International Journal of Clinical Medicine
Vol. 3 No. 7 (2012) , Article ID: 26179 , 6 pages DOI:10.4236/ijcm.2012.37115
Whole-Body 64-Slice Multidetector Computed Tomography (MDCT) Identifies High-Risk Myeloma
![]()
1Haematology Division and BMT Unit, Antonio Perrino Hospital Hospital, Brindisi, Italy; 2Radiology Division Antonio Perrino Hospital, Brindisi, Italy.
Email: *giuseppemele2007@gmail.com
Received September 14th, 2012; revised October 20th, 2012; accepted November 30th, 2012
Keywords: Asymptomatic Myeloma; MDCT; MRI; Conventional Radiography
ABSTRACT
Background: In Multiple Myeloma (MM) the individuation of bone lesions at baseline is mandatory because the detection of cortical damage reflects prognostic implications. Conventional Radiography (CR) shows osteolytic bone lesions only when the cortical bone damage is more than 30%. Whole-body 64-slice multidetector computed tomography (MDCT) has recently been employed for detecting early osteolytic disease. Patients and Methods: Twenty-height patients with Asymptomatic MM according to IMWG criteria underwent a 64 MDCT. Results: In our experience MDCT revealed osteolysis in 14/28 patients with normal skeletal survey and in 6 patients with normal Magnetic Resonance Imaging (MRI). Patients with radiological evidence of bone disease on MDCT were at high risk of progression with a median time to progression of 5 months (range 1 - 26 months) in comparison with patients without radiological evidence of bone disease who, conversely, showed a median time to progression of 20 months (range 8 - 40 months) (P = 0.0001). Conclusions: MDCT is able to identify MM patients with a high risk of progression, who might benefit from early therapy.
1. Introduction
Multiple Myeloma (MM) is an incurable malignancy characterized mainly by osteolytic bone destruction. Based on the currently available evidence compiled by the International Myeloma Working Group (IMWG) [1], Conventional Radiography (CR) still represents the “gold standard” of the staging procedure of newly diagnosed and relapsed MM patients, however, the sensitivity is not sufficient to detect early osteolytic lesions (CR shows osteolytic bone lesions only when the cortical bone damage is more than 30% - 50%). Since early myeloma may reveal no abnormalities on CR, Durie et al. introduced an update of their original system; the new system, named Durie and Salmon Plus staging system and published in 2005, integrates Magnetic Resonance Imaging (MRI) of the spine and whole body positron emission tomography into anatomic and functional system [2].
The lytic bone lesions as well as elevated levels of paraprotein, the presence of an immunoglobulin A isotype, and Bence-Jones protein have been proposed to distinguish between asymptomatic MM patients who are likely to remain stable without therapy for a long period and patients at high risk for a various complication who might benefit from early treatment [3].
The ability to detect both very small lytic lesions (<5 mm) and extramedullary involvement in a single examination, the short acquisition time (8 seconds), the 3D reconstruction algorithms permitting to explore particular and difficult anatomic regions (skull, clavicle, ribs), and the absence of contrast material make Whole-body 64- slice multidetector computed tomography (MDCT) an attractive diagnostic tool. The incapacity to detected osteolysis, essential criteria to treat patients, together with the incomplete field of view including only spine and pelvis, which account for only part of the bone involvement in MM patients, the long acquisition time, the severe claustrophobia, the presence of metallic prostheses, and, finally, the incapacity to distinguish easily between viable neoplastic tumours and treated bone marrow lesions represent the main limitations of conventional MRI.
It is not yet clear whether MDCT is equal to conventional MRI for the assessment of bony metastases. According to Lecouvet MDCT identifies bone destruction in cases where conventional MRI is negative [4]. Conversely, Baur-Melnyk had demonstrated that sensitivity of conventional MRI is superior to MDCT [5]. According to Horger, in analogy to conventional MRI, wholebody low-dose MDCT permits simultaneously the detection of all 3 categories of myeloma manifestations: bone marrow involvement, extramedullary manifestations and lytic bone destruction [6].
In our study, we have used the MDCT to rule out the diagnosis of active myeloma and to try to definitively diagnose asymptomatic MM. In addition, we have compared MDCT with conventional MRI of the spine and pelvis to assess which of these imaging modalities would be more suitable for detecting the presence of focal bone lesions that were not recognized on CR.
2. Patients and Methods
We study 28 patients (12 males and 16 females; mean age 60.5 years, range 33 - 81) with Asymptomatic MM according to standardized criteria (IMWG criteria), who were referred to our Haematology Unit. All patients in our study had unequivocal MM based on marrow plasmacytosis and sufficiently high levels of myeloma protein that excluded patients with Monoclonal Gammopathy of Uncertain Significance (MGUS). The term “Asymptomatic MM” is used to describe a stage of disease in which there are no symptoms or organ damages related-MM; CR was the “only” method employed for radiological screening at diagnosis, as requested to current evidence by IMWG. All patients underwent a 64 MDCT (Brilliance 64, Philips Medical Systems, Germany) without contrast from the skull to the pelvis, at the endinspiration breath-old in the cranial-caudal direction. Technical parameters were as follows: 120 kV; 210 mAs; tube rotation time 0.5; pitch 0.923; reconstruction width 1.5 mm; all images were evaluated using three different window/level settings: one specific for bone (3000; 1000 HU), and, to evaluation collateral report, one for mediastinum/abdomen (350; 50 HU), one for pulmonary parenchyma (1600; −600 HU). MDCT evaluation also included the skull, thigh-bone and shoulders. All radiological examinations were performed at least within 2 - 3 months. Our experience was performed with the informed consent of patients.
Exclusion criteria were patients with MM who had additional malignancies. A small number of patients with MGUS (7 patients) and symptomatic MM (11 patients) represented the control group to verify sensitivity and specificity of MDCT for detecting the presence of focal bone lesions. Symptomatic MM was defined by appearance of lytic bone lesions on CR, occurrence of hypercalcaemia, renal disease or progressive anaemia.
Experienced radiologists were blinded with respect to the clinical features of the disease and value of hematologic data. Radiological results were related to conventional biological markers for burden tumor myeloma (b2-microglobulin [β2m] and erythrocyte sedimentation rate [ESR]), therefore, the validation was achieved by the combined evaluation both laboratory data and radiological features. Statistical calculations were performed with MedCalc 9.2.0.1 (MedCalc software Maria Kerke, Belgium). The distribution of times to progression was estimated using the Kaplan-Meyer method and compared with the log-rank test. Currently the follow-up is between 1 and 40 months starting from the first MDCT.
3. Results
“Control group”. Seven patients with MGUS and 11 patients with symptomatic MM represented the control group to verify sensitivity and specificity of MDCT for detecting the presence of focal bone lesions. The 7 patients with MGUS (Table 1) presented completely negative both radiographic techniques (MDCT, conventional MRI); therefore, the CR was concordant with the results of MDCT and conventional MRI in all cases (100%). MDCT was positive in all patients with symptomatic MM (11/11) (Table 1), demonstrating in 5 patients a more extensive involvement in comparison with conventional MRI; conventional MRI was positive in 8/11 (73%) patients with symptomatic MM; therefore, in 3 patients with symptomatic MM (27%), MDCT detected osteolytic bone lesions that were not recognized on conventional MRI.
“Study group”. Regarding to 28 asymptomatic MM patients (Table 1), MDCT revealed lytic bone lesions in 14 patients (50%) that were not recognized on CR and in 6 patients that were not recognized on conventional MRI. In 8 patients with MDCT and conventional MRI positive, MDCT demonstrated a more extensive involvement in comparison with conventional MRI. In 4 patients conventional MRI showed bone lesions that were not recognized on MDCT. MDCT and conventional MRI completely were negative in 10/28 patients (36%). Patients with radiological evidence of bone disease on MDCT were at high risk of disease progression with a median time to progression of five months (range 1 - 26 months) in comparison with patients without radiological evidence of bone disease who, conversely, showed a median time to progression of twenty months (range 8 - 40 months). The times to progression for each risk category are depicted in Figure 1 and the differences were statistically significant (P = 0.0001).
In our study the results of MDCT correlated with biological markers related to tumour burden and disease activity (Table 2). Higher serum levels of β2m and ESR were recognized in patients in whom the MDCT


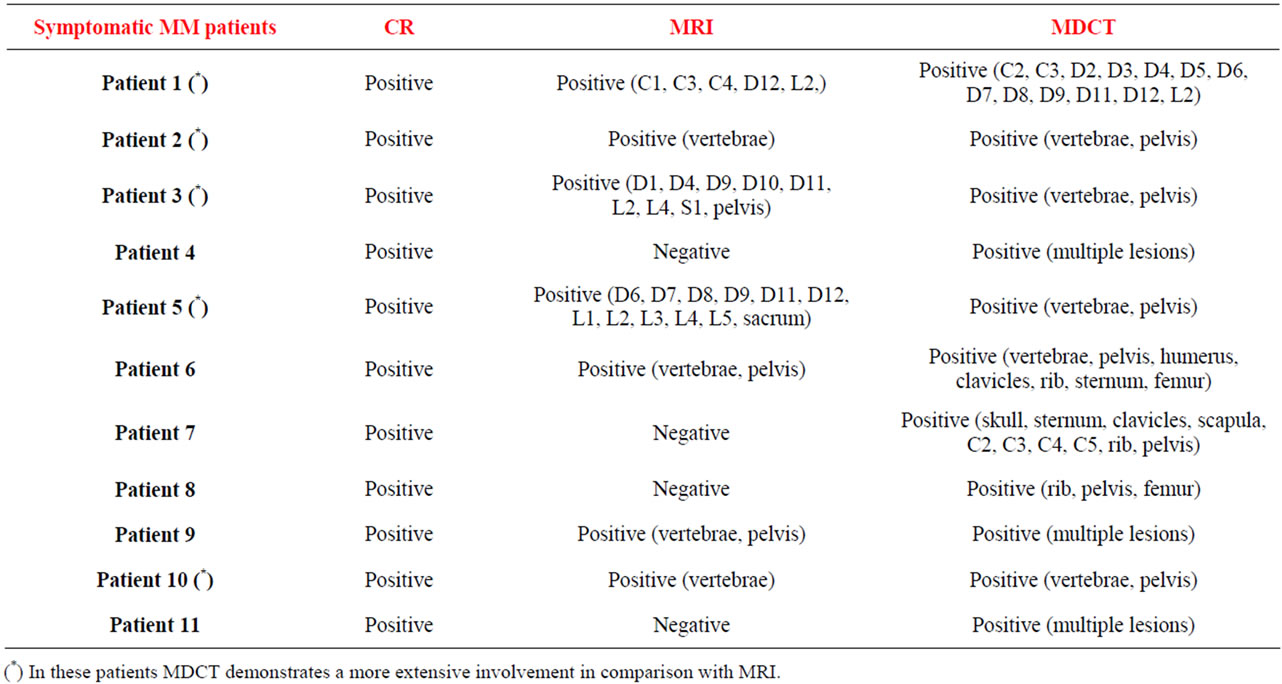
Table 1. Radiological results: conventional radiography, MDCT and conventional MRI.
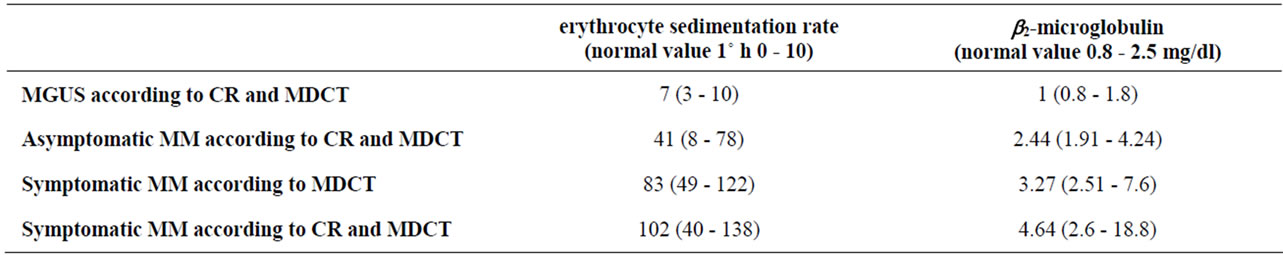
Table 2. Conventional biological markers related to disease activity.
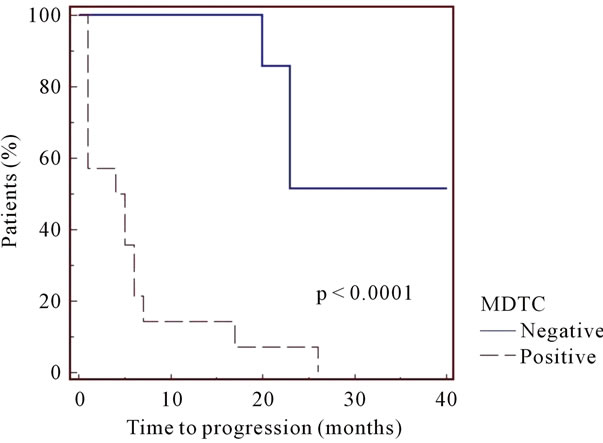
Figure 1. Patients (n. 14) with radiological evidence of bone disease on MDCT were at high risk of disease progression with a median time to progression of five months (range 1 - 26 months) in comparison with patients (n. 10) without radiological evidence of bone disease who, conversely, showed a median time to progression of twenty months (range 8 - 40 months).
demonstrated a more extensive and manifest involvement. Particularly, in MGUS patients, the median value of β2m and ESR was respectively 1 mg/dl (0.8 - 1.8) and 7 (3 - 10); in patients with Asymptomatic MM according to CR and MDCT, the median value of β2m and ESR was respectively 2.44 mg/dl (1.92 - 4.24) and 41 (8 - 78); in patients with Symptomatic MM according to MDCT, the median value of β2m and ESR was respectively 3.27 mg/dl (2.51 - 7.6) and 83 (49 - 122); in patients with Symptomatic MM according to CR and MDCT, the median value of β2m and ESR was respectively 4.64 mg/dl (2.8 - 9) and 102 (70 - 138).
In two patients, MDCT demonstrated unsuspected second non-haematologic malignancies involving the lung.
4. Discussion
New imaging techniques have been proposed both for MM staging and for monitoring response to chemotherapy; unfortunately, none of these modern technologies has been proven entirely reliable in this regard. In fact, basing on the currently available evidence compiled by the IMWG [1], CR remains the standard method to detect osteolytic bone lesions, although the false-negative rate is relatively high (30% - 70%) [5]; MRI or CT remain the diagnostic procedures of choice to assess suspected cord compression [1].
The individuation of bone lesions is mandatory both for the staging of monoclonal gammophaties and because it reflects pivotal prognostic implications. Patients who are clinically asymptomatic but have radiological evidence of bone disease are at high risk of progression with a median time to progression of 8 months [7]. Asymptomatic MM often presents with a multifocal cortical damage unfortunately not detectable by CR imaging because CR shows osteolytic bone lesions only when the cortical bone damage is more than 50% [8].
Conventional MRI is an appropriate tool for detecting bone marrow involvement and soft tissue disease but not skeletal destruction, which represents the major reason to treat patients with MM. MDCT is an appropriate tool for detect both early osteolysis (<5 mm) especially in the spine and associated soft tissue disease in a single examination. In addition, MDCT provides complementary information in comparison to that obtained with conventional MRI. In fact, a further significant advantage of MDCT, is its ability of demonstrating other unsuspected pathological processes—additional malignancies, especially those involving the lungs. In the context of a higher incidence of second malignancies associated with use of lenalidomide [9], the ability of detecting 2 unsuspected non-hematologic malignancies is very interesting. The mayor limitation of MDCT is the radiation dose delivered to patients.
On our initial experience, positive MDCT identified high-risk myeloma; patients with abnormal MDCT had significantly earlier disease progression (median 5 months) than patients with a normal pattern (median 20 months) (P = 0.0001) (Figure 1). Conversely, negative MDCT was a positive prognostic factor. In some selected cases, MDCT was superior to conventional MRI. The falsenegatives on MDCT might be due to early bone marrow replacement before any destruction of trabecular and cortical bone. The false-negatives on conventional MRI might be explained by the fact that very small lytic lesions can be visualized on MDCT before significant bone marrow disease.
In conclusion, MDCT is an excellent imaging instrument for early detection of skeletal destruction on high early disease. According to our opinion, 1) in patients without radiologically detectable osteolysis but with a reasonable suspicion of myeloma requiring therapy (elevated levels of paraprotein, extensive bone marrow plasmacytosis) we recommend the use of MDCT; 2) in the initial staging of MM, MDCT and conventional MRI are complementary imaging techniques; due to the direct visualization of the bone marrow, conventional MRI is appropriate for detecting early bone marrow involvements in absence of osteolysis, while MDCT is superior in detecting very small lytic lesions in absence of evident bone marrow disease, particularly in the long bones and in the difficult anatomic regions (skull, clavicle, ribs). Whole-body low-dose CT techniques (32 or 16-slice scanners) are being developed as very attractive and realistic alternative to conventional X-ray imaging [6]; radiation dose with a low-dose protocol is only slightly more than the radiation dose of CR [5]. Therefore, in the era of new imaging techniques that offer advanced diagnostic accuracy, CR represents an obsolete and out of use diagnostic procedure. Our experience can be criticized since our patients were retrieved from a single institution, and cohort is very small. A big number of patients that confirm the results of our study is necessary.
5. Contributions and Acknowledgements
Giuseppe Mele designed the study and drafted the article. Anna Grazia D’Agostino and Gianni Quarta contributed to the interpretation of data. Giacomo Loseto and Angela Melpignano were involved in the collection of clinical data. Maria Rosaria Coppi was involved in the collection of laboratory data.
The authors thank Giuseppe Lucarelli for technical assistance.
REFERENCES
- M. Dimopoulos, E. Terpos, R. L. Comenzo, et al., “International Myeloma Working Group Consensus Statement and Guidelines Regarding the Current Role of Imaging Techniques in the Diagnosis and Monitoring of Multiple Myeloma,” Leukemia, Vol. 23, 2009, pp. 1545-1556. doi:10.1038/leu.2009.89
- B. G. Durie, R. A. Kyle, A. Belch, et al., “Myeloma Management Guidelines: A Consensus Report from the Scientific Advisors of the International Myeloma Foundation,” Hematology Journal, Vol. 4, 2003, pp. 379-398. doi:10.1038/sj.thj.6200312
- D. M. Weber, M. Dimopoulos, L. A. Moulopoulos, et al., “Prognostic Features of Asymptomatic Multiple Myeloma,” British Journal of Hematology, Vol. 97, No. 4, 1996, pp. 810-814. doi:10.1046/j.1365-2141.1997.1122939.x
- F. E. Lecouvet, B. C. Vande Berg, J. Malghem and B. E. Maldague, “Magnetic Resonance and Computed Tomography Imaging in Multiple Myeloma,” Seminars in Musculoskeletal Radiology, Vol. 5, No. 1, 2001, pp. 43-55. doi:10.1055/s-2001-12920
- A. Baur-Melnyk, S. Buhmann, C. Becker, et al., “WholeBody MRI versus Whole-Body MDCT for Staging of Multiple Myeloma,” American Journal of Roentgenology, Vol. 190, No. 4, 2008, pp. 1097-1104. doi:10.2214/AJR.07.2635
- M. Horger, L. Kanz, B. Denecke, et al., “The Benefit of Using Whole-Body, Low-Dose, Nonenhanced, Multidetector Computed Tomography for Follow-Up and Therapy Response Monitoring in Patients Whit Multiple Myeloma,” Cancer, Vol. 109, No. 8, 2007, pp. 1617-1626. doi:10.1002/cncr.22572
- F. Wisloff, P. Andersen, T. R. Andersson, et al., “Incidence and Follow-Up of Asymptomatic Multiple Myeloma. The Myeloma Project of Health Region I in Norway,” European Journal of Haematology, Vol. 47, No. 5, 1991, pp. 338-341. doi:10.1111/j.1600-0609.1991.tb01857.x
- A. Baur-Melnyk and M. Reiser, “Staging of Multiple Myeloma with MRI: Comparison to MSCT and Conventional Radiography,” Radiologe, Vol. 44, No. 9, 2004, pp. 874-881.
- M. Dimopoulos, P. G. Richardson, N. Brandenburg, et al., “A Review of Second Primary Malignancy in Patients with Relapsed or Refractory Multiple Myeloma Treated with Lenalidomide,” Blood, Vol. 119, No. 12, 2012, pp. 2764-2767. doi:10.1182/blood-2011-08-373514
NOTES
*Corresponding author.

