28˚C and 31˚C. The temperature profile of conjugal transfer of IncQ plasmid [33] is more similar to that of crown gall tumor formation [30] than that of conjugal transfer of Ti plasmid [32]. The similarity of thermosensitivity profile between crown gall formation and conjugal transfer of IncQ plasmids suggests that the same machinery may involve in transfer of T-strands from the bacteria to plant cells, and in conjugal transfer of IncQ plasmids between bacteria.
The thermo-sensitivity of bacteria pilus assembly was observed by Fullner et al. [27]. The induction of vir genes by 200 μM of acetosyringone was essential for the pilus assembly. The pilated cells constituted 10% to 20% of cells observed under electron microscope at 19˚C and were over ten-fold more abundant than those at 28˚C. The lossand gain-of-function experiments demonstrated that the same collection of vir genes, virA, virG, virB1 through virB11, virD4 were required for the pilus production and conjugal transfer of plasmid. The additional vir gene, virE1 required for transport of virE2 was necessary for tumor induction on leaves of Kalanchoe daigremontiana. The results suggest that the assembly of pilus on A. tumefaciens is essential for both processes, transfer of T-strands from the bacteria to plant cells, and conjugal transfer of plasmid between bacteria.
Distinct profiles of temperature-dependence were evident between the expression of virB genes and conjugal transfer of plasmid. The induction of virB genes was found optimal at 25˚C and the conjugal transfer of plasmid optimal at 19˚C. Baron et al. [34] reported that highest amount of pili and pili component VirB were detected at 20˚C, and small amount of pili was detected at 26˚C while pili formation at 28˚C was strongly inhibited. They also reported that tumor formation was induced at 20˚C at wounded K. diagremontiana. It can be concluded that 20˚C is the optimal temperature to induce assembly, formation and structural stability of pili, as well as to maintain plant tissue viability.
Pilus is composed of two VirB proteins, major VirB2 and minor VirB5. Extracellular pili are anchored with a type IV secretion systems (T4SS) likely to locate in the bacterial periplasm, and to span both the inner and outer membranes. The three-dimensional structure of the type IV secretion system core complex was elucidated at 15 angstroms resolution by cryo-electron microscopy [35]. The core complex is composed of 14 copies of three proteins, VirB7, VirB9 and VirB10. The three proteins assemble into a 1.05 megadalton core complex structure consisting of two layers, the inner-membrane-spanning layer and ~0.6 megadalton outer-membrane spanning layer. The overall dimension of core complex is 85 and 100 angstroms in inner and outer layer heights, respecttively, and 185 angstroms in diameter. The outer-membrane complex was purified after chymotryptic cleavage of the T4SS and the three-dimensional structure was elucidated at 2.6 angstroms resolution by X-ray crystallography [36]. VirB10 protein forms the entirety of the inner wall of the outer-membrane channel structure, and VirB9 and VirB7 proteins encircle the exterior of the VirB10 protein channel. VirB6 and VirB8 complex are in the inner-membrane complex. VirB8 protein was localized in helical arrays around the circumference of virulenceinduced A. tumefaciens cells as reported by Aguilar et al. [37].
5. Acknowledgements
The authors wish to acknowledge the financial support partly from a USDA/ARS Specific Cooperative Agreement 58-6435-8-308 to N. Murai, and from the College of Agriculture, Louisiana State University, and LSU AgCenter.
REFERENCES
- E. F. Smith and C. O. Towsend, “A Plant Tumor of Bacterial Origin,” Science, Vol. 25, No. 643, 1907, pp. 671- 673. doi:10.1126/science.25.643.671
- I. Zaenen, N. Van Larebeke, H. Teuchy, M. Van Montagu and J. Schell, “Supercoiled Circular DNA in Crown-Gall Inducing Agrobacterium Strains,” Journal Molecular Biology, Vol. 86, No. 1, 1974, pp. 109-127. doi:10.1016/S0022-2836(74)80011-2
- M. D. Chilton, M. H. Drummond, D. J. Merlo, D. Sciaky, A. L. Montoya, M. P. Gordon and E. W. Nester, “Stable Incorporation of Plasmid DNA into Higher Plant Cells: The Molecular Basis of Crown Gall Tumorigenesis,” Cell, Vol. 11, No. 2, 1977, pp. 263-271. doi:10.1016/0092-8674(77)90043-5
- J. R. Zupan and P. Zambryski, “Transfer of T-DNA from Agrobacterium to the Plant Cell,” Plant Physiology, Vol. 107, No. 4, 1995, pp. 1041-1047. doi:10.1104/pp.107.4.1041
- J. Sheng and V. Citovsky, “Agrobacterium-Plant Cell DNA Transport: Have Virulence Proteins, Will Travel,” Plant Cell, Vol. 8, No. 10, 1996, pp. 1699-1710. doi:10.1105/tpc.8.10.1699
- S. B. Gelvin, “Agrobacterium-Mediated Plant Transformation: The Biology behind the ‘Gene-Jockeying’ Tool,” Microbiology Molecular Biology Reviews, Vol. 67, No. 10, 2003, pp. 16-37. doi:10.1128/MMBR.67.1.16-37.2003
- P. Zambryski, H. Joos, C. Genetello, J. Leemans, M. Van Montagu and J. Schell, “Ti Plasmid Vector for the Introduction of DNA into Plant Cells without Alteration of Their Normal Regeneration Capacity,” EMBO Journal, Vol. 2, No. 12, 1983, pp. 2143-2150.
-
N. Murai, D. W. Sutton, M. G. Murray, J. L. Slightom, D. J. Merlo, N. A. Reichert, C. Sengupta-Gopalan, C. A. Stock, R. F. Baker, J. D. Kemp and T. C. Hall, “Phaseolin Gene from Bean Is Expressed after Transfer to Sunflower via Tumor-Inducing Plasmid Vectors,” Science, Vol. 222, No. 4623, 1983, pp. 476-482. doi:10.1126/science.222.4623.476- °
- A. Hoekema, P. R. Hirsch, P. J. J. Hooykas and R. A. Schilperoort, “A Binary Plant Vector Strategy Based on Separation of virand T-Region of the Agrobacterium tumefaciens Ti Plasmid,” Nature, Vol. 303, No. 5913, 1983, pp. 179-180. doi:10.1038/303179a0
- R. Hellens, P. Mullineaux and H. Klee, “A Guide to Agrobacterium Binary Ti Vectors,” Trends in Plant Science, Vol. 5, No. 10, 2000, pp. 446-448. doi:10.1016/S1360-1385(00)01740-4
- T. Komori, T. Imayama, N. Kato, Y. Ishida, Y. Ueki and T. Komari, “Current Status of Binary Vectors and Superbinary Vectors,” Plant Physiology, Vol. 145, No. 4, 2007, pp. 1155-1160. doi:10.1104/pp.107.105734
- S. J. Clough and A. F. Bent, “Floral Dip: A Simplified Method for Agrobacterium-Mediated Transformation of Arabidopsis thaliana,” Plant Journal, Vol. 16, No. 6, 1998, pp. 735-743. doi:10.1046/j.1365-313x.1998.00343.x
- M. Burow, C. A. Chlan, P. Sen, A. Lisca and N. Murai, “High Frequency Generation of Transgenic Tobacco Plants after Modified Leaf Disk Cocultivation with Agrobacterium tumefaciens,” Plant Molecular Biology Reporter, Vol. 8, No. 2, 1990, pp. 153-168. doi:10.1007/BF02669766
- M. Burow, P. Sen, C. A. Chlan and N. Murai, “Developmental Control of the β-Phaseolin Gene Requires Positive, Negative, Temporal Seed-Specific Transcription Regulatory Elements and a Negative Element for Stem and Root Expression,” Plant Journal, Vol. 2, No. 4, 1992, pp. 534- 548. doi:10.1111/j.1365-313X.1992.00537.x
- W. Dillen, J. De Clercq, J. Kapila, M. Zambre, M. Van Montagu and G. Angenon, “The Effect of Temperature on Agrobacterium tumefaciens-Mediated Gene Transfer to Plants,” Plant Journal, Vol. 12, No. 6, 1997, pp. 1459- 1463. doi:10.1046/j.1365-313x.1997.12061459.x
- R. Chakrabarty, N. Viswakarma, S. R. Bhat, P. B. Kirti, B. D. Singh and V. L. Chopra, “Agrobacterium-Mediated Transformation of Cauliflower: Optimization of Protocol and Development of Bt-Transgenic Cauliflower,” Journal of Bioscience, Vol. 27, No. 5, 2002, pp. 495-502. doi:10.1007/BF02705046
- G. Sunilkumar and K. Rathore, “Transgenic Cotton: Factors Influencing Agrobacterium-Mediated Transformation and Regeneration,” Molecular Breeding, Vol. 8, No. 1, 2001, pp. 37-52. doi:10.1023/A:1011906701925
- S. Jin, X. Zhang, S. Liang, Y. Nie, X. Guo and C. Huang, “Factors Affecting Transformation Efficiency of Embryogenic Callus of Upland Cotton (Gossypium hirsutum) with Agrobacterium tumefaciens,” Plant Cell, Tissue & Organ Culture, Vol. 81, No. 2, 2005, pp. 229-237. doi:10.1007/s11240-004-5209-9
- T. Kondo, H. Hasegawa and M. Suzuki, “Transformation and Regeneration of Garlic (Allium sativum L.) by Agrobacterium-Mediated Gene Transfer,” Plant Cell Report, Vol. 19, No. 10, 2000, pp. 989-993. doi:10.1007/s002990000222
- W. Dang and Z.-M. Wei, “An Optimized AgrobacteriumMediated Transformation for Soybean for Expression of Binary Insect Resistance Genes,” Plant Science, Vol. 173, No. 4, 2007, pp. 381-389. doi:10.1016/j.plantsci.2007.06.010
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures,” Physiologia Plantarum, Vol. 15, No. 3, 1962, pp. 473-497. doi:10.1111/j.1399-3054.1962.tb08052.x
- S. Lee, “New Binary Ti Vectors with the Co-Directional Replicons for Agrobacterium tumefaciens-Mediated Transformation of Higher Plants,” Ph.D. Dissertation, Louisiana State University, Baton Rouge, 2010.
- S. J. Park, “Agrobacterium tumefaciens-Mediated Transformation of Tobacco (Nicotiana tabacum L.) Leaf Disks: Evaluation of the Co-Cultivation Conditions to IncreaseGlucuronidase Gene Activity,” M.S. Thesis, Louisiana State University, Baton Rouge, 2006.
- G. Su, “Low Co-Cultivation Temperature at 20˚C Improved Agrobacterum tumefaicens Mediated Transformation of Tobacco Leaf Disks,” M.S. Thesis, Louisiana State University, Baton Rouge, 2010.
- R. A. Jefferson, “Assaying Chimeric Genes in Plants: The GUS Gene Fusion System,” Plant Molecular Biology Reporter, Vol. 5, No. 4, 1987, pp. 387-405. doi:10.1007/BF02667740
- Z. Zheng, K. Sumi, K. Tanaka and N. Murai, “The Bean Seed Storage Protein b-Phaseolin Is Synthesized, Processed, and Accumulated in the Vacuolar Type-II Protein Bodies of Transgenic Rice Endosperm,” Plant Physiology, Vol. 109, No. 3, 1995, pp. 777-786.
- K. J. Fullner, J. C. Lara and E. W. Nester, “Pilus Assembly by Agrobacterium T-DNA Transfer Genes,” Science, Vol. 273, No. 5278, 1996, pp. 1107-1109. doi:10.1126/science.273.5278.1107
- A. J. Riker, B. Henry and B. M. Duggar, “Growth Substance in Crown Gall as Related to Time after Inoculation, Critical Temperature, and Diffusion,” Journal of Agriculture Research, Vol. 63, No. 7, 1941, pp. 395-405.
- J. De Clercq, M. Zambre, M. Van Montagu, W. Dillen and G. Angenon, “An Optimized Agrobacterium-Mediated Transformation Procedure for Phaseolius acutifolius A. Gray,” Plant Cell Report, Vol. 21, No. 4, 2002, pp. 333-340. doi:10.1007/s00299-002-0518-0
- A. J. Riker, “Studies in the Influence of Some Environmental Factors on the Development of Crown Gall,” Journal Agriculture Research, Vol. 32, 1926, pp. 83-89.
- A. C. Braun, “Thermal Studies on the Factors Responsible for Tumor Initiation in Crown Gall,” American Journal of Botany, Vol. 34, No. 4, 1947, pp. 234-240. doi:10.2307/2437424
- J. Tempe, A. Petit, M. Holsters, M. Van Montagu and J. Schell, “Thermosensitive Step Associated with Transfer of the Ti Plasmid during Conjugation: Possible Relation to Transformation in Crown Gall,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 74, No. 7, 1977, pp. 2848-2849. doi:10.1073/pnas.74.7.2848
- K. J. Fullner and N. W. Nester, “Temperature Affects the T-DNA Transfer Machinery of Agrobacterium tumefaciens,” Journal of Bacteriology, Vol. 178, No. 6, 1996, pp. 1498-1504.
- C. Baron, N. Domke, M. Beinhofer and S. Hapfelmeier, “Elevated Temperature Differentially Affects Virulence, VirB Protein Accumulation, and T-Pilus Formation in Different Agrobacterium tumefaciens and Agrobacterium vitis Strains,” Journal of Bacteriology, Vol. 183, No. 23, 2001, pp. 6852-6861. doi:10.1128/JB.183.23.6852-6861.2001
- V. Chandran, R. Fronzes, S. Duquerroy, N. Cronin, J. Navaza and G. Waksman, “Structure of the Outer Membrane Complex of a Type IV Secretion System,” Nature, Vol. 462, No. 7276, 2009, pp. 1011-1015. doi:10.1038/nature08588
- R. Fronzes, E. Schafer, L. Wang, H. R. Saibil, E. V. Orlova and G. Waksman, “Structure of a Type IV Secretion System Core Complex,” Science, Vol. 323, No. 5911, 2009, pp. 266-268. doi:10.1126/science.1166101
- J. Aguilar, J. Zupa, T. A. Cameron and P. C. Zambryski, “Agrobacterium Type IV Secretion System and Its Substrates form Helical Arrays around the Circumference of Virulence-Induced Cells,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 107, No. 8, 2010, pp. 3758-3763. doi:10.1073/pnas.0914940107
NOTES
*The first two authors contributed equally to this work and both are considered to be senior authors.

