Open Journal of Social Sciences
Vol.03 No.03(2015), Article ID:54792,8 pages
10.4236/jss.2015.33025
Cytotoxicity and Antimicrobial Property of the Leaf Extract of Euphorbia hirta (Tawa-Tawa)
Lorna T. Enerva1,2, Theresita V. Atienza1,3, Zenaida R. Glifonea1, Ofelia B. Villamor1, Normita A. Villa2,3
1College of Science, Polytechnic University of the Philippines, Manila, Philippines
2Taguig City University, Taguig, Philippines
3Graduate School, Polytechnic University of the Philippines, Manila, Philippines
Email: bettyenerva@yahoo.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 23 December 2014; accepted 15 March 2015; published 18 March 2015
ABSTRACT
Tawa-tawa is usually abundant throughout the Philippines in waste places and open grasslands. The plant is an annual hairy herb, usually much-branched from the base. These branches are simple or forked, ascending or spreading up to 40 centimeters long and often reddish or purplish. The leaves are opposite, distichous, oblong-elliptic to oblong-lanceolate, 1 to 2.5 centimeters long, toothed at the margin, and usually botched with purple in the middle. The plant deserves special attention because of its medicinal properties. Local tradition credits that this plant can help patients stricken with dengue fever. While it does not fight with the virus, it promotes the development of blood platelets and softens the effect of the viruses which attack the blood. However the department of health advises the public not to be much dependent on the said herbal medicine, despite its proven efficacy. This study was conducted to elucidate the structure, antimicrobial and cytotoxicity of the extract. Twenty grams of air-dried tawa-tawa were percolated in ethyl acetate of 200 ml for one week, rota-evaporated and purified by wet column chromatography. The phytochemical tests revealed the presence of alkaloids and tannins. The structure of the constituents was partially elucidated by ultraviolet spectra and gas chromatography-mass spectra analysis. The λmax was at 420 nm with an absorbance of 1.233 and GC-mass spectra characterized 100 compounds. The antimicrobial analysis gave positive inhibition of P. aeruginosa, S. aureus, C. albicans and T. mentagrophytes with activity index of 0.2, 0.3, 0.4 and 0.2 respectively. The cytotoxicity test showed that a concentration of 36.7185 grams in water of 350 ml of the leaf extract was toxic based on the t-test of the live and dead cells by in-vitro analysis on the lymphocytes from normal blood cells.
Keywords:
Tawa-Tawa, Antimicrobial, Cytotoxicity, Alkaloids, Tannins

1. Introduction
Tawa-tawa, gatas-gatas, magatas or botonis is abundant in open grasslands and waste places. The scientific name is Euphorbia hirta; it is classified as a weed and is a native of India. This is widely distributed in the Philippines, from sea level to an altitude of 500 meters. This weed also occurs in Borneo, Indonesia and New Guinea.
The botanical description is as follows. This weed can grow up to 60 cm long with a hairy stem which produces plentiful latex. The leaves are hairy and elliptical with slender dentate margin. The plant has many flowers about 5 to 8 centimeters found in axillary cymes at each leaf note. The fruit is a capsule with three valves and produces tiny, oblong, four-sided red seeds. It has a white or brown main root.
The chemical constituents of this plant are the following: gallic acid, quercetin and a phenoilc substance C28H18O15. These substances are responsible for its medicinal properties. Some of its medicinal values are as follows. In Indonesia, paste of psuedoball is put on sores or applied to stomach to expel worms and malignant tumors. The plant can be boiled and drank to expel worms. The plant can be eaten to cure dysentery.
In the Philippines there are many testimonies that tawa-tawa can cure dengue. There are testimonies that upon drinking the extract of this plant, the blood platelets of dengue patients are increased. The department of science and technology of the Philippine Council for Health and Development is conducting several studies on this plant to determine the curative component of tawa-tawa for dengue and tuberculosis [1] . The study is one of DOST’s researches with the purpose of producing drugs from herbal sources.
The department of health is discouraging dengue patients to dink this plant most especially for critical dengue patients and prescribes oral rehydration in this case. Dr. Jaime Tan Galvez [2] , the former health secretary, is in favor of promoting herbal medication. This study was undertaken to determine the cytotoxicity and antibacterial property of the plant.
2. Methodology
Tawa-tawa plant (Figure 1) were collected from Elvinda Subdivision, San Pedro Laguna. The plant samples were submitted to the Bureau of Plant Industry for authentication and identification. After identification twenty grams of the plant were washed, air dried, cut and placed in a closed container. Ethyl acetate was used in the percolation of the leaves for one week. The extract was concentrated using a rotary evaporator. The crude extract was purified by column chromatography and subjected to several tests.
3. Analysis of the Crude Extract
The chemical test done was test for alkaloids. The extract samples were placed in test tubes and the following reagents were used for the determination of the alkaloids. Alkaloid reagents are used to identify quantities of the natural bases of their derivatives and can be divided into precipitants and colored reagents. The precipitating agents combine with alkaloids to give highly colored insoluble complexes. The colored reagents are usually dehydrating or oxidizing reagents that give characteristic colored solution upon reaction with alkaloids. These two tests used are the Dragendorff’s and Wagner’s Tests.
Figure 1. Tawa-tawa plant [3] .
4. Dragendorff’s Test
Dragendorff’s reagent was prepared from 0.85 grams of bismuth nitrate in a mixture of 10 ml of acetic acid and 40 ml of distilled water. Solution B was prepared from 8 grams of potassium iodide in 20 ml of distilled water. The two mixtures were mixed and added to the crude extract. An orange color indicated the presence of alkaloid.
5. Wagner’s Test
Wagner’s reagent was made from 1.275 grams of ioddine and 3.6 grams of KI dissolved in enough distilled water to make 100 ml. This was added to the crude extract, and the formation of a red precipitate indicated the presence of an alkaloid.
6. Purification of the Crude Extract
In a column with silica gel 60 (0.63 - 0.200 mm), the crude extract was eluted using 9:1 proportion of dichloromethane and ethyl acetate. This procedure was repeated in a smaller column.
7. Instrumentation
Two instruments were used to elucidate the structures of the semi-purified extract. Hitachi UV-Vis spectrophotometer model and Clarus 500 Gas chromatography-mass spectrometer. The analyses were done at De La Salle University, Taft Avenue.
8. Antimicrobial Assay
The antimicrobial assay was used to determine the potential antimicrobial property of the tawa-tawa extract [4] .
Microbial suspension containing approximately 60 million cells per ml (McFarland No 2) were prepared from 24 hour old culture of the bacteria and yeasts and 5 - 7 days old culture of the mold. The suspending medium used was 0.1% peptone water.
One tenth (0.1) ml aliquots of the suspension of the bacteria and fungi were transferred into pre-poured Nutrient Agar (NA), Glucose Yeast Peptone (GYP) and Potato Dextrose Agar (PDA) respectively, about 5 ml of the corresponding medium, melted and cooled to about 45˚C, was poured into the plate. The plate was swirled to distribute the microbial cells evenly on the plate and the agar overlay was allowed to solidify. Alternatively, the incoula was streaked onto the surface with a sterile swab. Three equidistant points were born on the plates with a cork borer and 200 microliters of the sample was applied to each well. The size of each well was 10 mm.
The plates were incubated at room temperature. NA, GYP and PDA plates were incubated at room temperature for 24 - 48 hours for bacteria and yeast and 4 - 7 days for fungi, respectively. The clearing zones were measured in millimeters and the average diameter of the clearing zones were calculated. The Antimicrobail Index (AI) was computed using the following formula:
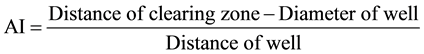
9. Cytotoxicity Test
The procedure for the cytotoxicity test used [5] .
10. Lymphocyte Culture
Blood from a healthy individual was collected in a green-top vacutainer tubes. Whole blood was carefully overlaid onto 4 ml of Histopaque in two centrifuge tubes and then centrifuged at 2000 rpm for 30 minutes at room temperature. The yellow top layer was transferred to fresh centrifuge tubes. Ten ml of serum-free media were added to each tube and the tubes were inverted several times to wash the lymphocytes. The tubes were centrifuged at 1000 rpm for 10 minutes at RT. The supernatant was removed and pellets were combined into one tube. Ten ml of serum-free media were again added. The tube was inverted several times to suspend and wash the pellet and then centrifuged at 1000 rpm for 10 min at RT. The supernatant was removed, leaving about 0.5 ml, and supplemented media was added such that the final cell density was 2 × 106 cells/ml. The pellet was resuspended by inverting the tube several times. The culture was incubated at 37˚C for 1 hr.
11. Cytotoxicity Assay
Fifty µl of supplemented media was dispensed into each of three microcentrifuge tubers. Tawa-tawa leaf extract was passed through a 0.2 µm filter and 50 µl of filtrate was dispensed into the six microcentrifuge tubes containing the supplemented media and tawa-tawa leaf extract. Cultures were mixed thoroughly and incubated at 37˚C for 24 hours.
12. Cell Count
After 24 hrs of incubation, treated cultures were obtained for cell counting. Eight µl of trypan blue, and 8 µl of mixed solution was placed in a hemocytometer. The number of live lymphocytes and the number of dea lymphocytes were counted in all 25 squares within the 1 mm center grid. Cell density (number of cells per ml) is computed using the formula:
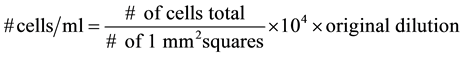
Original dilution = 2
13. Results and Discussion
Twenty grams of air dried tawa-tawa leaves were percolated using 200 ml of ethyl acetate as solvent. The crude extract was dark green liquid. The extract was tested for the presence of alkaloids. The extract was purified by column chromatography. The semipurified extracts were elucidated by UV-Vis spectrophotometer and gas chromatography-mass spectra analysis.
The crude extract was positive for alkaloids. An orange precipitate was obtained for the Dragendorf test [6] and a red precipitate for the Wagner test [7] . The mechanism of action is proposed to happen via coupling of the Dragendorf reagent heavy metal atom in the reagent with the nitrogen in the alkaloid to form ion pairs. The ion pairs form an insoluble solid. For Wagner’s test an aqueous solution of iodine and potassium iodide is used for microchemical analysis of alkaloids. The presence of a red precipitate is indicative of the presence of alkaloids.
A tannin compound is widely distributed in many species of plants, where they are used in protection from predators. This is an astringent bitter plant polyphenolic that binds to and form precipitate proteins and other compounds. Ferric chloride is one of the tests used for tannins and a green precipitate is formed with this reagent (Table 1).
One instrument used to elucidate the structure of the constituents of tawa-tawa was ultraviolet spectroscopy. Table 2 presents the results of the analysis. The λmax was at 420 nm with an absorbance of 1.40 (Figure 2). The absorption in the visible region indicates the presence of auxochromes and chromophores in the structure.
Table 1. Tests for alkaloids and tannins.
Table 2. UV-VIS spectra result.
The last instrument used to elucidate the structure of tawa-tawa is gas chromatography-mass spectra instrument (Figure 3). This instrument separated the constituents of the semi-purified extract and the mass spectra bombarded the constituents with electron to form charged ions. Gas chromatography identified the different retention time for the possible identification of the compounds present in the plant extract. Eight compounds were identified which are tabulated in Table 3.
Antimicrobial resistance is a growing problem in medicine, and many studies have tried to understand the molecular basis of such resistance in an attempt to render the bacteria susceptible once again. There is a need to continuously search for plants which exhibits antimicrobial property. One of this herbal plant is tawa-tawa which we found to have antimicrobial properties with two bacteria and fungi.
Table 4 presents the results of the antimicrobial properties of the tawa-tawa leaf. The first bacterium inhibited by the plant extract was P. aeruginosa (Figure 4) which has a low antibiotic susceptibility. This is a gram-negative aerobic rod which produces a green pigment pyocyanin. This bacterium is found in soil, water, plants and animals. The tawa-tawa leaf extract is a primary herbal medicine that can solve this problem of drug resistance. As bacterial antibiotic resistance continue to exhaust the supply of effective antibiotics, a global public health disaster appears likely.
Figure 2. UV-Vis spectra of tawa-tawa.
Figure 3. Gas chromatography-mass spectra of tawa-tawa plant.
Table 3. Gas chromatography-mass spectra analysis.
Table 4. Antimicrobial analysis of the plant extract.
Figure 4. Pseudomonas aeruginos [8] .
The extract also inhibited Staphyloccus aureus with an activit index of 0.2. S. aureus (Figure 5) is gram- positive, cluster-forming coccus, nonspore forming facultative anaerobe. This organism is colored yellow in agar plates and found in the nasal passages, anatomical locales, skin, oral cavity and gastrointestinal tract. This bacteria can lead to a disease of the skin that results in an abscess, boil and when ingested can cause food poisoning.
The extract inhibited the growth of C. albicans and T. mentagrophytes. Candida albicans (Figure 6) is a diploid fungus and a causal agent of opportunistic oral and genital infections in human. This is a budding yeast present in the mucous membranes of the mouth, intestinal tract, and vagina of healthy people.
There was also an inhibition to Trichopyton mentagrophytes (Figure 7) a zoophilic small-spored ectothrix species of fungi that causes infection of the hair, skin and nails. This is a cosmopolitan fungus dermatophyte that needs keratin for growth.
The last analysis performed was the cytotoxicity test (Figure 8). Cytotoxicity measurements are performed for screening for compounds with cytotoxic or cytostatic potential in clinical research. This method makes toxicity testing quicker, less expensive, and more directly relevant to human exposures. This method also reduced the use of animals in safety testing by replacing animal tests with in vitro tests based on cells, cellular components and tissues, preferably of human origin. This became important in the political, ethical and legal context
Figure 5. Staphyloccus aureus [9] .
Figure 6. Candida albicans [10] .
where the highest safety standards and the implementation of the so-called “3R Principle” of Replacement, Refinement and Reduction of experimental animals by alternatives were highest priorities [10] . Table 5 presents the cytotoxicity test.
The results of the study showed that the concentration of 36.7185 grams in 350 ml of water of the leaf extract was toxic based on the t-test. The computed t-value was 17.059 compared to the t = 2.776 at 0.05 level of significance. Plants have defense mechanisms against predators that can have adverse effects on humans. There is a need for proper double-blind clinical trials to determine the safety and efficacy of plant before they can be recommended for medical use. Although many consumers believe that herbal medicines are safe because they are “natural”, herbal medicines and synthetic drugs may interact, causing toxicity to the patient.
Figure 7. Trichopyton mentagrophytes [11] .

Figure 8. Cytotoxicity test of tawa-tawa. (a) Cells from untreated culture (treated with supplemented rpm). The dead cells (arrows) are blue and bloated while live cells are unpogmented; (b) Live and dead cells from culture treated with tawa-tawa extract. Dead cell is blue, live cells are not pigmented.
Table 5. Cytotoxicity test.
14. Conclusion
People still rely on traditional plant-based medicine as their primary form of health care. There is a need to perform cytotoxicity test as a preclinical phase to ensure the safety of the consumer. The tawa-tawa leaf extract gave positive results for the tests for alkaloids and tannins. The leaf extract gave the λmax at 420 nm in the visible region. The GC-mass spectra identified eight compounds. The extract inhibited the growth of P. aeruginosa, Staphyloccus aureus, Candida albicans and Trichopyton mentagrophytes with activity index of 0.2, 0.3, 0.4 and 0.2 respectively. The extract was toxic at 36.7185 grams in water of 350 ml based on the t-test. There is a need to use different concentrations of the leaf extract for a further cytotoxicity test.
Cite this paper
Lorna T.Enerva,Theresita V.Atienza,Zenaida R.Glifonea,Ofelia B.Villamor,Normita A.Villa, (2015) Cytotoxicity and Antimicrobial Property of the Leaf Extract of Euphorbia hirta (Tawa-Tawa)。 Open Journal of Social Sciences,03,162-170. doi: 10.4236/jss.2015.33025
References
- 1. Marchadesch, B. (2012) Local Tawa-Tawa Herbs May Cure Tuberculosis, Dengue. www.gmanetwork.com/news/story/254965/science/local-tawa-tawa-herbs-maycure-tuberculosisdengue
- 2. Tan, J.G. Philippines: Hotspot of Biodiversity? http://thephilippines.ph/tag/dr-jaime-galvez-tan/
- 3. http://en.wikiperia.org/wiki/Euphorbia-hirta#mediaviewer/File: Euphorbia-hirta-NP.JPG
- 4. University of the Philippines Natural Science Research Institute (2013) Procedures for Antimicrobial Assay. University of the Philippines Natural Science Research Institute, Quezon.
- 5. University of the Philippines Natural Science Research Institute (2013) Procedures for Cytotoxicity Assay. University of the Philippines Natural Science Research Institute, Quezon.
- 6. http://wiki.answers.com/Q/what-is-Dragendorff%E2%80%99s_reagent
- 7. www.scrid.com/doc/51100924/alkaloids-Table-Format#scribd
- 8. www.picsearchcom/pseudomonas.bacteria.picture.html
- 9. http://photobucket.com.images/staphylococcus+aureus/?page=
- 10. http://en.wikipedia.org/wiki/Main_Page
- 11. www.doctorfungus.org@2005










