Paper Menu >>
Journal Menu >>
 Graft copolymerization of N,N- Dimethylacrylamide to cellulose in homogeneous media using atom transfer radical polymerization for hemocompatibility Graft copolymerization of N,N- Dimethylacrylamide to cellulose in homogeneous media using atom transfer radical polymerization for hemocompatibility * Li-Feng Yan, Wei Tao Hefei National Laboratory for Physical Science at Microscale, and Department of Chemical Physics, University of Science and Technology of China, Hefei, 230026, P.R.China. * Correspondence should be addressed to Li-Feng Yan (lfyan@ustc.edu.cn). of the cellulose backbone during the formation of ABSTRACT free radical grafting sites, and the presence of a con- siderable amount of ungrafted cellulose in the prod- In homogeneous media,N,N-Dimethylacrylamideuct. In addition, these techniques usually results in (DMA) wasgrafted copolymerization to cellulose the graft copolymer with poor control over the com- by a metal-catalyzed atom transfer radical poly-position, such as molecular weight and the merization (ATRP) process. First, cellulose waspolydispersity of the grafted chains [3]. Recently, dissolved in DMAc/LiCl system, and it reacted controlled/“living”radical polymerization methods with 2-bromoisobutyloyl bromide (BiBBr) to pro-have been developed [4], which is able to minimize duce macroinitiator (cell-BiB). Then DMA was chain transfer and to control the molecular weight polymerized to the cellulose backbone in a and polydispersity.Among them atom transfer radi- homogeneous DMSO solution in presence of cal polymerization (ATRP) and reversible addition the cell-BiB. Characterization with FT-IR, NMR,fragmentation transfer polymerization (RAFT) are and GPC measurements showed that there the two convenient methods to prepare well-defined obtained a graft copolymer with cellulose back-polymers. Using living free radical polymerization bone and PDMA side chains (cell-PDMA) in well-methods to prepare cellulose graft copolymer is an defined structure. The proteins adsorption stud-attractive topic and some investigations had been car- ies showed that the cellulose membranes modi-ried out. Perrier,et al. reported a preparation of poly- fied by the as-prepared cell-PDMA copolymer styrene graft cellulose by a RAFT process [5]. own good protein adsorption resistancet. Carlmark and Malmstromsynthesized a poly(2- hydroxyethyl methacrylate) graft cellulose using an ATRP process [6]. However, in both the studies, the graft copolymerization occurs only on the surface of cellulose fiber due to the heterogeneous process. Huang, et al. reported a homogeneous ATRP process 1. INTRODUCTIONto prepare cellulose graft copolymers with different Cellulose is the most fluent feedstock in the worldmonomers; the reason why ethyl cellulose was that could be used to prepare new kinds of materials, selected as the feedstock is its easily dissolving abil- and cellulose derivatives have potential applicationity in many solvents[8, 9, 25, 26, 27]. By now there as functional polymers. Graft copolymers are the are still less reports to synthesize cellulose graft important topic for their novel properties. Today,copolymer through a living radical polymerization “grafting from” method has been widely used to pre-directly from cellulose in its homogeneous solution, pare cellulose copolymers. Ceric ion initiation,and it is important to prepare well-defined structures Fenton's reagent and-radiation are the widely used of the graft copolymer. methods to graft monomers to cellulose [1,2]. How-Poly(N,N-dimethylacrylamide) (PDMA) is well- ever, there are some drawbacks of these methods,known for its remarkable water solubility and such as the production of unwanted homopolymer biocompatibility [10]. Recently, well-defined PDMA together with the graft copolymer, chain degradation has been prepared by both RAFT [11] and ATRP pro- Keywords:Cellulose; Atom transfer radical polymerization (ATRP); Homogeneous; Graft copolymerization; Hemocompatibility J. Biomedical Science and Engineering, 2008, 1, 37-43Scientific Research Publishing JBiSE Published Online May 2008 in SciRes.http://www.srpublishing.org/journal/jbise SciRes Copyright ©2008 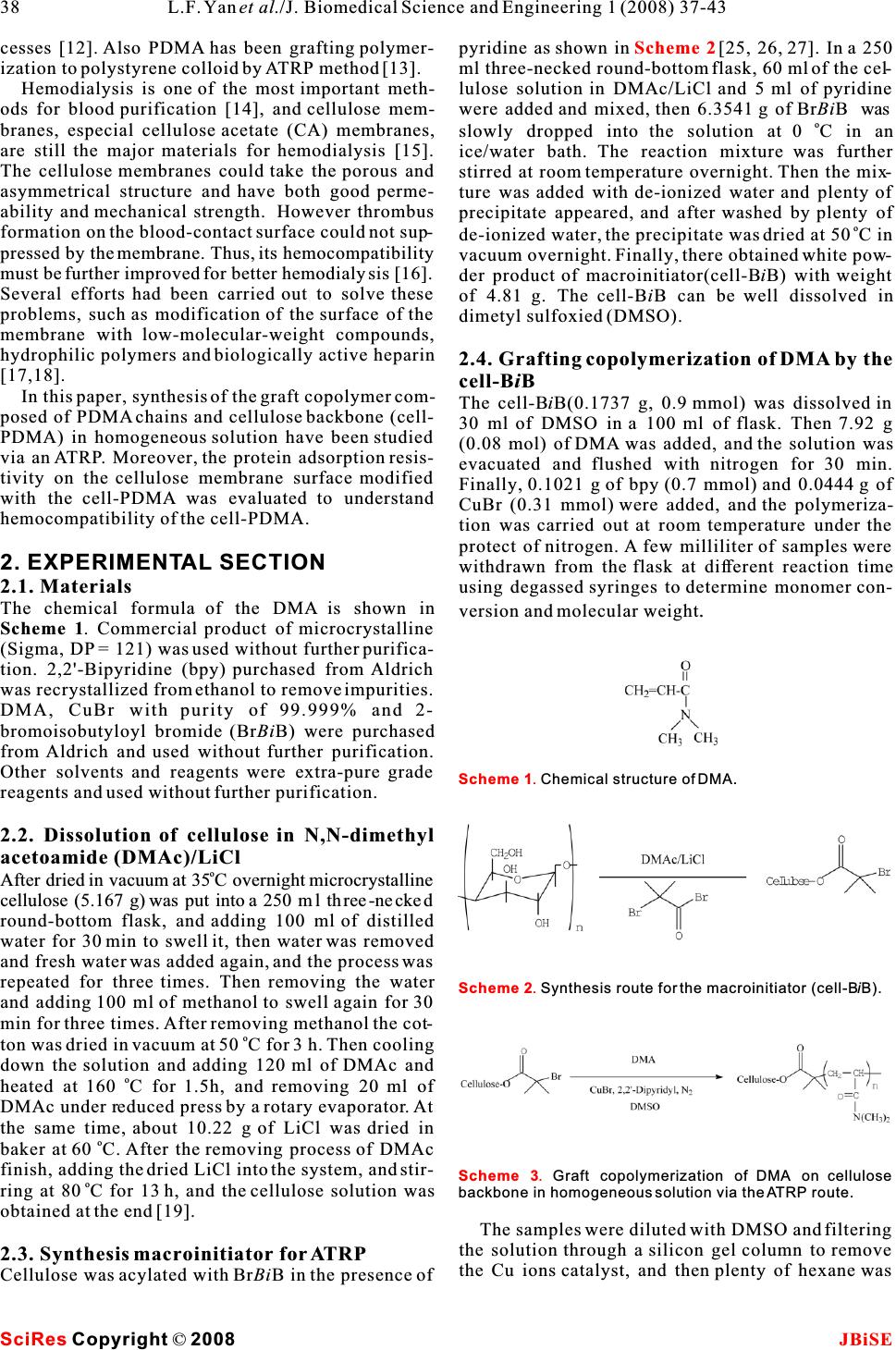 cesses [12]. Also PDMA has been grafting polymer-pyridine as shown in [25, 26, 27]. In a 250 ization to polystyrene colloid by ATRP method [13].ml three-necked round-bottom flask, 60 ml of the cel- Hemodialysis is one of the most important meth-lulose solution in DMAc/LiCl and 5 ml of pyridine ods for blood purification [14], and cellulose mem-were added and mixed, then 6.3541 g of BrBiB was o branes, especial cellulose acetate (CA) membranes,slowly dropped into the solution at 0 C in an are still the major materials for hemodialysis [15].ice/water bath. The reaction mixture was further The cellulose membranes could take the porous andstirred at room temperature overnight. Then the mix- asymmetrical structure and have both good perme-ture was added with de-ionized water and plenty of ability and mechanical strength. However thrombusprecipitate appeared, and after washed by plenty of o formation on the blood-contact surface could not sup-de-ionized water, the precipitate was dried at 50C in pressed by the membrane. Thus, its hemocompatibility vacuum overnight. Finally, there obtained white pow- must be further improved for better hemodialysis [16].der product of macroinitiator(cell-BiB) with weight Several efforts had been carried out to solve these of 4.81 g. The cell-BiB can be well dissolved in problems, such as modification of the surface of the dimetyl sulfoxied (DMSO). membrane with low-molecular-weight compounds, hydrophilic polymers and biologically active heparin2.4. Grafting copolymerization of DMA by the [17,18]. cell-BiB In this paper, synthesis of the graft copolymer com-The cell-BiB(0.1737 g, 0.9 mmol) was dissolved in posed of PDMA chains and cellulose backbone (cell-30 ml of DMSO in a 100 ml of flask. Then 7.92 g PDMA) in homogeneous solution have been studied (0.08 mol) of DMA was added, and the solution was via an ATRP. Moreover, the protein adsorption resis-evacuated and flushed with nitrogen for 30 min. tivity on the cellulose membrane surface modified Finally, 0.1021 g of bpy (0.7 mmol) and 0.0444 g of with the cell-PDMA was evaluated to understandCuBr (0.31 mmol) were added, and the polymeriza- hemocompatibility of the cell-PDMA.tion was carried out at room temperature under the protect of nitrogen. A few milliliter of samples were 2. EXPERIMENTAL SECTIONwithdrawn from the flask at different reaction time 2.1. Materialsusing degassed syringes to determine monomer con- The chemical formula of the DMA is shown inversion and molecular weight. Scheme 1. Commercial product of microcrystalline (Sigma, DP = 121) was used without further purifica- tion. 2,2'-Bipyridine (bpy) purchased from Aldrich was recrystallized from ethanol to remove impurities. DMA, CuBr with purity of 99.999% and 2- bromoisobutyloyl bromide (BrBiB) were purchased fromAldrich and used without further purification. Other solvents and reagents were extra-pure grade reagents and used without further purification. 2.2. Dissolution of cellulose in N,N-dimethyl acetoamide (DMAc)/LiCl o After dried in vacuum at 35C overnight microcrystalline cellulose (5.167 g) was put into a 250 ml three-necked round-bottom flask, and adding 100 ml of distilled water for 30 min to swell it, then water was removed and fresh water was added again, and the process was repeated for three times. Then removing the water and adding 100 ml of methanol to swell again for 30 min for three times. After removing methanol the cot- o ton was dried in vacuum at 50 C for 3 h. Then cooling down the solution and adding 120 ml of DMAc and o heated at 160 C for 1.5h, and removing 20 ml of DMAc under reduced press by a rotary evaporator.At the same time, about 10.22 g of LiCl was dried in o baker at 60 C.After the removing process of DMAc finish, adding the dried LiCl into the system, and stir- o ring at 80C for 13 h, and the cellulose solution was obtained at the end [19]. The samples were diluted with DMSO and filtering the solution through a silicon gel column to remove 2.3. Synthesis macroinitiator for ATRPthe Cu ions catalyst, and then plenty of hexane was Cellulose was acylated with BrBiB in the presence of Scheme 2 SciRes JBiSE Copyright © 2008 L.F. Yanet al./J. Biomedical Science and Engineering 1 (2008) 37-43 38 Scheme 1.Chemical structure of DMA. Scheme 2. Synthesis route for the macroinitiator (cell-BiB). Scheme 3. Graft copolymerization of DMA on cellulose backbone in homogeneous solution via the ATRP route.  added to produce the precipitate of the products. ThefromtheXPSelementalanalysis. o products were dried at 40 C in vacuum overnight. 2.8. Protein adsorption on the membrane sur- face 2.5. Isolation of the grafted PDMA chains by Amount of proteins adsorbed on the membrane was hydrolysis measured by almost the same method reported previ- The copolymers were hydrolyzed by 70% H SO for 24 ously [20]. The round (diameter: 1.5 cm) cellulose 8h at boiling point. At the end, the residual polymermembranes were placed into a 24-well plate. To was participated into plenty of hexane and was driedequilibrate the membrane surface, phosphate buffer by freeze drying, then the products were analyzed by solution (PBS, pH 7.4, ionic strength : 0.15 mol/l) GPC. was added into each well and allowed to remain for 15 h at room temperature. Protein solutions were pre- 2.6. Characterizationpared in the concentration of 4.5 mg/ml of albumin, The chemical structure was confirmed using an FT-1.6 mg/ml of-globulin, and 0.3 mg/ml of fibrinogen, 113 IR (FT/IR-615, JASCO, Tokyo, Japan).H- andC-which are 10% of the concentration of the human NMR spectra were obtained on a NMR spectrometer plasma level. After removing the PBS, 1.0 ml of each (-300, JEOL, Tokyo, Japan) with D Oas the sol-protein solution was poured onto each membrane and 2 o vent. The molecular weights of these polymers wereallowed to remain at 37 C for 3 h. After rinsing the determined bygel permeationchromatography (GPC).membrane three times with PBS, the membrane was The mixture of methanol/water = 7/3 containing 10 taken out of the 24-well plate, and was rinsed again mmol/L of lithium bromide was used as an eluent forsufficiently with the 50 ml of PBS. The membrane the GPC measurement at a flow rate of 0.4 ml/min was placed into a glass bottle with a 1 wt% aqueous (Column:SB-804HQ,Shodex, Tokyo, Japan). The solution of sodium dodecyl sulfate (SDS) and shaken number-averaged molecular weight (M) and weight-(150 rpm) in a shaking bath for 3 h at room tempera- nture to detach the adsorbed protein on the surface. A averaged molecular weight (M) were calculated wprotein analysis kit (Micro BCA protein assay using poly(ethylene glycol) standards.reagent kit, #23235, Pierce, Rockford, IL, USA) X-ray photoelectron spectroscopy (XPS) was con-based on the bicinchoninic acid method was used to ducted on an AXIS-HSi (Shimadzu/KRATOS, Kyoto,determine the protein concentration in the SDS solu- Japan) employing Mg Kexcitation radiation (1253.6 tion. eV). The take-off angle of the photoelectron for each atom was fixed at 90 deg.3. RESULTSAND DISCUSSION ForAtomic force microscopy (AFM) measurement, The cell-BiB was prepared by partial esterification of the sample was dissolved in DMF at a concentrationthe hydroxyl groups of the glucose units of cellulose -6 of 810 g/m. Then a droplet (20l) of the solutionwith BiBBr in the presence of pyridine. The reaction was deposited onto freshly cleaved mica, and it waswas carried out homogeneously in DMAc/LiCl solu- spin-coated at speed of 900 rpm for 8 s and then 4000tion at room temperature for 23 h. The formation of rpm for 30s. The height image of the copolymer onthe ester bond resulted in the appearance of the char- mica were measured by an AFM (Nanoscope IIIa, D.I.) -1 acteristic peaks at 1743 cm for the C=O stretching in tapping mode with silicon TESP cantilevers. Theband in the FTIR spectrum, as shown in . scanning rate ranged from 0.5 Hz to 1.0 Hz, andThe substitution of the hydroxyl groups on the cel- 512512 pixels images were record.lulose backbone with BiBBr was also confirmed by 2.7. Coating of the cell-PDMA on cellulose membrane (TM) The regenerated cellulose membrane, Cuprophan, was obtained from Enka, A. G. (Wappertal-Barmen, Germany). The thickness of the membranes was 20m. First the cellulose membranes were cut into pieces with diameter of 1.5cm, and they were immersed into deionized water for 30 min, and then o were dried at 35 C in vacuum for 15h. Then the cellu- lose membranes were immersed into the 0.5 wt% aqueous solution of the cell-PDMA for 3 min, and the membranes were took out and dried under atmo- o spheric conditions for 2h, and then was dried at 35C in vacuum for 15 h. The structure of the grafted DMA on the cellulose membranes were confirmed using XPS and FT-IR. The ratio of nitrogen atom (N) in the DMA unit versus carbon atom (C) was determined Figure 1 SciRes JBiSE Copyright © 2008 39 L.F. Yanet al./J. Biomedical Science and Engineering 1 (2008) 37-43 Figure 1. FT-IR spectra of cotton (1), cell-BiB (2), DMA (3) and cell-PDMA (4). Ccell-PDMA DMA Ccell-B/B Ccellulose -1 Wavelength(cm ) Transmittance(%)  113 PDMA was compared with that of the cell-BiB and both the H-NMR and C-NMR.As shown in-1 , there appears a new single peak at 1.8 ppm (peak DMAmonomer,theabsorptionsat1642cm a) for methyl protons in the ester group of BiB,andappeared after grafting, which was assigned to the the peaks at = 2.8-5.6 ppm (peak b) for the methy-freeC=OofPDMA,andthepeaksatabout3100- -1 lene protons and hydroxyl protons in the glucose3500 cmwas assigned to the OH group of cellulose units of cellulose[21]. The total substitution degree[23]. 1 (DS) of BiB is obtained by the ratio of the integral of shows a H-NMR spectrum for the cell- the methyl groups to the integral of protons of glu-o PDMA in methanol-d at 25C, the spectra is about 13 4 cose, and the DS is 0.2. shows the C-the same as that of PDMA. The resonance bands NMR of the cell-BiB, and clearly both the methyl car-observed at 2.9-3.1 ppm are attributed to the bon from BiB (peak a) and the carbon in glucosedimethyl group, and those observed at 1.3~1.8 ppm is (peak b) appear, and the peak c at 176 ppm attributedattributed to the methyl amd methylene protons of to the C=O carbon of BiB [22].PDMA [24]. Part of the resonance bands of cellulose The as-prepared cell-BiB can be dissolved well inprotons are overlapped with that of PDMA while DMSO. The graft copolymerization of DMA to cellu-there appear peaks at 2.9-4.0 ppm for the characteris- o13 lose was carried out in DMSO at 100 C,tics of cellulose.shows a C-NMR spec- o [DMA]:[cell-BiB]:[CuBr]:[bpy] = 88:1:2.9:1.3, andtrum for cell-PDMA in DO at 25C. The characteris- 2 [DMA] = 2.7 M. shows the kinetic plot of 0tic of the resonance peak for PDMA was observed at the reaction, and the variation of ln([M]/[M]) is lin- 035 ppm, which is attributed to the dimethyl moiety ear with time, indicating a constant concentration of[25]. The weak peaks appear at 75-85 ppm are attrib- propagating radicals which is the characteristic of theuted to the carbon for cellulose back bone, and the controlled/“living” radical polymerization.peak appear at 182 ppm is attributed to the carbon for The chemical structure of the cell-PDMA was iden-the carbonyl groups. tified by FT-IR spectroscopy, NMR and GPC. AsThe grafted PDMA chains were converted to indi- shown in , when the FT-IR spectrum of cell-vidual molecules through hydrolysis of the backbone Figure 2a Figure 2c Figure 2b Figure 2d Figure 3 Figure 1 SciRes JBiSECopyright ©2008 40L.F. Yanet al./J. Biomedical Science and Engineering 1 (2008) 37-43 Figure 2.113 H-NMR and C-NMR spectra of cell-BiB (a, b) and cell-PDMA (c, d). Aa DMSO Aa Ab AbAb Abb ppm 0 12 3 4 56 7 Ab Ab Aa Ac ppm 020406080100120 140160180 ppm 020406080100120 140160180200220 CH3 C=O Ccellulose ppm 0 12 3 4 56 Ac Ad Ab+c H2O Cellulose OCBr Aa C H b H3 CH3 OCellulose OBr C Aa C C b H3 CH3 O C C Cellulose OH2CNn H ) Ab (C C CO N CH3CH3 Aa Ad C 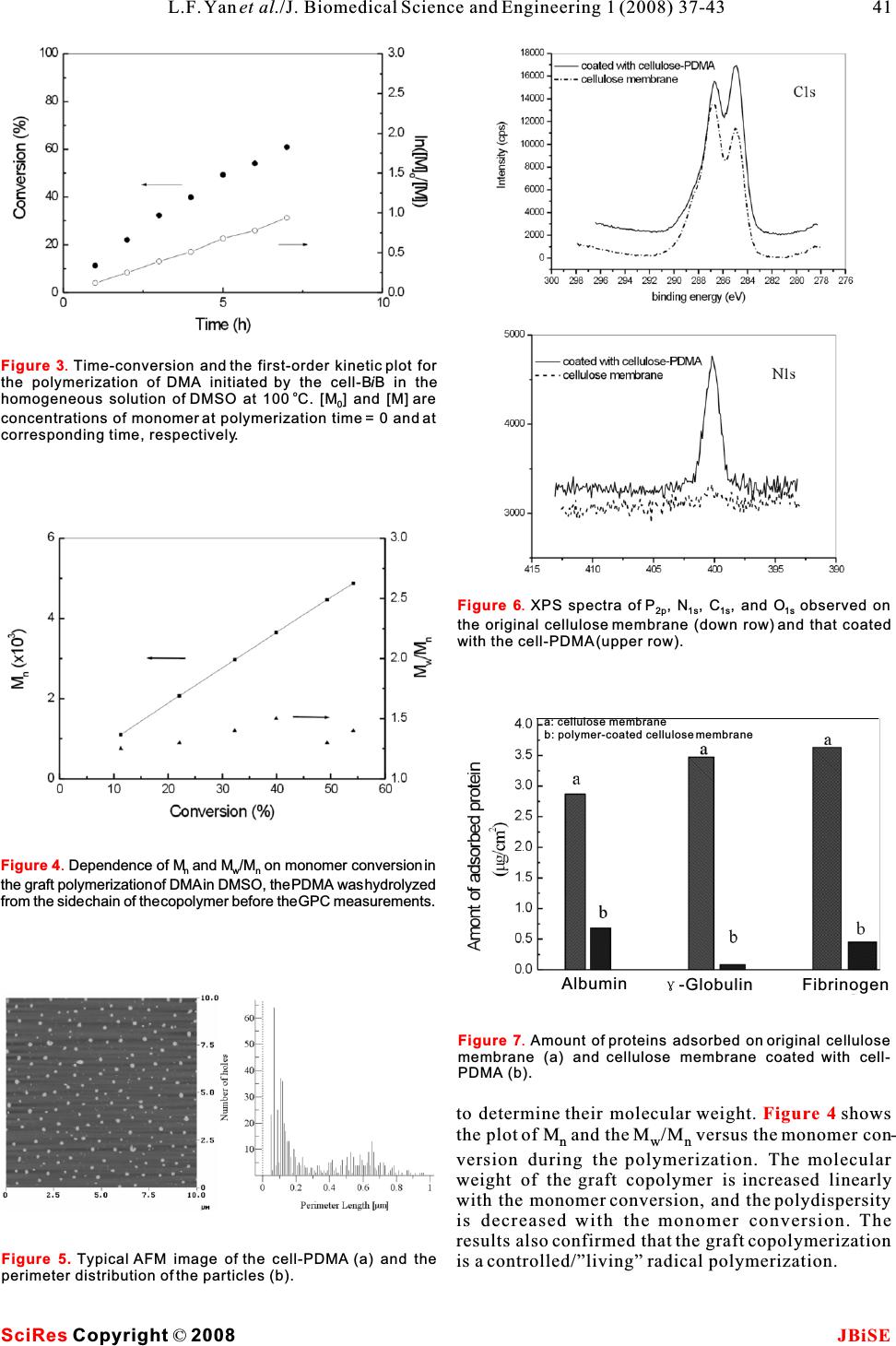 SciRes JBiSE Copyright © 2008 41 L.F. Yanet al./J. Biomedical Science and Engineering 1 (2008) 37-43 Figure 3. Time-conversion and the first-order kinetic plot for the polymerization of DMA initiated by the cell-BiB in the o homogeneous solution of DMSO at 100C. [M] and [M] are 0 concentrations of monomer at polymerization time = 0 and at corresponding time, respectively. Figure 4.Dependence of M and M/M on monomer conversion in nwn the graft polymerization of DMA in DMSO, the PDMA was hydrolyzed from the side chain of the copolymer before the GPC measurements. Figure 5. TypicalAFM image of the cell-PDMA (a) and the perimeter distribution of the particles (b). Figure 6. XPS spectra of P, N, C, and O observed on 2p 1s 1s1s the original cellulose membrane (down row) and that coated with the cell-PDMA (upper row). Figure 7. Amount of proteins adsorbed on original cellulose membrane (a) and cellulose membrane coated with cell- PDMA (b). to determine their molecular weight. shows the plot of M and the M/M versus the monomer con- nwn version during the polymerization. The molecular weight of the graft copolymer is increased linearly with the monomer conversion, and the polydispersity is decreased with the monomer conversion. The results also confirmed that the graft copolymerization is a controlled/”living” radical polymerization. Figure 4 a: cellulose membrane b: polymer-coated cellulose membrane Albumin -Globulin Fibrinogen  SciRes JBiSE Copyright © 2008 42L.F. Yanet al./J. Biomedical Science and Engineering 1 (2008) 37-43 Figure 5 Figure 5b Figure 6 Figure 7 shows the AFM image of the cell-PDMAcontrolled/”living” radical polymerization. The char- copolymer deposited on surface of the new cleaved acterizations indicate that the graft copolymerization mica. Many nanoparticles appear with a homoge-is efficient and the obtained copolymer owns well- neous size, and gives the perimeter distri-defined structures.After coated the cell-PDMA onto bution of the particles. Clearely, there are two kindsthesurface of commercial cellulose membrane, there of particles exist, one with the diameter about 200 nm, obtained membrane with good hemocompatibility, and the other about 38 nm in diameter. Huang alsowhich was confirmed by the protein adsorption reported similar result when they measrued the size experiments. This provides a new chance to modify of celluose-PS graft copolymer by AFM, and they con-the surface of polysaccharide materials to improve cluded that the smaller particles are the graft copoly-their hemocompatibility. The cell-PDMA has a strong mer and the bigger one are the micelles of the graft potential application on surface treatment to enhance copolymer when comparing the AFM data to dynamic separation ability and selectivity on every cellulose laser light scattering results. Here, we believe that membrane including CA and nitrocellulose, which the smaller particles result from the cell-PDMAare applied in biotechnology research and bioengi- copolymer while the bigger one is the aggregates or neering field. micelle of the graft copolymer. The as-prepared cell-PDMA was a water-soluble polymer having both affinities to the cellulose baseACKNOWLEDGEMENT membrane, and its potential blood compatibilityThis work is supported by the National Basic Research Program of China (No. 2007CB210201) and the National Key Technology R&D could improve the surface blood compatibility of theProgram (No. 2006BAF02A09). cellulose membrane by a convenient technique, such as coating by its aqueous solution. Coating of cellu- lose membrane with the cell-PDMA was carried out REFERENCE [1]D.W. Jenkins & S.M. Hudson. Review of vinyl graft copolymerization by immersing the membrane into its aqueous solutionfeaturing recent advances toward controlled radical-based reac- following a dry process under vacuum. The amounttions and illustrated with chitin/chitosan trunk polymers. Chem of the copolymer immobilized on the membrane was Rev.2001,101: 3245-3273. measured by XPS. shows the XPS chart of[2]Hu, Z.H. & Zhang, L.M. Water-soluble ampholytic grafted poly- saccharides. II. Synthesis and characterization of graft both the original cellulose membrane and the copoly-terpolymers of starch with acrylamide and [2-(methacryloylox)] mer coated cellulose membrane (upper row). The ethyl dimethyl (3-sulfopropyl) ammonium hydroxide. J peaks attributed to nitrogen (400 eV) atoms was Macromol Sci Pure Appl Chem 2002, A39: 419-430. observed on the surface of membrane coated with the [3]D. Roy, J. T. Guthrie & S. Perrier. Graft polymerization: graft- ing poly(styrene) from cellulose via RAFT polymerization. cell-PDMA. For the membrane coated with the copol-Macromolecules2005, 38:10363-10372. ymer, the atomic concentrations of nitrogen and car-[4]Qiu, J., B. Charleux & K. Matyjaszewski. Controlled/living rad- bon are 1.82% and 66.16%, respectively. The mole ical polymerization in aqueous media: homogeneous and heter- fraction of DMA on the membrane surface is 0.028 by ogeneous system. Prog Polym Sci 2001, 26:2083-2134. [5]D. Roy, J.T. Guthrie & S. Perrier. RAFT graft polymerization of calculation, which defined as [number of DMA unit 2-(Dimethylaminoethyl) Methacrylate onto cellulose fibre. (mol)]/[number of DMA unit (mol) + number of cel-Aust J Chem2006, 59: 737-741. lulose unit (mol)] was calculated from the value of [6]A. Carlmark & E.E. Malmstrom. ATRP grafting from cellulose N/C. fibers to create block-copolymer grafts. Biomacromolecules2003, 4: 1740-1745. The adsorption of proteins during contact with [7]T.Aoki, H.K. Kawashima, H. Katono, K. Sanui, N. Igata, T. blood on artificial surface is the initials step in a Okano & Y. Sakurai. Temperature-Responsive Interpentrating sequence of events which cause activation of several Polymer Networks Constructed with Poly (Acrylic Acid) and cascades of proteolysis systems in the plasma, e.g.,Poly (N,N-Dimethylacrylamide). Macromolecules 1994, 27: 947-952. complement, coagulation pathway, etc. therefore, the [8]M.S. Donovan, T.A. Sanford, A.B. Lowe, B.S. Sumerlin, Y. amount of proteins adsorbed on the surface is one ofMitsukami & C.L. McCormick. RAFT polymerization of N,N- the important factors for evaluating the hemocompatibilitydimethylacrylamide in water. Macromolecules 2002, 35: 4570- of materials. Here, the adsorption of three typical 4572. [9]Ding, S.J., M. Radosz & Shen, Y.Q. Atom transfer radical poly- plasma proteins such as albumin,-globulin, andmerization of N,N-dimethylacrylamide.Macromole Rapid fibrinogen on the cell-PDMA coating cellulose mem-Commun 2004, 25: 632-636. branes and original cellulose membranes were mea-[10]J. N. Kizhakkedathu & D. E. Brooks. Synthesis of poly (N,N- sured.As shown in, the amounts of each dimethylacrylamide) brushes from charged polymeric sur- faces by aqueous ATRP: Effect of surface initiator concentra- absorbed protein on the membrane coated with cell-tion. Macromolecules2003, 36: 591-598. PDMA was 70-80% reduced by comparison with[11]T. Nishimura. Biomedical Applications of Polymeric Materi- those on the original cellulose membrane for all of als, Eds., CRC Press, Boca Raton, 1993. the proteins examined in this study. That is, grafting[12]P. Delanaye, B. Lambermount, J. M.Dongne, B.Dubois, A.Ghuysen, N. Janssen, T. Desaive, P. Kolh, V.D'Drio & J. M. of PDMA chains on the cellulose plays an important Krzesinki. Confirmation of high cytokine clearance by role to reduce protein adsorption.hemofiltration with a cellulose triacetate membrane with large pores: an in vivo study.Int. J. Artif. Organs2006, 29:944. 4. CONCLUSION[13]H.D. Humes, W.H. Fissell & K. Tiranathanagul. The future of In this study, we have successfully synthesized the hemodialysis membranes. Kidney Int 2006, 69:1115-1119. cell-PDMA in homogeneous media using an ATRP[14]Kung, F.C., Chou, W.L. & Yang, M.C. In vitro evaluation of  cellulose acetate hemodialyzer immobilized with heparin. Polym Adv Tech2006, 17: 453-462. [15]Yuan, J., Zhang, J., Zang, X.P., Shen, J. & Lin, S. Improve- ment of blood compatibility on cellulose membrane sur- face by grafting betaines. Colloids Surf B Biointerf 2003, 30:147-155. Capitani & V. Crescenzi. Versatile grafting of polysaccharides in homogeneous mild conditions by using atom transfer radical polymerization. Biomacromolecules 2006, 7:2154-2161. [22]E. Meaurio, L.C. Cesteros & I. Katime. FTIR study of hydrogen bonding of blends of poly(mono n-alkyl itaconates) with poly(N,N-dimethylacrylamide) and poly(ethyloxazoline). [16]T. Furuzono, K. Ishihara, N. Nakabayashi & Y. Tamada. Chem-Macromolecules1997, 30:4567-4573. ical modificationofsilk fibroin with 2-methacryloyloxyethyl[23]Huynh-Ba-Gia & J.E. McGraph. High resolution NMR spectra of phosphorylcholine. II. Craft-polymerization onto fabric polyn,n-dimethylacrylamideinCDCl solution.Polym Bull 1980, 3 through 2-methacryloyloxyethyl isocyanate and interaction 2:837-840. between fabric and platelets. Biomaterials 2000, 21:327-333.[24]B.L.Rivas, S.A. Pooley, M. Soto, H.A. Maturana & K.E. Geckeler. [17]K. Ishiahra, R. Aragaki, T. Ueda, A. Watanabe & N. Poly(N,N'-dimethylacrylamide-co-acrylic acid): Synthesis, char- Nakabayashi.Reduced thrombogenicity of polymers hav-acterization, and application for the removal and separation of ing phospholipid polar groups. J Biomed Mater Res 1990, inorganic ions in aqueous solution. J Appl Polym Sci 1998, 24:1069-1077. 67:93-100. [18]Qi, Z. H.Synthesis of CA by solid acid catalyst. BS Thesis,[25]Shen, D.W., Yu, H. & Huang, Y. Densely grafting copolymers of University of Science and Technology of China, 2001.ethyl cellulose through atom transfer radical polymerization. J [19]K. Fukumoto, K. Ishihara, R. Takayama, J. Aoki & N.Polym Sci Part A Polym Chem 2005, 43:4099-4108. Nakabayashi. Improvement of blood compatibility on cel-[26]Shen, D., Yu, H. & Huang, Y.Synthesis of graft copolymer of lulose dialysis membrane.2.blood compatibility of ethyl cellulose through living polymerization and its self- phospholipid polymer grafted cellulose membrane.assembly. Cellulose 2006, 13:235-244. Biomaterials 1992, 13: 235-239.[27]Kang, H., Liu, W., He, B., Shen, D., Ma, L. & Huang, Y. Synthesis [20]P. Vlcek, M. Janata, P. Latalova, J. Kriz, E. Cadova, L.of amphiphilic ethyl cellulose grafting poly (acrylic acid) copol- Toman. Controlled grafting of cellulose diacetate. Polymer ymers and their self-assembly morphologies in water. Polymer 2006, 47:2587-2595.2006, 47:7927-7934. [21]D. Bontempo, G. Masci, P.D. Leonardis, L. Mannina, D. SciRes JBiSE Copyright © 2008 43 L.F. Yanet al./J. Biomedical Science and Engineering 1 (2008) 37-43 |

