Journal of Cancer Therapy
Vol. 3 No. 1 (2012) , Article ID: 17230 , 7 pages DOI:10.4236/jct.2012.31013
Cytotoxic Activity of Thelesperma megapotamicum Organic Fractions against MCF-7 Human Breast Cancer Cell Line*
![]()
1Center of Excellence in Products and Processes, Córdoba, Argentina; 2National Research Council of Argentina, Córdoba, Argentina; 3Institute of Cell Biology, Faculty of Medical Sciences, National University Córdoba, Córdoba, Argentina; 4Agricultural Biology Department, Faculty of Agriculture and Veterinary Medicine, University of Río Cuarto, Córdoba, Argentina.
Email: #anafigueroa05@yahoo.com.ar
Received November 5th, 2011; revised December 15th, 2011; accepted December 29th, 2011
Keywords: Thelesperma megapotamicum; Cancer Breast; MCF-7 Cells; Apoptosis; Membrane Syalization; Gama-Glutamyltranspeptidase Activity
ABSTRACT
Thelesperma megapotamicum (Asteraceae) is commonly used in Argentine to treat various diseases (renal, digestive affections, and as anaesthesia). The present study showed the mechanisms involved “in vitro” cytotoxicity of T. megapotamicum Fractions. Five Fractions (F1 - F5) were separated by column chromatography (Silica gel) using hexane:diethyl ether as eluents. Viability was evaluated in Human breast carcinoma cell line (MCF-7) by staining with crystal violet. With respect to F1 Fraction treatment, the cell survival was 49.14% ± 8.87%, while the F2 and F3 ones exhibited a strong reduction of cell viability to only 26.35% ± 1.63% and 23.3%1 ± 0.53% of the control cell at 50 µg/ml, respectively. Apoptotic effect of these Fractions was detected using FITC-labeled Annexin V and propidium iodide binding assays and was confirmed by a higher proportion of apoptotic cells due to F2 and F3 treatments. T. megapotamicum active Fractions could facilitate the tumoral cells death by decreasing the activity of the enzyme Gamma-glutamyltranspeptidase and causing alteration in cell membrane sialoglycoconjugates and others involved anticancer mechanisms including apoptosis.
1. Introduction
Breast cancer is the most commonly occurring non-skin malignancy among women and the second leading cause of cancer-related death. Approximately 1 in 8.2 women will receive a diagnosis of breast cancer during her lifetime, and 1 in 30 will die of the disease [1].
More than 80% of the world populations use the traditional medicine to satisfy their needs of primary care of the health and that great part of the traditional treatments implies the use of extracts plants or their active principles [2]. In recent years, phytochemicals have been recognized as a new prevention and therapeutic approach for breast cancer [3,4].
Argentine medicinal plants have a long-standing history in many indigenous communities and are still useful resources for treating diseases. Subsequently, pharmacological studies (activity reports) are necessary in order to extend the scarce ethnopharmaceutical data, which have given empirical support to the medicinal use of these regional plants based on traditional knowledge [5].
Since the native flora may contain some anticancer compounds, it has become a valuable resource looking for a chemopreventive agent. Regarding this, the medical potential of Argentine plants has been recorded based on their traditional use, with an ethnobotanical survey of central Argentina being carried out over a 26-year period (1979-2005) [6].
Thelesperma megapotamicum belongs to Asteraceae family, it is known as Pampa tea or Indian tea [7]. Several compounds were isolated from this specie as stigmasterol, flavonoids (luteolin, 7-O-Glucosido, marein) and phenylpropanoids [8,9].Various studies are in line with the hypothesis that flavonoids [10,11] and phenylpropanoids [12] prevent or inhibit cancer development. Although, is known that luteolin has inhibitor activity of topoisomerase I in eukaryotic DNA [13] and DNA protector effect against oxidant agents [14]. Although these mechanisms have been already reported, the precise of antitumoral mode of action of T. megapotamicum compound had not been yet thoroughly studied. Probably, the cytotoxic effect produced by the extract cannot be clearly assigned to a single mechanism or a specific cellular target yet. Concerning this, several mechanisms are able to modify cellular membrane integrity that play a critical role in cell functionality, viability and apoptosis, which is relevant in carcinogenesis and oncological pharmacology [15,16].
Accordingly, the aim of this work was to elucidate the mechanisms involved in T. megapotamicum Fractions in an “in vitro” cytotoxicity representative model and also to study the effect over some membrane components.
2. Materials and Methods
2.1. Plant Material
Plant recollection was carried out at Santa María de Punilla, “Sierras de Córdoba”. This side is located at approximately 45 km north of “Córdoba”, Argentine. Plant samples were collected during spring 2008, immediately transported to the lab and a voucher specimen was deposited in the International Herbarium of the National University of Rio Cuarto, Argentina (RIOC420).
The rhizome (12 g) was thoroughly extracted with 50 ml of hexane: ethyl ether (1:1 V/V) by maceration at room temperature for 24 hours under stirring. A supernatant was obtained by filtration, and concentrated under reduced pressure.
2.2. Bioassay-Directed Fractionation
The hexane:diethyl ether rhizome extract was subjected to Silica gel-60 column chromatography (15.5 cm length, 0.9 cm i.d.) using hexane: ethyl ether as eluents; 50 fractions of 5 ml were checked by thin layer chromatography (TLC) and observed without chemical treatment, under UV 254 nm light. On the basis of the similar TLC profile the fractions were pooled into 5 groups: F1 - F5.
For the “in vitro” viability test, the dried Fractions were dissolved in DMSO at a concentration of 50 mg/ml.
2.3. Cell Culture
Human breast carcinoma cell line (MCF-7, cell line ATCC HTB-22), were cultured in Dulbecco’s Modified Eagle’s medium with 10% (V/V) fetal bovine serum, 100 IU/ml penicillin G and 40 μg/ml gentamycin sulphate, incubated in a humidified atmosphere of 5% CO2 at 37˚C.
2.4. In Vitro Viability Test
After 48 hours attachment in a 96 well-plates (2 × 104 cells/well) viable cells were incubated with 0 and 50 μg/ml of each Fraction (F1 - F5) for 6 hours. Afterwards, the medium containing extracts was removed and cell viability was determined by measuring the absorbance at 570 nm using crystal violet as followed. Viable cells were stained with 0.5% crystal violet in 50% methanol for 15 min. After washing with 50% methanol three times, the staining cells were solubilized with 20% methanol in sodium citrate solution (0.1 M, pH 5.4). Results, consistent with cellular density, were recorded by an Anthos Labtec 2010 micro ELISA reader at 570 nm. The viability percentage was defined as the relative absorbance of treated versus untreated control cells (100%). To compare the effectiveness of the extracts with a clinical drug, a concentration of 0.1 µM of Paclitaxel and 40 µM of Luteolin (Sigma Chemical Co.) were also tested as positive controls.
2.5. Determination of Cell Death and Apoptosis
Apoptosis was analyzed by flow cytometry using fluorescein isothiocyanate (FITC)-labeled Annexin V and propidium iodide (PI) staining. Briefly, the cells were seeded in 6-well plates at a density of 1 × 106 cells/ml and incubated for 24 hours at 37˚C as above. The medium was then replaced with fresh medium containing the different Fractions (0, 50 µg/ml) and the cells were incubated at 37˚C for 6 hours. The cells that had become detached from the surface of the flask (floating cells), together with those which remained attached (harvested by trypsinization), were pelleted by centrifugation. Cell pellets were resuspended in complete medium (5 × 105 cells/ ml), with binding buffer and incubated with FITC-labeled Annexin V and PI (at a final concentration of 1 and 2 μg/ml, respectively) for 15 min. The stained cells were analyzed on a COULTER EPICS® flow cytometer. Cells negative for both FITC-labeled Annexin V and PI staining are live cells. Annexin V-positive and PI-negative staining cells are undergoing early stages of apoptosis. PIand Annexin V-positive staining cells are late apoptotic cells, and PIpositive and Annexin V-negative staining cells are necrotic cells [17].
2.6. Sialic Acid Membrane Content
The MCF-7 cells were seeded at a density of 1 × 106 cells/well onto 6-well culture plates and incubated for 24 hours at 37˚C, humidified air and 5% CO2. Then 50 μg/ml of the F1, F2 and F3 Fractions was added to the wells and the cells were incubated for 6 hours in the same conditions. After enzymatic harvesting with porcine trypsin, cells were homogenized at 20.000 rpm for 30 seconds in 1 ml of 10 mM HEPES buffer (pH 7.4, containing 2 μg/ml leupeptin and 1mM EDTA). They were centrifuged at 100.000 g for 1 h at 4˚C to recover the pellet, which was then resuspended in 200 μl of 10 mM HEPES buffer. The protein content was determined according to the Bradford method, and each suspension (150 μl) was mixed with 750 μl chloroform/methanol
(2:1 V/V, Fölch extraction). The mixture was centrifuged at 1.000 g for 10 min, and then the upper layer was used for sialic acid determination.
2.7. Gamma Glutamyltranspeptidase Activity
To determinate gamma glutamyltranspeptidase (γ-GT) activity the modified Szasz method was used following the γ-GT-test kinetic AA kit manufacturer’s instructions [18]. Samples were mixed with 1% Triton X-100 and 100 mM Tris-HCl substrate buffer, pH 8.5 (containing 2.9 mM L-γ-glutamyl-3-carboxi-4-nitroanilide and 100 mM GLY-GLY) in the proportion 1:1:9 (v/v/v). Then, the absorbance at 405 nm was recorded. Results were expressed in mIU/mg of proteins (1 IU = 1 μmole of product/min at pH 8.5 and 25˚C).
2.8. Sialic Acid Content Measurement (Membrane Sialylation)
After Fölch extraction, the Sialic acid (SA) content (nmol/mg of protein) was measured in the upper phase at 580 nm according to Miettinen and Takki-Luukkainen [19].
2.9. Chemicals
Culture reagents and other chemicals were obtained from the Sigma-Aldrich Co. (USA). The γ-G-test kinetic AA kit™ for in vitro diagnosis was from Wiener Laboratories.
2.10. Statistical Analysis
Data were expressed as mean ± standard deviation (SD) from four separate experiments performed in triplicate, unless otherwise noted. ANOVA models were used to evaluate differences of cellular viability and membrane parameters (γ-GT and SA) among treatments. The association between these variables was determined using the correlation coefficient (CC). Analytical probes were performed using the InfoStat 2007e.1 software.
3. Results
In order to evaluate the Fractions cytotoxic effect, different experiments with the MCF-7 cell line were run. The survival rates in the Fractions treated MCF-7 cells, determined by Crystal violet staining, are shown in Figure 1. With regard to the results obtained, at a concentration of 50 µg/ml of the F1 Fraction, following 6 hours of incubation, cell survival was 49.14% ± 8.87%, while the F2 and F3 ones exhibited a strong reduction of cell viability to only 26.35% ± 1.63% and 23.31% ± 0.53% of the control cell at 50 µg/ml, respectively. On the other

Figure 1. Cell viability was measured with violet crystal. Cells were incubated under the following conditions: none (Control), 50 µg/ml of each Fraction (F1 - F5) of T. megapotamicum and 40 µM of Luteolin. Data are expressed as % of controls (mean ± SD). Significant decrease compared with the corresponding untreated controls (p ≤ 0.05).
hand, Fractions F4 and F5 had lower cytotoxic effect (77.09% ± 20.00% and 73.21% ± 7.73% survival compared to control at 50 µg/ml, respectively). With regard to 0.1 µM of Paclitaxel, this antitumoral drug was not cytotoxic towards the MCF-7 after 6 hours of incubation (data not shown), these results are in agreement with previous reports [17]. Note that by this procedure 40 µM of Luteolin exhibited a 55.04% ± 24.24% survival relative to the control.
The apoptotic nature of T. megapotamicum Fractionsinduced cell death was confirmed using FITC-labeled Annexin V and PI binding assays as useful markers of early apoptotic and necrotic events, respectively. Stained cells were identified by flow cytometric analysis and expressed as a percentage of the total number of cells in each case.
This included cells that had become detached from the surface of the flask, as a result of poor health of the cells (including apoptotic and necrotic cells), but which had remained intact. The flow cytometric analysis excluded debris and highly condensed cells by gating on intact cells, based on light scatter. Consistent with the data shown in Figure 2, not higher percentages of early apoptotic cells were detected in the different Fractions treatment and control cells. The highest percentages of advanced apoptosis were detected in cells treated with Fractions F2 and F3. A slight increase in necrosis was also seen on Fractions F2 and F3 treatments.
The lipid sialylation and the γ-GT were studied as markers of the cell membrane status. The specific activeity of γ-GT, depicted in Figure 3 had quantifiable basal values in MCF-7. The cells treated with Fractions F1 - F3 decreased the γ-GT activity with respect to control. A decreased activity was seen with the Fraction F1 (4.8 fold lower; p ≤ 0.02), Fraction F2 (15.55 fold lower;
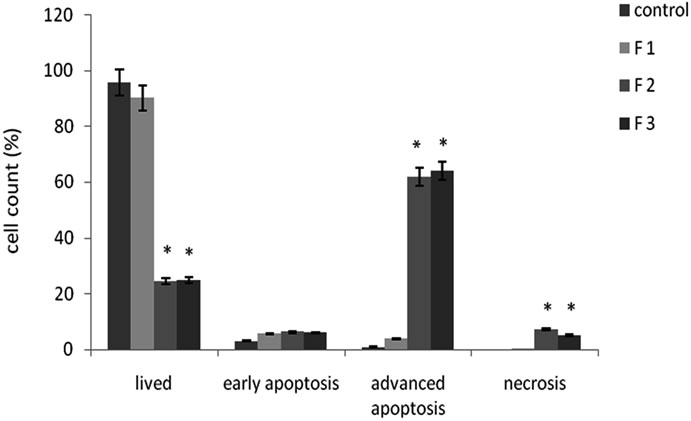
Figure 2. Apoptosis in T. megapotamicum Fractions treated MCF 7 cells. Attached as well detached FITC labeled Annexin V and/or propidium iodide stained cells were identified by flow cytometric analysis. Annexin V and PI negative staining cells were considered lived cells. Annexin V positive and PI negative staining cells are undergoing early stages of apoptosis, Annexin V and PI positive staining cells are late apoptotic cells, and PI positive and Annexin V negative staining cells are necrotic cells. Bars indicate the percentage of the entire population of cells in each stage, summarized from 2 experiments (means). *p < 0.05 for each stage with respect to that in the control.
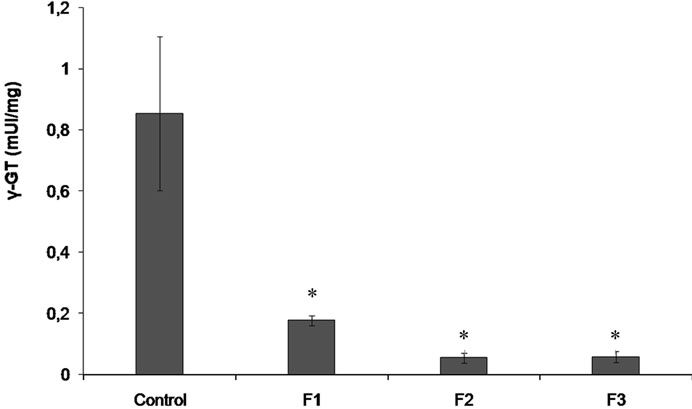
Figure 3. γ-GT specific activity in MCF-7 cells. Cells were incubated under the following conditions: none (Control), Fractions F1 - F3, 50 µg/ml. Data (mean ± SD). Values significantly different from those of the control group: *p < 0.05.
p ≤ 0.02), and Fraction F3 (14.7 fold lower; p ≤ 0.02) with respect to control. MCF-7 cells had basal levels of SA in their membranes of 232.2 ± 64.3 pmole of SA/mg of proteins, being the SA content in MCF-7 incubated with Fractions F1, F2 and F3 lower than control (p ≤ 0.05): 2.7, 3.6 and 5.7 folds respectively (Figure 4).
4. Discussion
Carcinogenesis involves a growing accumulation of genetic and epigenetic aberrations, leading to the deregulation of cellular homeostasis, followed by neoplastic progression [20]. As a new approach in cancer therapy, we have investigated the effects “in vitro” of the T. megapo-
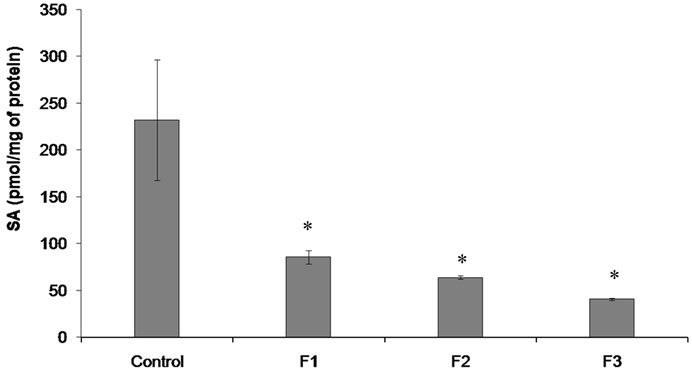
Figure 4. SA content in MCF-7 cells. Cells were incubated under the following conditions: none (Control), Fractions F1-F3. Data are mean ± SD. Values significantly different from those of the control group: *p < 0.05.
tamicum active Fractions on cells viability, γ-GT activity and membrane sialylation in MCF-7 cell culture.
The results reported herein reveal that T. megapotamicum exerts growth inhibition in cultured human breast cancer MCF-7 cells; this cell line showed a high sensibility to T. megapotamicum active organic Fractions at a very low incubation times (6 hours). We further demonstrate that these Fractions are much more effective in decreasing the cell viability compared to the commonly used chemotherapeutic agent, Paclitaxel. Previous reports shown that Paclitaxel at 0.01 and 0.1 µM, did not induce significant death of these cancer cells, within 48 hours [21].
Our results suggest that F2 and F3 Fractions could facilitate the tumoral cells death by apoptosis, as indicated by the FITC-labeled Annexin and Propidium Iodide Fluorescence Assay. At the 50 μg/mL, for Fractions F2 and F3 on MCF-7 cells, FITC-labeled Annexin V fluorescence was detected substantially, as early as 6 hours post treatment. This showed that the plasma membrane of MCF-7 cells had incorporated FITC-labeled Annexin V, indicating that PS had been translocated from the inner to the outer leaflet of the plasma membrane (early step in apoptosis).
The γ-GT, a key enzyme of glutathione metabolism, can modulate crucial redox-sensitive functions, such as antioxidant/antitoxic defenses and cellular proliferative/ apoptotic balance, with potential implications in tumor progression and drug resistance [22,23].
Previous studies have shown the possible role of membrane γ-GT enzyme for evaluating anticancer drugs. This enzyme is located on the outer aspect of plasma membrane of most cell types, and is often expressed at high levels in malignant tumors and their metastases [24]. The administration of the T. megapotamicum Fractions to MCF-7 cells was reflected in a decrease of γ-GT activity. Since, γ-GT expression is regulated by the cytosolic glutathione pool and steroids [25,26], we speculated that the decreased Fractions-induced activity might generate a fewer intracellular glutathione to sustain the enzymatic activity level necessary into the cells. So, a γ-GT reduction may participate in the mechanism of Fractions sensibility and increase the apoptosis on MCF-7 cells.
Gangliosides (sialylglicolipids) have previously been found to be involved in cellular surface-relates regulation and in biomembrane resistance to oxidation [27,28]. Furthermore, since some cancer cells present aberrant glycolsilation, with the SA content being a useful tumor marker [29]. SA, the end moieties of the carbohydrate chains are biologically important and essential for functions of glycoconjugates and are reported to be altered in cancer patients. A positive correlation between serum levels of different forms of SA and extent of malignant disease was observed [30]. Glycosylation has been demonstrated to play a critical role during malignant transformation. Patients with breast cancer had significantly higher levels of different forms of SA as compared to the controls [31,32].
The basal levels of SA in cells membranes incubated with different Fractions of T. megapotamicum responded decreasing its values corresponding to a decrease in the rate of cell viability.
These data suggest that the active Fractions of T. megapotamicum, apart from their effects on cellular growth, could inhibit the expression of membrane SA.
In this work, the pharmacological regulation of Fractions could be important, since ganglioside molecular interactions are involved in cancer growth due to their role in the immune response and in the metastatic process [33,34].
Taking together, T. megapotamicum active Fractions could regulate the MCF-7 cells death by decreasing the activity of the enzyme γ-GT, and causing alteration in cell membrane sialoglycoconjugates and others involved anticancer mechanisms including apoptosis.
T. megapotamicum might contain flavonoids, such as Luteolin, and phenylpropanoids [7]. Previous investigators have shown that both family compounds have anticancer effects [12,35]. Meanwhile, we have not yet specifically looked into the active ingredient(s) of the T. megapotamicum that inhibits the growth of breast cancer.
In this work, organic “Pampa tea” Fractions have shown pronounced activity. Further evaluation will be done in the future for possible isolation of active anti-tumor compounds.
5. Acknowledgements
This work was partially supported by grants from the Consejo Nacional de Investigaciones científicas y Técnicas (CONICET-AVCR) No. 962/07-05-2009.
REFERENCES
- J. D. Yager and N. E. Davidson, “Estrogen carcinogenesis in breast cancer,” The New England Journal of Medicine, Vol. 354, No. 3, 2006, pp. 270-282. doi:10.1056/NEJMra050776
- A. Bermúdez, M. A. Oliveira-Miranda and D. Vazquez, “La investigación etnobotánica sobre plantas medicinales: Una revisión de Sus objetivos y enfoques actuales,” Interciencia, Vol. 30, No. 8, 2005, pp. 453-459.
- J. M. Cline and C. L. Hughes Jr., “Phytochemicals for the prevention of breast and endometrial cancer,” Cancer Treatment Research, Vol. 94, 1998, pp. 107-134. doi:10.1007/978-1-4615-6189-7_7
- L. S. Einbond, Y. Wen-Cai, K. He, H. Wu, E. Cruz, M. Roller and F. Kronenberg, “Growth inhibitory activity of extracts and compounds from Cimicifuga species on human breast cancer cells,” Phytomedicine, Vol. 15, No. 6-7, 2008, pp. 504-511. doi:10.1016/j.phymed.2007.09.017
- E. L. Ratera and M. O. Ratera, “Plantas de la Flora Argentina Empleadas en Medicina Popular,” Hemisferio Sur S.A., Buenos Aires, 1980.
- M. Goleniowski, G. A. Bongiovanni, L. Palacio, C. O. Nuñez and J. J. Cantero, “Medicinal plants from the ‘Sierra de Comechingones,’ Argentina,” Journal of Ethnopharmacoly, Vol. 107, No. 3, 2006, pp. 324-341. doi:10.1016/j.jep.2006.07.026
- E. L. Ariza, “Tratamiento taxonómico: descripción de Familias, Géneros y Especies; Asteraceae,” In: G. E. Barboza, J. J. Cantero, C. O. Nuñez and L. Ariza Espinar, Eds., Flora medicinal de la Provincia de Córdoba (Argentina), Pteridófitas y Antófitas silvestres o naturalizadas, Museo Botánico, Córdoba, 2006, pp. 475-477.
- A. Ateya, T. Okarter, J. Knapp, P. Schiff Jr. and D. Slatkin, “Flavonoids of Thelesperma megapotamicum,” Planta Medica, Vol. 45, No. 8, 1982, pp. 247-248. doi:10.1055/s-2007-971384
- V. Pathak, F. Bohlmann, R. King and H. Robinson, “Chemotaxonomy of the Genus Thelesperma,” Revista Latinoamericana de Quimica, Vol. 18, 1987, pp. 28-29.
- M. Hertog, P. Hollman and V. De Putte, “Content of Potentially Anticarcinogenic flavonoids of tea infusions, wines and fruit juices,” Journal of Agricultural and Food Chemistry, Vol. 41, No. 8, 1993, pp.1242-1246. doi:10.1021/jf00032a015
- P. Hollman, M. Hertog and M. Katan, “Analysis and health effects of flavonoids,” Food Chemistry, Vol. 57, No. 1, 1996, pp. 43-46. doi:10.1016/0308-8146(96)00065-9
- L. G. Korkina, “Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health,” Cellular and Molecular Biology, Vol. 53, No. 1, 2007, pp. 15-25.
- A. R. Chowdhury, S. Sharma, S. Mandal, A. Goswami, S. Mukhopadhyay and H. K. Majumder, “Luteolin, an Emerging Anti-Cancer Flavonoid, Poisons Eukaryotic DNA Topoisomerase I,” Biochemical Journal, Vol. 366, No. 2, 2002, pp. 653-661. doi:10.1042/BJ20020098
- K. Horváthová, L. Novotný, D. Tóthová and A. Vachálková, “Determination of free radical scavenging activity of quercetin, rutin, luteolin and apigenin in H2O2- treated human ML cell K562,” Neoplasma, Vol. 51, No. 5, 2004, pp. 395-399.
- K. Hossain, A. A. Akhand, M. Kato, J. Du, K. Takeda, J. H. Wu, K. Takeuchi, W. Liu, H. Suzuki and I. Nakashima, “Arsenite induces apoptosis of murine T lymphocytes through membrane raft-linked signaling for activation of c-Jun amino-terminal kinase,” Journal of Immunology, Vol. 165, No. 8, 2000, pp. 4290-4297.
- M. E. Pasqualini, V. L. Heyd, P. Manzo and A. R. Eynard, “Association between E-cadherin expression by human colon, ladder and breast cancer cells and the 13-HODE: 15-HETE ratio. A possible role of their metastatic potential,” Prostaglandins, Leukotrienes, and Essential Fatty Acids, Vol. 68, No. 1, 2003, pp. 9-16. doi:10.1016/S0952-3278(02)00230-2
- G. A. Bongiovanni, J. J. Cantero, A. Eynard and M. E. Goleniowski, “Organic extracts of Larrea divaricata Cav. induced apoptosis on tumoral MCF7 cells with an higher cytotoxicity than nordihydroguaiaretic acid or Paclitaxel,” Journal of Experimental Therapeutics & Oncology, Vol. 7, No. 1, 2008, pp. 1-7.
- G. Szasz, “A Kinetic Photometric Method for Serum Gamma-Glutamyltranspeptidase,” Clinical Chemistry, Vol. 15, No.2, 1969, pp. 124-136.
- T. Miettinen and I. T. Takki-Luukkainen, “Use of butyl acetate in the determination of sialic acid,” Acta Chemical Scandinava, Vol. 13, 1959, pp. 856-858. doi:10.3891/acta.chem.scand.13-0856
- P. L. Quiroga, A. R. Eynard, E. A. Soria and M. A. Valentich, “Interaction between Retinoids and Eicosanoids: Their Relevance to Cancer Chemoprevention,” Current Nutrition & Food Science, Vol. 5, No. 2, 2009, pp. 126- 133. doi:10.2174/157340109788185553
- N. S. Yaacob, N. Hamzah, N. Nursyazni, N. M. Kamal, S. A. Zainal Abidin, C. S. Lai, V. Navaratnam and M. N. Norazmi, “Anticancer activity of a sub-fraction of dichloromethane extract of Strobilanthes crispus on Human Breast and Prostate Cancer Cells in Vitro,” BMC Complementary and Alternative Medicine, Vol. 10, 2010, pp. 42-55. doi:10.1186/1472-6882-10-42
- M. H. Hanigan, H. F. Frierson Jr., P. E. Swanson and B. R. De Young, “Altered expression of gamma-glutamyltranspeptidase in human tumors,” Human Pathology, Vol. 30, No. 3, 1999, pp. 300-305. doi:10.1016/S0046-8177(99)90009-6
- A. Pompella, A. Corti, A. Paolicchi, C. Giommarelli and F. Zunino, “g-Glutamyltransferase, redox regulation and cancerdrug resistance,” Current Opinion in Pharmacology, Vol. 7, No. 4, 2007, pp. 360-366. doi:10.1016/j.coph.2007.04.004
- S. Dominici, L. Pieri, M. Comporti and A. Pompella, “Possible role of membrane gamma-glutamyltransferase activity in the facilitation of transferrin-dependent and -Independent Iron Uptake by Cancer Cells,” Cancer Cell International, Vol.3, No. 7, 2003, pp. 1-8. doi:10.1186/1475-2867-3-7
- T. H. Rasmussen, S. J. Teh, P. Bjerregaard and B. Korsgaard, “Anti-Estrogen Prevents Xenoestrogen-Induced Testicular Pathology of Eelpout (Zoarces viviparus),” Aquatic Toxicology, Vol. 72, No. 3, 2005, pp.177-194. doi:10.1016/j.aquatox.2004.12.003
- S. J. Chinta, J. M Kumar, H. Zhang, H. J. Forman and J. K. Andersen, “Upregulation of Gamma-Glutamyltranspeptidase Activity Following Glutathione Depletion Has a Compensatory Rather Than an Inhibitory effect on Mitochondrial Complex I Activity: Implications for Parkinson’s Disease,” Free Radical Biology & Medicine, Vol. 40, No. 9, 2006, pp. 1557-1563. doi:10.1016/j.freeradbiomed.2005.12.023
- R. L. Proia, “Glycosphingolipid Functions: Insights from Engineered Mouse Models,” Philosophical Transactions of the Royal Society B: Biological Sciences, Vol. 358, No. 1433, 2003, pp. 879-883. doi:10.1098/rstb.2003.1268
- O. Sergent, M. Pereira, C. Belhomme, M. Chevanne, L. Huc, D. Lagadic-Gossmann, “Role for Membrane Fluidity in Ethanol-Induced Oxidative Stress of Primary Rat Hepatocytes,” The Journal of Pharmacology and Experimental Therapeutics, Vol. 313, No. 1, 2005, pp. 104- 111. doi:10.1124/jpet.104.078634
- S. Narayanan, “Sialic Acid as a Tumor Marker,” Annals of Clinical and Laboratory Science, Vol. 24, No. 4, 1994, pp. 376-384.
- G. N. Raval, L. J. Parekh, D. D. Patel, F. P. Jha, R. N. Sainger and P. S. Patel, “Clinical usefulness of alterations in sialic acid, sialyltransferase and sialoproteins in breast cancer,” Indian Journal of Clinical Biochemistry, Vol. 19, No. 2, 2004, pp. 60-71. doi:10.1007/BF02894259
- A. Kobata and S. Takasaki, “Structural Characterization of Oligo-Saccharides from Glycoproteins,” In: M. Fukuda, and A. Kobata, Eds., Glycobiology: A Practical Approach, Oxford University Press, New York, 1993, pp. 165-185.
- A. Varki, “Biological roles of oligosaccharides: All of the theories are correct,” Glycobiology, Vol. 3, No. 2, 1993, pp. 97-130. doi:10.1093/glycob/3.2.97
- S. Hakomori, “Glycosylation defining cancer malignancy: new wine in an old bottle,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 99, No. 16, 2002, pp. 10231-10233. doi:10.1073/pnas.172380699
- N. M Varki and A. Varki, “Diversity in Cell Surface Sialic Acid Presentations: Implications for Biology and Disease,” Laboratory Investigation, Vol. 87, No. 9, 2007, pp. 851-857. doi:10.1038/labinvest.3700656
- H. L. Liu, W. B. Jiang and M. X. Xie, “Flavonoids: Recent Advances as Anticancer Drugs,” Recent Patents on Anti-Cancer Drug Discovery, Vol. 5, No. 2, 2010, pp. 152-164. doi:10.2174/157489210790936261
Abbreviations:
TLC: thin layer chromatography;
γ-GT: gamma glutamyltranspeptidase;
SA: sialic acid;
FITC: fluorescein isothiocyanate;
PI: propidium iodide;
PS: phosphatidylserine.
NOTES
*There is no conflict of interest with regard to this manuscript.
#Corresponding author.

