 Vol.2, No.2, 85-90 (2010) Natural Science http://dx.doi.org/10.4236/ns.2010.22013 Copyright © 2010 SciRes. OPEN ACCESS Metal ion-binding properties of L-glutamic acid and L-aspartic acid, a comparative investigation S. A. A. Sajadi Sharif University of Technology, Institute of Water & Energy, Tehran, Iran; sajadi@sharif.ac.ir Received 14 November 2009; revised 9 December 2009; accepted 30 December 2009. ABSTRACT A comparative research has been developed for acidity and stability constants of M(Glu)1, M(Asp)2 and M(Ttr)3 complexes, which have been determined by potentiometric pH titration. Depending on metal ion-binding properties, vital differences in building complex were observed. The present study indicates that in M(Ttr) com- plexes, metal ions are arranged to the carboxyl groups, but in M(Glu) and M(Asp), some metal ions are able to build chelate over amine groups. The results mentioned-above demonstrate that for some M(Glu) and M(Asp) complexes, the stability constants are also largely determined by the affinity of metal ions for amine group. This leads to a kind of selectivity of metal ions, and transfers them through building complexes accompanied with glutamate and aspartate. For heavy metal ions, this building complex helps the absorption and filtration of the blood plasma, and consequently, the excursion of heavy metal ions takes place. This is an important method in micro-dialysis. In this study the different as- pects of stabilization of metal ion complexes regarding to Irving-Williams sequence have been investigated. Keywords: Glutamic Acid; Aspartic Acid; Tartaric Acid; Divalent Metal Ions; Potentiometric Titration; Acidity and Stability Constants. 1. INTRODUCTION It is known that metal ions are important for numerous biochemical reactions. For example, enzymes work only in the presence of such metal ions. The metal ion com- plexes of many amino acids have been investigated [1-6]. Functionalized membranes represent a field with multi- ple applications. Examination of specific metal-macro- molecule interactions on these surfaces indicates an ex- cellent method for characterization of these materials. Ion exchange, chelation, and electrostatic interactions form the basis of metal sorption. The behavior of various materials functionalized with polypeptides and other molecules is a topic of interest because of its applica- tions in affinity separations, biosensors, and other appli- cations including site-specific interactions [7]. An exam- ple of the latter involves the removal of heavy metals from aqueous solutions [8-11]. These sorbents are made of a variety of materials containing different functional groups. The advantage of affinity separations is that they may be tailored for the desired selectivity and capacity. The functionalization of materials is of vital importance for the production of new materials with specific proper- ties. The characterization of these new materials is also critical. Previous investigations showed that for the “harder” ligands, the ionic term dominates and their binding energies are affected by changes in covalency over the series. It is the competition between these be- haviors that produces the Irving-Williams series in sta- bility constants [12]. L-glutamate and L-aspartate are key molecules in cellular metabolism (Figure 1). In hu- mans, dietary proteins are broken down by digestion into amino acids, which serve as metabolic fuel for other functional roles in the body. Based on above-mentioned, the essential role of Glu is interesting to study the interaction between other metal ions with Glu and related compounds. 2. EXPERIMENTAL 2.1. Materials The L-glutamic acid and L-aspartic acid (extra pure) was purchased from Merck, Darmstadt, Germany. The nitrate salt of Na+, Ca2+, Mg2+, Mn2+, Co2+, Cu2+, and Zn2+ (all pro analysis) were from Merck. All the starting materials were of reagent grade and used without further purifica- tion. Potassium hydrogen phthalate and standard solu- tions of sodium hydroxide (titrasol), nitric acid, EDTA and of the buffer solutions of pH 4.0, 7.0 and 9.0 were all from Merck. All solutions were prepared with deion- ized water. Water was purified by Milil-Q water purifi- cation system, de-ionized and distillated. 1L-Glutamic acid; 2L-As artic acid; 3L-Tartaric acid  S. A. A. Sajadi / Natural Science 2 (2010) 85-90 Copyright © 2010 SciRes. OPEN ACCESS 86 (b) (a) (d) (c) Figure 1. Chemical structures of (a, b) L-glutamic acid; (c) L-aspartic acid and; (d) tartaric acid. 2.2. Ph Titrations Reagents Carbonate-free sodium hydroxide 0.03 M was pre- pared and standardized against sodium hydrogen phtha- late and a standard solution of nitric acid 0.5 mM. M(II) nitrate solution (0.03 M) was prepared by dissolving the above substance in water and was standardized with standard solution of EDTA 0.1 M (triplex). 2.3. Apparatus All pH titrations were performed using a Metrohm 794 basic automatic titrator (Titrino), coupled with a thermo- stating bath Hero at 25C (±0.1C) and a Metrohm com- bined glass electrode (Ag/AgCl). The pH meter was calibrated with Merck standard buffer solutions (4.0, 7.0 and 9.0). 2.4. Procedure For the determination of acid dissociation constants of the ligand L, an aqueous solution (0.03 mM) of the pro- tonated ligand was titrated with 0.03 M NaOH at 25C under nitrogen atmosphere and ionic strength of 0.1 M, NaNO3. For the determination of binary (a ligand and Cu2+) system, the ratios used were 1:1, Cu(II) : Ligand and 1:1, Cu(II) : L, 0.3 mM. This solution was titrated with 0.03 M NaOH under the same conditions men- tioned above. Each titration was repeated seven times in order to check the reproducibility of the data. 2.5. Calculation The acid dissociation constants, 2() H L Kand () H L K for H2(L) were calculated by an algebraic method. The equilibria involved in the formation of 1:1 complex of L and a divalent metal ion may be expressed as Eqs.(7- 10). 3. RESULTS AND DISCUSSION The potentiometric pH-titrations (25C, 0.1 M, NaNO3) were carried out to obtain the acidity and stability con- stants which are summarized in Tables 1 and 2. Acidity constants L-glutamate O2CCH2CH2CH(NH2)CO2 and L- aspartate ions (L2-), O2CCH2CH(NH2)CO2, are two-basic species, and thus they can accept two protons, given H2(L), for which the following de-protonation equilibriums are hold: H2(L) H++H(L) (1) 2() H L K= [H(L)-][H+]/[H 2(L)] (2) H(L)H++L2 (3) () H L K= [L2][H+]/[H(L)] (4) The two proton in H2(L) are certainly bound at the terminal acetate and amino groups, i.e., it is released from HO2CCH2CH2CH(NH3 +)CO2 – H 2Glu according to Equilibriums (1) and (2). It is known as zwitter-ion, and is also closed to the de-protonation of acetate groups which occurs at the terminal acetate groups of tartaric acid [6,13]. L2 can release one more proton from the terminal acetate group. Hence, here due addition to Equilibrium (3) should be considered, which takes place above a pH ≈ 2 (see Figure 2). H3(L)+H++H2(L) (5) 3() H L K=[H2(L)][H+]/[H3(L)+] (6) Here, the aforementioned reaction is not considered further. 3.1. Stability of Binary and Ternary Complexes If we abbreviate for simplicity associating with Ca2+, Mg2+, Mn2+, Co2+, Cu2+, and Zn2+ with M2+, then one may write the following two Equilibriums (4) and (5): M2++H(L)M(H;L)+ (7) (;) M HL K= [M(H; L)+]/[M2+][ H(L) ] (8) M2++(L)2M(L) (9) () M L K= [M(L)]/[M2+][L2] (10) 3.2. Potentiometric Analyses These results are summarized in Tables 1 and 2. The data 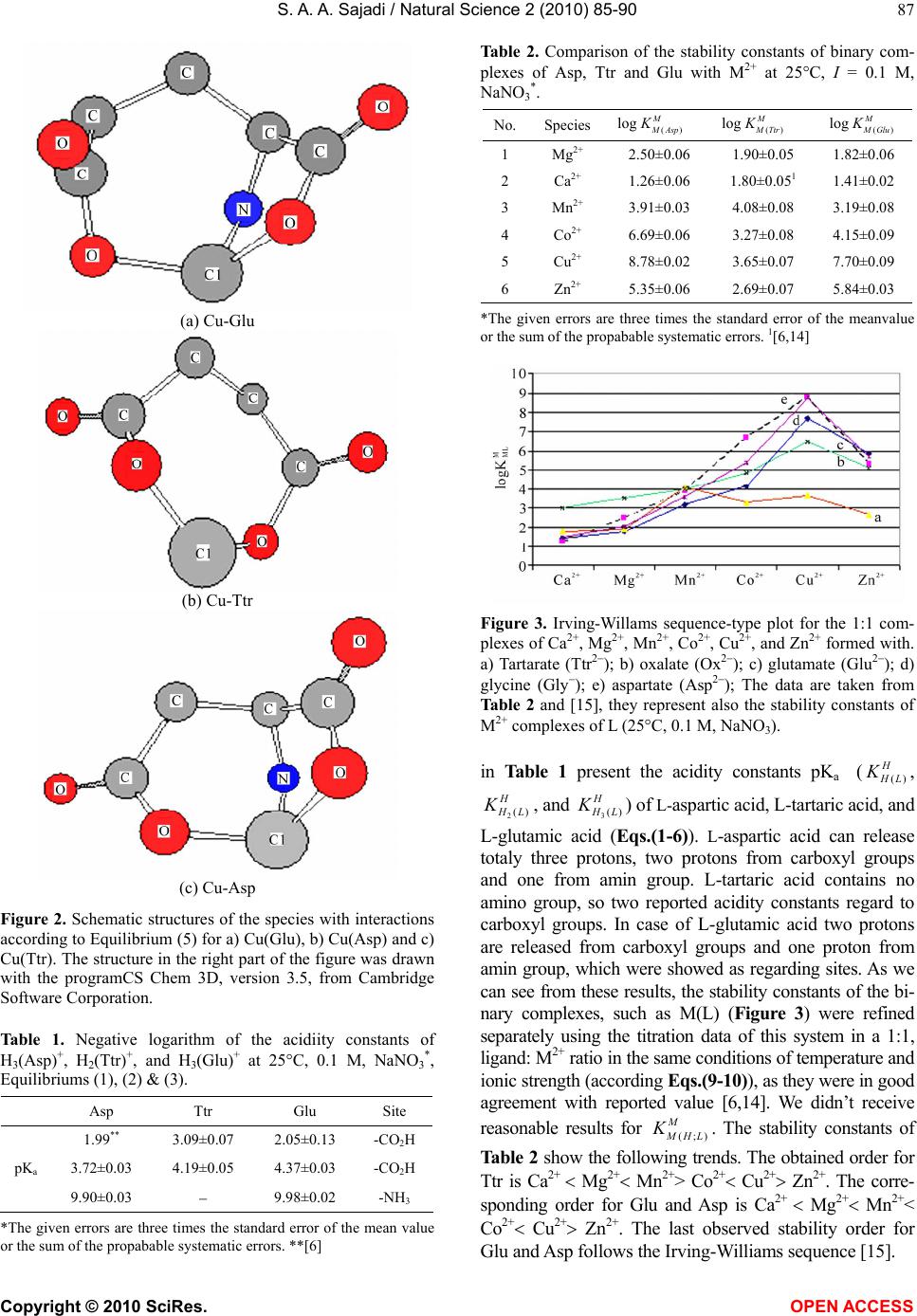 S. A. A. Sajadi / Natural Science 2 (2010) 85-90 Copyright © 2010 SciRes. OPEN ACCESS 87 (a) Cu-Glu (b) Cu-Ttr (c) Cu-Asp Figure 2. Schematic structures of the species with interactions according to Equilibrium (5) for a) Cu(Glu), b) Cu(Asp) and c) Cu(Ttr). The structure in the right part of the figure was drawn with the programCS Chem 3D, version 3.5, from Cambridge Software Corporation. Table 1. Negative logarithm of the acidiity constants of H3(Asp)+, H2(Ttr)+, and H3(Glu)+ at 25C, 0.1 M, NaNO3 *, Equilibriums (1), (2) & (3). Asp Ttr Glu Site 1.99** 3.09±0.07 2.05±0.13 -CO2H pKa 3.72±0.03 4.19±0.05 4.37±0.03 -CO2H 9.90±0.03 9.98±0.02 -NH3 *The given errors are three times the standard error of the mean value or the sum of the propabable systematic errors. **[6] Table 2. Comparison of the stability constants of binary com- plexes of Asp, Ttr and Glu with M2+ at 25C, I = 0.1 M, NaNO3 *. No.Species log () M Asp K log() M Ttr K log() M Glu K 1 Mg2+ 2.50±0.06 1.90±0.05 1.82±0.06 2 Ca2+ 1.26±0.06 1.80±0.051 1.41±0.02 3 Mn2+ 3.91±0.03 4.08±0.08 3.19±0.08 4 Co2+ 6.69±0.06 3.27±0.08 4.15±0.09 5 Cu2+ 8.78±0.02 3.65±0.07 7.70±0.09 6 Zn2+ 5.35±0.06 2.69±0.07 5.84±0.03 *The given errors are three times the standard error of the meanvalue or the sum of the propabable systematic errors. 1[6,14] Figure 3. Irving-Willams sequence-type plot for the 1:1 com- plexes of Ca2+, Mg2+, Mn2+, Co2+, Cu2+, and Zn2+ formed with. a) Tartarate (Ttr2); b) oxalate (Ox2); c) glutamate (Glu2); d) glycine (Gly); e) aspartate (Asp2); The data are taken from Table 2 and [15], they represent also the stability constants of M2+ complexes of L (25C, 0.1 M, NaNO3). in Table 1 present the acidity constants pKa (() H L K, 2() H L K, and 3() H L K) of L-aspartic acid, L-tartaric acid, and L-glutamic acid (Eqs.(1-6)). L-aspartic acid can release totaly three protons, two protons from carboxyl groups and one from amin group. L-tartaric acid contains no amino group, so two reported acidity constants regard to carboxyl groups. In case of L-glutamic acid two protons are released from carboxyl groups and one proton from amin group, which were showed as regarding sites. As we can see from these results, the stability constants of the bi- nary complexes, such as M(L) (Figure 3) were refined separately using the titration data of this system in a 1:1, ligand: M2+ ratio in the same conditions of temperature and ionic strength (according Eqs.(9-10)), as they were in good agreement with reported value [6,14]. We didn’t receive reasonable results for (;) M HL K. The stability constants of Table 2 show the following trends. The obtained order for Ttr is Ca2+ Mg2+ Mn2+> Co2+ Cu2+ Zn2+. The corre- sponding order for Glu and Asp is Ca2+ Mg2+ Mn2+< Co2+ Cu2+ Zn2+. The last observed stability order for Glu and Asp follows the Irving-Williams sequence [15]. 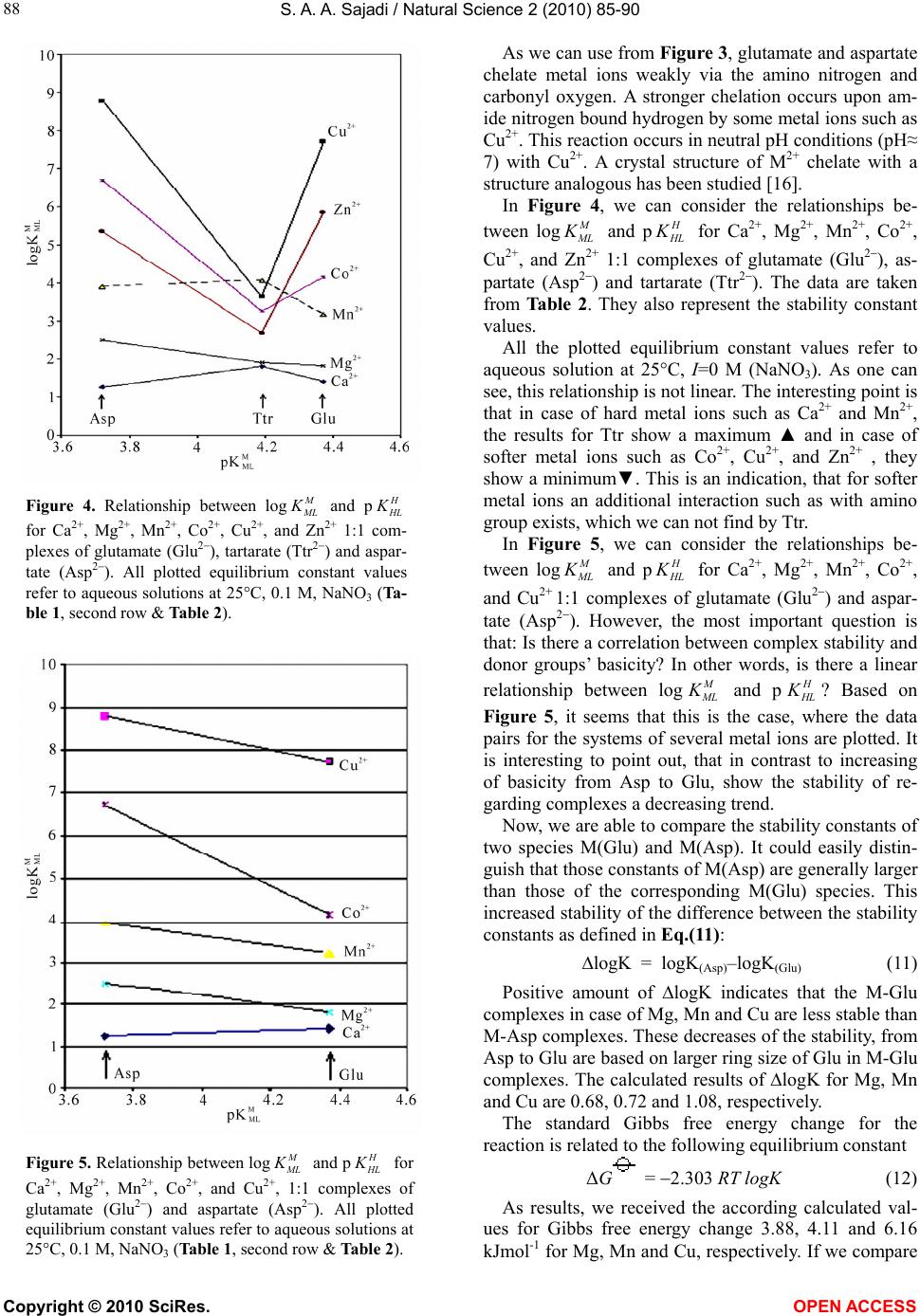 S. A. A. Sajadi / Natural Science 2 (2010) 85-90 Copyright © 2010 SciRes. OPEN ACCESS 88 Figure 4. Relationship between log L and p L for Ca2+, Mg2+, Mn2+, Co2+, Cu2+, and Zn2+ 1:1 com- plexes of glutamate (Glu2), tartarate (Ttr2) and aspar- tate (Asp2). All plotted equilibrium constant values refer to aqueous solutions at 25C, 0.1 M, NaNO3 (Ta- ble 1, second row & Table 2). Figure 5. Relationship between log L and p L for Ca2+, Mg2+, Mn2+, Co2+, and Cu2+, 1:1 complexes of glutamate (Glu2) and aspartate (Asp2). All plotted equilibrium constant values refer to aqueous solutions at 25C, 0.1 M, NaNO3 (Table 1, second row & Table 2). As we can use from Figure 3, glutamate and aspartate chelate metal ions weakly via the amino nitrogen and carbonyl oxygen. A stronger chelation occurs upon am- ide nitrogen bound hydrogen by some metal ions such as Cu2+. This reaction occurs in neutral pH conditions (pH≈ 7) with Cu2+. A crystal structure of M2+ chelate with a structure analogous has been studied [16]. In Figure 4, we can consider the relationships be- tween log L and p L for Ca2+, Mg2+, Mn2+, Co2+, Cu2+, and Zn2+ 1:1 complexes of glutamate (Glu2), as- partate (Asp2) and tartarate (Ttr2). The data are taken from Table 2. They also represent the stability constant values. All the plotted equilibrium constant values refer to aqueous solution at 25°C, I=0 M (NaNO3). As one can see, this relationship is not linear. The interesting point is that in case of hard metal ions such as Ca2+ and Mn2+, the results for Ttr show a maximum ▲ and in case of softer metal ions such as Co2+, Cu2+, and Zn2+ , they show a minimum▼. This is an indication, that for softer metal ions an additional interaction such as with amino group exists, which we can not find by Ttr. In Figure 5, we can consider the relationships be- tween log L and p L for Ca2+, Mg2+, Mn2+, Co2+, and Cu2+ 1:1 complexes of glutamate (Glu2) and aspar- tate (Asp2). However, the most important question is that: Is there a correlation between complex stability and donor groups’ basicity? In other words, is there a linear relationship between log L and p L ? Based on Figure 5, it seems that this is the case, where the data pairs for the systems of several metal ions are plotted. It is interesting to point out, that in contrast to increasing of basicity from Asp to Glu, show the stability of re- garding complexes a decreasing trend. Now, we are able to compare the stability constants of two species M(Glu) and M(Asp). It could easily distin- guish that those constants of M(Asp) are generally larger than those of the corresponding M(Glu) species. This increased stability of the difference between the stability constants as defined in Eq.(11): ∆logK = logK(Asp)–logK(Glu) (11) Positive amount of ∆logK indicates that the M-Glu complexes in case of Mg, Mn and Cu are less stable than M-Asp complexes. These decreases of the stability, from Asp to Glu are based on larger ring size of Glu in M-Glu complexes. The calculated results of ∆logK for Mg, Mn and Cu are 0.68, 0.72 and 1.08, respectively. The standard Gibbs free energy change for the reaction is related to the following equilibrium constant ΔG = 2.303 RT logK (12) As results, we received the according calculated val- ues for Gibbs free energy change 3.88, 4.11 and 6.16 kJmol-1 for Mg, Mn and Cu, respectively. If we compare 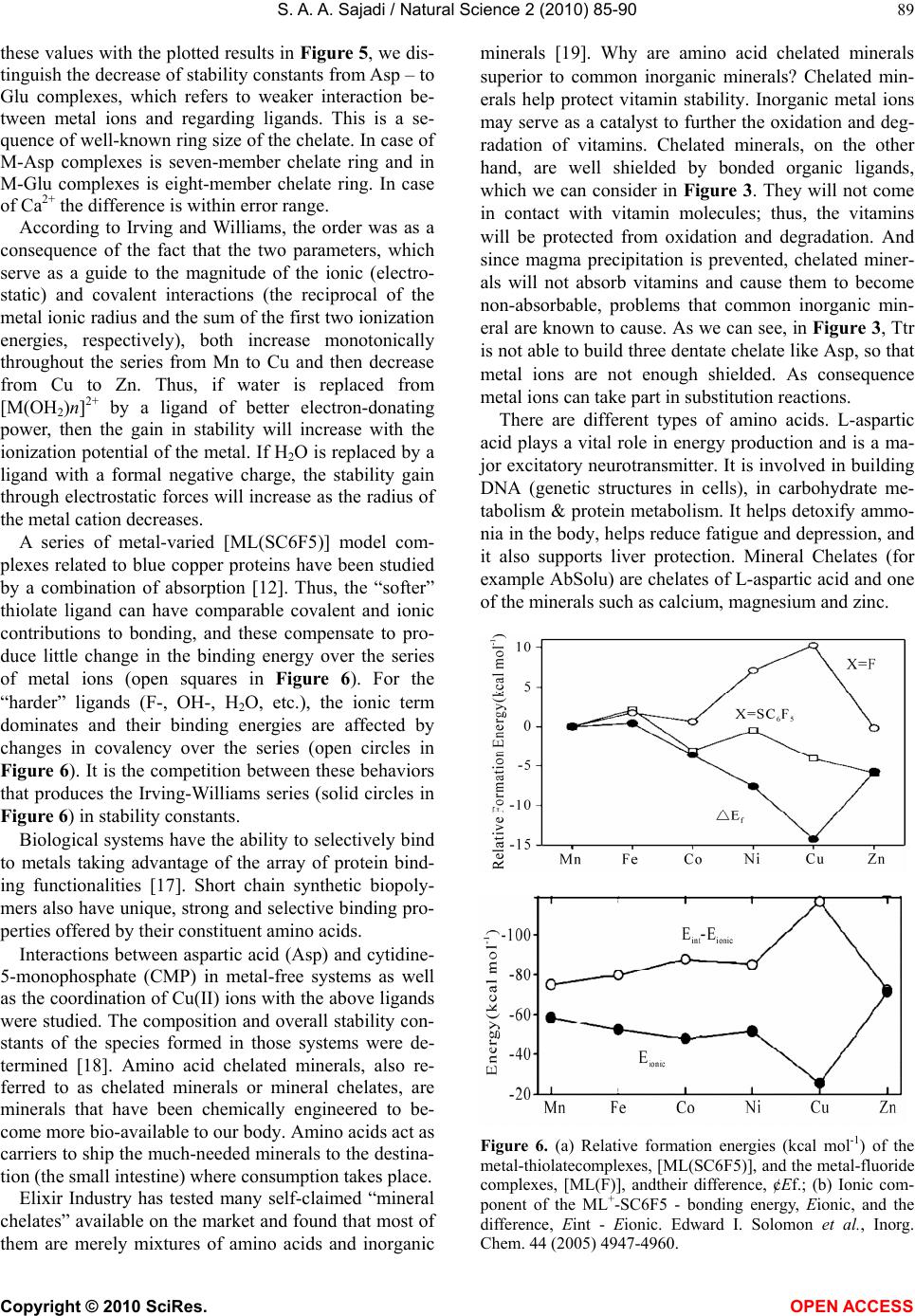 S. A. A. Sajadi / Natural Science 2 (2010) 85-90 Copyright © 2010 SciRes. OPEN ACCESS 89 these values with the plotted results in Figure 5, we dis- tinguish the decrease of stability constants from Asp – to Glu complexes, which refers to weaker interaction be- tween metal ions and regarding ligands. This is a se- quence of well-known ring size of the chelate. In case of M-Asp complexes is seven-member chelate ring and in M-Glu complexes is eight-member chelate ring. In case of Ca2+ the difference is within error range. According to Irving and Williams, the order was as a consequence of the fact that the two parameters, which serve as a guide to the magnitude of the ionic (electro- static) and covalent interactions (the reciprocal of the metal ionic radius and the sum of the first two ionization energies, respectively), both increase monotonically throughout the series from Mn to Cu and then decrease from Cu to Zn. Thus, if water is replaced from [M(OH2)n]2+ by a ligand of better electron-donating power, then the gain in stability will increase with the ionization potential of the metal. If H2O is replaced by a ligand with a formal negative charge, the stability gain through electrostatic forces will increase as the radius of the metal cation decreases. A series of metal-varied [ML(SC6F5)] model com- plexes related to blue copper proteins have been studied by a combination of absorption [12]. Thus, the “softer” thiolate ligand can have comparable covalent and ionic contributions to bonding, and these compensate to pro- duce little change in the binding energy over the series of metal ions (open squares in Figure 6). For the “harder” ligands (F-, OH-, H2O, etc.), the ionic term dominates and their binding energies are affected by changes in covalency over the series (open circles in Figure 6). It is the competition between these behaviors that produces the Irving-Williams series (solid circles in Figure 6) in stability constants. Biological systems have the ability to selectively bind to metals taking advantage of the array of protein bind- ing functionalities [17]. Short chain synthetic biopoly- mers also have unique, strong and selective binding pro- perties offered by their constituent amino acids. Interactions between aspartic acid (Asp) and cytidine- 5-monophosphate (CMP) in metal-free systems as well as the coordination of Cu(II) ions with the above ligands were studied. The composition and overall stability con- stants of the species formed in those systems were de- termined [18]. Amino acid chelated minerals, also re- ferred to as chelated minerals or mineral chelates, are minerals that have been chemically engineered to be- come more bio-available to our body. Amino acids act as carriers to ship the much-needed minerals to the destina- tion (the small intestine) where consumption takes place. Elixir Industry has tested many self-claimed “mineral chelates” available on the market and found that most of them are merely mixtures of amino acids and inorganic minerals [19]. Why are amino acid chelated minerals superior to common inorganic minerals? Chelated min- erals help protect vitamin stability. Inorganic metal ions may serve as a catalyst to further the oxidation and deg- radation of vitamins. Chelated minerals, on the other hand, are well shielded by bonded organic ligands, which we can consider in Figure 3. They will not come in contact with vitamin molecules; thus, the vitamins will be protected from oxidation and degradation. And since magma precipitation is prevented, chelated miner- als will not absorb vitamins and cause them to become non-absorbable, problems that common inorganic min- eral are known to cause. As we can see, in Figure 3, Ttr is not able to build three dentate chelate like Asp, so that metal ions are not enough shielded. As consequence metal ions can take part in substitution reactions. There are different types of amino acids. L-aspartic acid plays a vital role in energy production and is a ma- jor excitatory neurotransmitter. It is involved in building DNA (genetic structures in cells), in carbohydrate me- tabolism & protein metabolism. It helps detoxify ammo- nia in the body, helps reduce fatigue and depression, and it also supports liver protection. Mineral Chelates (for example AbSolu) are chelates of L-aspartic acid and one of the minerals such as calcium, magnesium and zinc. Figure 6. (a) Relative formation energies (kcal mol-1) of the metal-thiolatecomplexes, [ML(SC6F5)], and the metal-fluoride complexes, [ML(F)], andtheir difference, ¢Ef.; (b) Ionic com- ponent of the ML+-SC6F5 - bonding energy, Eionic, and the difference, Eint - Eionic. Edward I. Solomon et al., Inorg. Chem. 44 (2005) 4947-4960.  S. A. A. Sajadi / Natural Science 2 (2010) 85-90 Copyright © 2010 SciRes. OPEN ACCESS 90 There are different types of amino acids. Nutrition scientists selected L-aspartic acid based on many addi- tional benefits that come with it. Most of the competi- tors’ products contain two or more crystalline water in their molecules. Theoretically those products should not be referred to as “chelates” and the bonding (if indeed exists) of amino acid molecules to mineral ions is vul- nerable. These products are anhydrous chelates of two L- aspartic acid molecules and a single metal ion. Based on the results of this work, we can draw the conclusion, that hard metal ions just with identical stability constants- could have similar interaction with glutamate. Even based on these, results of acidity constants reported (Ta- ble 2) glutamamic acid occurs in high organism in form of glutamate. Earlier works have reported the structure of glutamate complexes with some metal ions [20] such as Co2+, Cu2+ and Zn2+. These metal ions are able to have additional interactions with glutamate. This leads to building of macro-chelate (Figure 3). 4. CONCLUSIONS It is shown, that Glu has identical complex properties as Asp i.e. the both amino acids have similar structure. The only difference is also the size of amino acids. The pre- vious studies have shown, that Glu has special applica- tions [21]. The glutamate industry have found that manufactured free glutamic acid, in the form of monosodium gluta- mate (MSG), hydrolyzed vegetable proteins, etc., etc., when added to the processed foods, masks off flavors and makes the blandest and cheapest foods taste won- derful. An other interesting point is the pharma- cologi- cal application of new generation of glutamate and as- partate complexes and it seems essential to understand their reaction mechanisms. REFERENCES [1] IUPAC-IUBMB Joint Commission on Biochemical No- menclature. Nomenclature and Symbolism for Amino Acids and Peptides. Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Retrieved on 2007–05–17. [2] Nelson, D.L., Cox, M.M., “Lehninger, principles of bio- chemistry” 3rd Edition, Worth Publishing: New York, 2000. ISBN 1–57259–153–6. [3] Stryer, L. (1995) Biochemistry, 4th Edition, W.H. Free- man and Company New York. [4] Chen, P.E., Geballe, M.T., Stansfeld, P.J., Johnston, A.R., Yuan, H., Jacob, A.L., Snyder, J.P., Traynelis, S.F. and Wyllie, D.J.A. (2005) Structural features of the glutamate binding site in recombinant NR1/NR2A N-Methyl-D- Lartate receptors determined by site-directed mutagene- sis and molecular modeling. Molecular Pharmacology, 67, 1470-1484. [5] Dunn, M.S. and Smart, B.W. (1963) “DL-Lartic, Acid” Organic Syntheses, 4, 55. [6] Martel, A.E. (2006) Critical stability constants of metal complexes. 26. [7] Xiao, S., Textor, M., Spencer, N.D., Sigrist, H. and Langmuir, (1998) 14, 5507. [8] Konishi, Y., Shimaoka, J. and Asai, S. (1998) React. Func. Polym., 36, 197. [9] Reichert, J. and Binner, J.G.P.J. (1996) Mater. Sci., 31, 231. [10] Bonn, G., Reiffenstuhl, S., Jandik, P. and Chromatogr, J. (1990) 499, 669. [11] Bhattacharyya, D., Hestekin, J.A., Brushaber, P., Cullen, L., Bachas, L.G. and Sikdar, S.K. (1998) Membr. J. Sci. 141, 121. [12] Solomon, E.I. et al., Inorg. Chem., 44(2005), 4947-4960. [13] Sajadi, S.A.A. Alamolhoda, A.A. and Nazari Alavi, A. (2009) Scientica Iranica, in press. [14] IUPAC Stability Conatants Database, Release 3, version 3.02, coplied by L.D. Pettit, H.K. J. Powel, Academic Software Timble, UK, (1998). [15] Irving, H. and Williams, R.J.P.J. (1953) Chem. Soc., 3192-3210. [16] Tsukihara, T. Katsube, Y. Bull. (1972) Chem. Soc. Jpn., 45, 1367. [17] Guo, M.H., Zou, Wang, H. Kong, L. and Anal, J.N. (2001) Chim. Acta, 443, 91. [18] Romualda Bregier-Jarzebowska, Anna Gasowska, and Lechosław Lomozik, Bioinorganic Chemistry and Ap- plications, 2008, Article ID 253971. [19] The technology of achieving this structure is patented and was jointly developed by Elixir Industry, Las Vegas, NV 89104-7900, USA, and the Chinese National Insti- tute of Pharmaceutical Industry. [20] Miranda, J.L. and Felcman, J. (2003) Polyhedron, 22, 225-233. [21] Reeds, P.J. et al., (2000) J. Nutrition, 130(45), 978S- 982S.
|