Health
Vol. 4 No. 7 (2012) , Article ID: 21193 , 5 pages DOI:10.4236/health.2012.47069
Risk assessment of alcohol and obesity on liver enzymes (transaminases, cholestatic)
![]()
1Department of Zoology, University of Karachi, Karachi, Pakistan; *Corresponding Author: gr8_dezirez@hotmail.com
2Institute of Biochemistry, University of Balochistan, Quetta, Pakistan
3Center for Environmental Studies, PCSIR Labs Complex, Karachi, Pakistan
Received 30 March 2012; revised 14 April 2012; accepted 27 April 2012
Keywords: Moderate Alcohol; Body Mass Index; Obesity; Liver Enzymes
ABSTRACT
Background: This study was designed to investigate the BMI and alcohol consumption effects on hepatic enzymes. The degree of alteration among moderate drinkers is still unclear. Objective: To determine causes of liver failure due to alcohol and obesity. We observed the association between moderate alcohol consumption, body mass index (BMI; in kg/m2) and transaminase, cholestatic enzymes. Design: Serum alanine aminotransferase (ALT), alkaline phosphate (ALP), aspartate aminotransferase (AST), and gamma-glutamyltransferase (GGT) were examined in 995 healthy persons. In this study 400 persons were reported as abstainers and 595 participants involved as a moderate drinkers. The study population was further split into according to BMI as follows: <19 (underweight), ≥19 and <25 (normal weight), ≥25 and <30 (overweight), and ≥30 (obese). Results: Serum ALT (P < 0.002), GGT (P < 0.001) and ALP (P < 0.001) but not AST (P < 0.883) activities in moderate drinkers were higher than those in abstainers. Mean ALT activity is higher in obese and over weight in alcohol consumers and abstainers as compared to mean AST activity in the same groups. ALP activity was increased with BMI in moderate drinkers. In abstainers activity of ALP shows weak relation in order to BMI. Conclusion: The result of moderate alcohol use raises activity of hepatic enzymes with increasing BMI. Most participants with alcohol consumption have an AST/ALT ratio above 1.
1. INTRODUCTION
Alcoholic hepatic syndrome is a world extensive health problem [1,2]. There are three most common type of alcoholic liver diseases are fatty liver/steatosis, alcoholic hepatitis and liver cirrhosis. The steatosis has been reported 80% in heavy drinkers, 10% - 35% alcoholic hepatitis, and about 10% liver cirrhosis [3,4]. Alcoholic hepatitis is liver inflammation cause by alcohol intake. The alcoholic hepatitis mostly develops in those people who use alcohol heavily from many years, the correlation between alcoholic hepatitis and drinking is complex. Not all heavy drinkers develop alcoholic hepatitis, this can also occur in moderate drinkers [5,6]. The alcohol in beer, wine and liquor consist of extremely lethal chemicals, like acetaldehyde. These chemicals cause inflammation that devastates liver cells. At this point, web-like scars and small loops of tissue replace with healthy liver tissue, which effects on the ability of liver function. This scarring called cirrhosis, is the last stage of alcoholic liver disease [6]. The swiftly increasing of obesity is a major hazard to modern health care. Overweight or obesity is a major threat in most of the industrialized countries [7]. During the last few decades the ethanol consumption medical disorders have increased quickly [8]. Moderate alcohol consumption with obesity may increase fat accumulation in hepatic tissue and changes in liver enzymes. The serum-liver enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyltransferase (GGT) are broadly used as indicator to assess the level of liver injury [9].
The aim of the present study was to observe the associations between alcohol consumption, BMI, and different liver enzymes in a healthy abstainers and drinkers.
2. SUBJECTS AND METHODS
2.1. Subjects and Study Protocol
The subjects of this study were residents of Karachi city, Pakistan. The population of healthy volunteers was 995 men and their range of age was (34 ± 17). The participants were classified as abstainers (n = 400, age 34 ± 17) and drinkers (n = 595, age 35 ± 19) and further classified according to BMI as shown in Table 1. The BMI value defined as weight (kg)/height (m2), was used as a weight index. Abstainers were those participants who used no alcohol during since last 3 to 4 months. Those participants who consumed alcohol during past 1 to 2 days before sampling were categorized as drinker. None of the participants had any history of medical disorders. In this study, 65% participants were smokers and 35% participants were nonsmokers. Smoking was not allowed for one hour before sampling. Hepatic enzymes (AST, ALT ALP and GGT) were measured with the help of Micro-Vita-Lab.
A Performa (survey and questionnaire form) was designed to collect the detail information from the donors indicating their name, age, residence, health status, alcohol intake and smoking habit.
2.2. Collection of Serum
About 10 cc of blood was collected by venepuncture using disposable sterilized syringes. It was collected in dry and clean test tubes and centrifuged at 3000 rpm for ten minutes to separate serum and kept at -70c. It was further used to study above mentioned parameters.
3. RESULTS
The key features of the participants according to weight status are shown in Table 1. Overweight and obesity were common in study population. The underweight participants were younger (P < 0.01) than participants of other BMI groups. The normal weight participants mean age was (35 ± 7), also younger than other groups of BMI. Mean alanine aminotransferase (ALT or SGPT), aspartate aminotransferase (AST or SGOT), γ- glutamyltransferase (GGT) and alkaline phosphate (ALP) values in the present study population noted either no alcohol use (abstainers; n = 400) or alcohol consume (n = 595). Hepatic enzyme ALT (P < 0.002), GGT (P < 0.001) and ALP (P < 0.001), enzymes activities in drinker were significantly higher than abstainers accept AST activity (P < 0.883) as presented in Figure 1. Mean distributions of alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), aspartate amino-transferase (AST) and alkaline phosphatase (ALP) activities in abstainers and moderate drinkers as further divided according to BMI (in kg/m2). Significant main effects of drinking status were observed (P < 0.001 for ALT, ALP and GGT). Significant main effects of BMI (P < 0.05) were noted for all enzymes. BMI < 19 was considered underweight, BMI ≥ 19 and < 25 was considered normal weight, BMI ≥ 25 and < 30 was considered overweight and BMI ≥ 30 was considered obese (Figure 2). The highest activities of hepatic enzymes were reported in those participants who had drinking status with obesity. In the present analyses between BMI and drinking status, liver enzymes effect in population as a dependent variable. Significant effects of BMI showed for all enzymes while drinking status was associated with ALT, GGT and ALP. ALP showed a marked difference among AST, ALT and GGT with regards to alcohol consumption and BMI.
Mean ALT activity is higher in obese and over weight in alcohol consumers and abstainers as compared to mean AST activity in the same groups. ALP activity was increased with BMI in moderate drinkers. ALP activity in abstainers shows weak relation in relation to BMI.
The values of AST: ALT above or below in the total population was also found to vary according to drinking status. Among the BMI subgroups, the distributions showed significant differences according to drinking status in participants with underweight and overweight (P < 0.05) among moderate drinkers and obese (P < 0.001). But no significance difference in participants with normal weight (Figure 3).
The significance correlations between the body mass index and hepatic enzyme in the current study population were observed for ALT, GGT and ALP among moderate drinkers than abstainers (Table 2).
Statistical Methods
Values are expressed as mean ± SD. Comparisons were made with the Kruskal-Wallis test or the MannWhitney test when compare two groups. Correlations were calculated with Pearson’s product-moment correla-
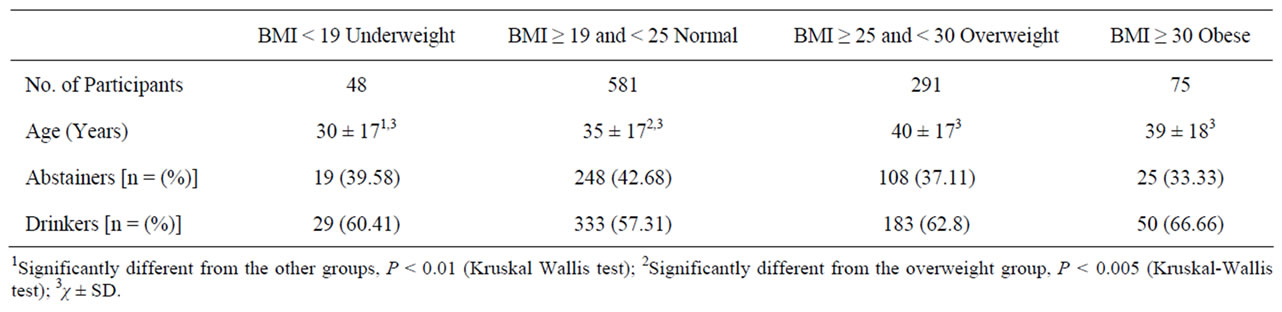
Table 1. Key Characteristics of the study participants.
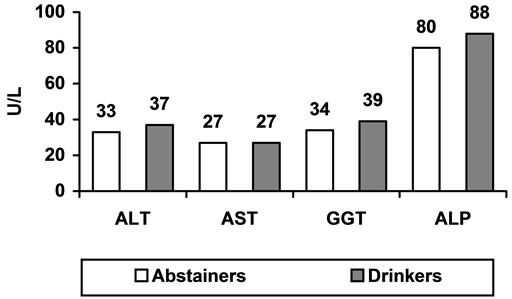
Figure 1. Mean values of liver enzymes in abstainers and moderate drinkers.
tion coefficients or with Spearman’s rank correlation, as required. The analyses were obtained with the help of SPSS statistical software after transformation of the raw data to obtain symmetrical distributions.
4. DISCUSSION
The present statistics shows that effect of moderate drinking on hepatic enzymes increased with increases of BMI. On the contrary, it may be said that increased drinking increases the effect of adiposity on liver activity.The impacts of alcohol or obesity on liver were re-
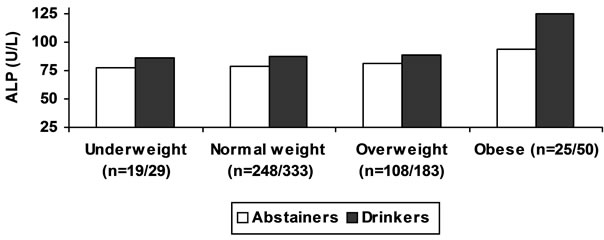
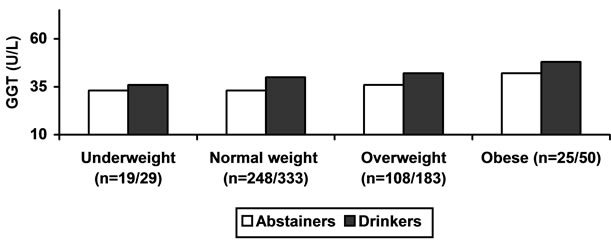
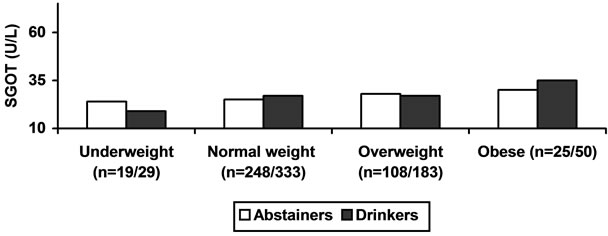
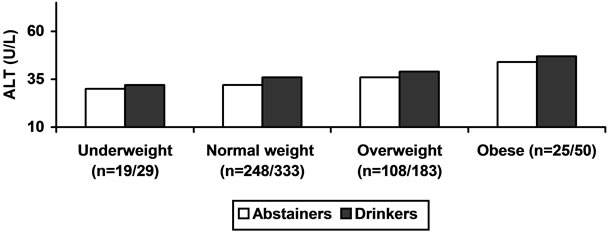
Figure 2. ALCOHOL, BMI and LIVER ENZYMES.
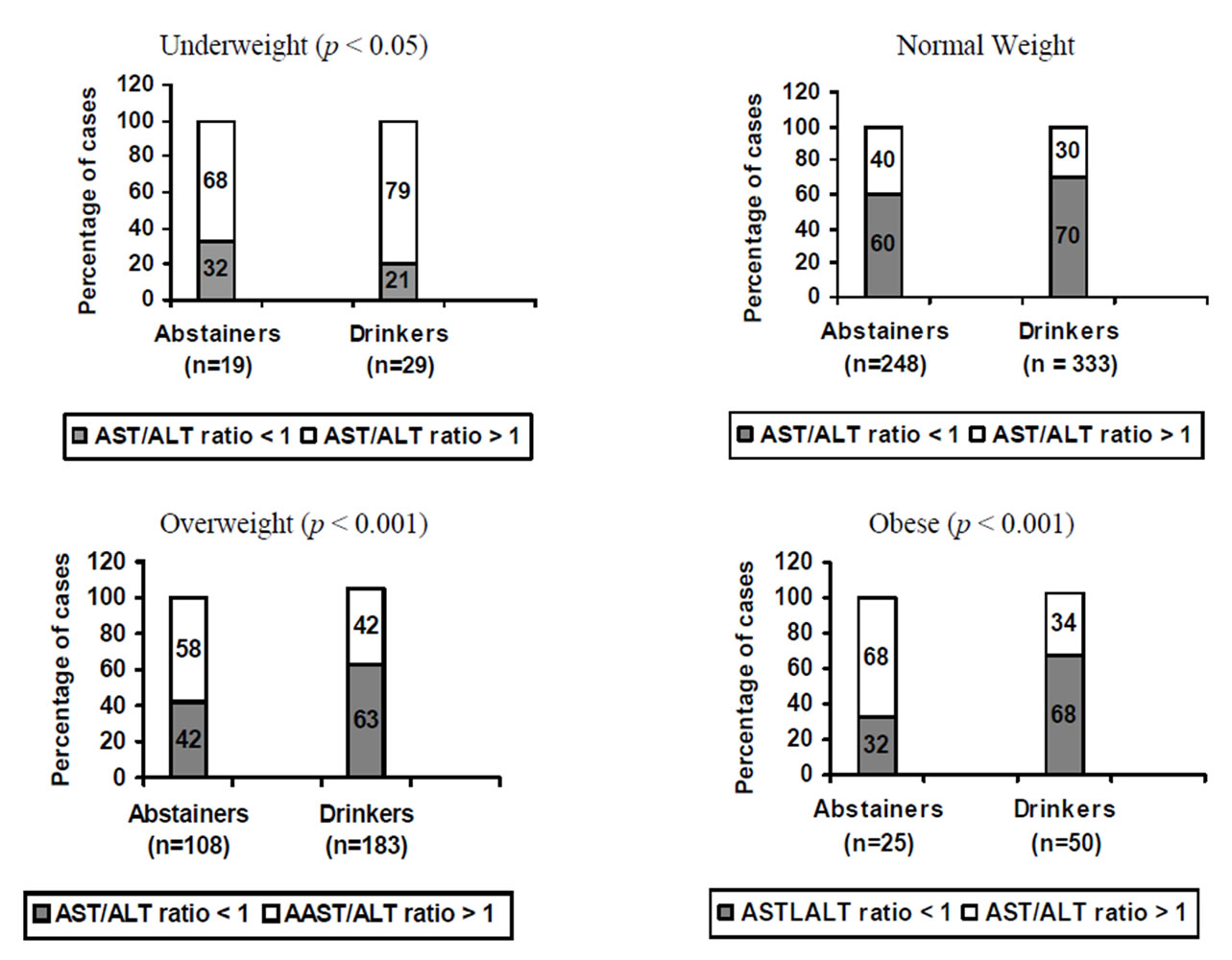
Figure 3. ALCOHOL, BMI and AST/ALT ratio.
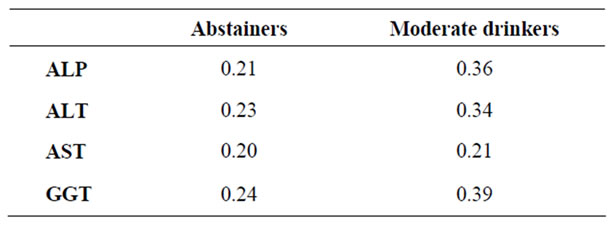
Table 2. Spearman’s correlation between BMI and hepatic enzymes in abstainers and moderate drinkers.
ported in many studies [8,10] but few researchers have reported the combined effects of moderate alcohol consumption and obesity on the biochemical changes that reflect health status [10,11]. In previous investigation alcohol consumption and obesity may raise serum GGT [12]. High risk of alcohol is observed related to be irregular aminotransferase activities in overweight and obese persons [13]. The current investigation in a huge populace of abstainers and moderate drinkers showed distinctive effects of moderate alcohol consumption and overweight on GGT enzyme activities. These findings show that obesity along with alcohol consumption may lead to raise metabolic burden and risk of liver problems. Even though the prime function of liver enzyme GGT is to metabolize extracellular reduced glutathione and GGT enzyme induction may also be strongly related with the generation of reactive oxygen species [14-16]. AST and ALT had no relationship with alcohol consumption although there is association between alcohol consumption with GGT [17] but in our study AST and GGT showed association with alcohol consumption. There is significant relationship between alcohol and GGT [18]. The serum GGT level showed a strong correlation between BMI changes [17] same as present findings. The significance of BMI change means that the effects of adiposity may be momentary and the change in BMI was the strongest determinant of change in GGT [18]. Present observation proposed that hepatic enzymes could be directly proportional with weight change. Normal or faintly abnormal LFTs do not rule out abundant liver diseases even advanced cirrhosis [19]. Among liver enzymes, the activities of ALT were found to be related with BMI [20]. Among overweight and obese females mean ALT value did not show any significant change from normal, whereas in overweight and obese males it was significantly high [21]. In current study ALT shows significant difference (P < 0.001) in obese male from normal. This is comparable to prior findings that obese persons may have higher levels of serum transaminases than their corresponding person [22].
In present study, most of the participants with alcohol consumption have an AST/ALT ratio above 1. The current study shows that there may be particular changes in the enzyme ratios also among healthy persons with only little changes in ALT and AST activities, signifying that the ratio of AST/ALT may depend on drinking status and BMI (Figure 2).
In patients with increased serum aminotransferase activity, the predominance of AST over ALT in alcoholic liver disease was first studied by Harinasuta. in 1967 [23]. The investigative significance of a high AST/ALT ratio for alcohol-related hepatic disease was reported [24]. The high AST/ALT ratio in alcoholic liver disease: 1) a decrease hepatic ALT activity [25]; 2) pyridoxal 5- phosphate diminution in the livers of alcoholics [26]; and 3) damage of mitochondrial in patients with high alcohol consumption may increase activity of mitochondrial aspartate in serum [27]. In early report high AST/ALT ratio with increased serum aminotransferase activity has also been correlated with the growth of cirrhosis in Nonalcoholic Steatohepatitis patients [28]. A high AST/ALT ratio in patients with increased serum aminotransferases has been observed in chronic viral hepatitis [29-33].
In current study mean ALT values in overweight and obese male abstainers and drinkers remained higher than the mean AST values in the same groups (Figure 3). While the ALT levels are higher than AST levels in most cases of nonalcoholic steatohepatitis (NASH) [34], the AST level may sporadically be higher than the ALT level, particularly in the presence of cirrhosis [35].
Enzyme levels often do not correlate with the severity of histological abnormalities [36] that varies from alcoholic hepatitis in which the ALT activity is higher than AST [35].
In current study observed that ALP is gradually increase with BMI in both groups but significantly higher in obese moderate alcohol consumers and ALP serum levels are higher in obese than in lean subjects. In contrast to previous study in which serum ALP levels were measured in 32,329 subjects [37]. It is possible that the higher level of liver ALP in obese than in lean subjects is a result of ALP release from adipose tissue. Liver ALP is a tissue non-specific alkaline phosphate (TNALP) isoenzyme (as are bone and kidney ALP), and it is known that TNALP is present in human preadipocytes16 and in the murine preadipocyte cell, 3T3-L1.15 Therefore, the association of liver but not bone serum ALP levels with obesity suggests that the TNALP isoform in adipose tissue may be the liver form.
In conclusion, although alcohol is certainly one of the most serious causes of high serum activities of the hepatic enzymes in subjects examined during study, marked overweight is potentially another important risk factor in the increase of liver enzymatic activities.
![]()
![]()
REFERENCES
- Kumar, V., Cotran, R. and Robbins, S. (2003) Robbins Basic Pathology. 7th Edition, Saunders, Philadephlia.
- Kim, J., Chu, S.K., Kim, K. and Moon, R.J. (2011) Alcohol use behaviors and risk of metabolic syndrome in South Korean middle-aged men. BMC Public Health, 11, 489-496.
- Thurman, R.G. (1998) Alcoholic liver injury involves activation of Kupffer cells by endotoxin. American Journal of Physiology, 275, 605-611.
- Tsukamoto, H. and Lu, S.C. (2001) Current concepts in the pathogenesis of alcoholic liver injury. The FASEB Journal, 15, 1335-1349. doi:10.1096/fj.00-0650rev
- Kaplan, M.M. (1972). Alkaline phosphatase. Gastroenterology, 62, 452-468.
- Salz, J.L., Daum, F. and Cohen, M.I. (1973) Serum alkaline phosphatase activity during adolescence. Journal of Pediatric, 8, 536-537. doi:10.1016/S0022-3476(73)80140-4
- Conway, B. and Rene, A. (2004) Obesity as a disease: No lightweight matter. Obesity Review, 5, 145-151. doi:10.1111/j.1467-789X.2004.00144.x
- Room, R., Babor, T. and Rehm, J. (2005) Alcohol and public health. Lancet, 365, 519-530.
- Niemela, O. (2007). Biomarkers in alcoholism. Clinica Chimica Acta, 377, 39-49. doi:10.1016/j.cca.2006.08.035
- Stranges, S., Dorn, J.M., Muti, P., et al. (2004) Body fat distribution, relative weight, and liver enzyme levels: A population-based study. Hepatology, 39, 754-763. doi:10.1002/hep.20149
- Rohrer, J.E., Rohland, B.M., Denison, A. and Way, A. (2005) Frequency of alcohol use and obesity in community medicine patients. BMC Family Practice, 6, 17. doi:10.1186/1471-2296-6-17
- Puukka, K., Hietala, J., Koivisto, H., Anttila, P., Bloigu, R. and Niemela O. (2006) Additive effects of moderate drinking and obesity on serum gammaglutamyl transferase activity. The American Journal of Clinical Nutrition, 83, 1351-1354.
- Ruhl, C.E. and Everhart, J.E. (2005) Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clinical Gastroenterology and Hepatology, 3, 1260-1268. doi:10.1016/S1542-3565(05)00743-3
- Browning, J.D. and Horton, J.D. (2004) Molecular mediators of hepatic steatosis and liver injury. The Journal of Clinical Investigation, 114, 147-152.
- Furukawa, S., Fujita, T., Shimabukuro, M., et al. (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of Clinical Investigation, 114, 1752-1761.
- Lee, D.H., Blomhoff, R. and Jacobs, D.R. (2004) Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radical Research, 38, 535-539. doi:10.1080/10715760410001694026
- Lee, D.H., Ha, M.H. and Christiani, D.C. (2001) Body weight, alcohol. Consumption and liver enzyme activity—A 4-years follow-up study. International. Journal of Epidemology, 30, 766-770. doi:10.1093/ije/30.4.766
- Nilsen, O. and Forde, O.H. (1994) Seven years longitudinal population study of change in gamma-glutamyltransferase: The Tromos study. American Journal of Epidemology, 139, 787-792.
- Jessic, R., Krstic, M. and Tomic, D. (2001) Modern functional diagnostics in liver diseases. Journal of Archive Gastroenterology Hepatology, 20, 1-6.
- Burns, C.J., Boswell, J.M. and Olsen, G.W. (1996) Liver enzyme activity and body mass index. Journal of Occupational and Environmental Medicine, 38, 1248-1252. doi:10.1097/00043764-199612000-00010
- Qureshi, I.Z, Shabana, A. and Fareeha, A. (2006) Effect of overweight and obesity on liver function in a samples from Pakistani population. Journal of Zoology, 38, 49-54.
- Ravussin, E., Burnand, B., Schutz, Y. and Jequier, E. (1982) Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. The American Journal of Clinical Nutrition, 35, 566-573.
- Harinasuta, U., Chomet, B., Ishak, K. and Zimmerman, H.J. (1967) Steatonecrosis-Mallory body type. Medicine, 46, 141-162. doi:10.1097/00005792-196703000-00008
- McCullough, A.J. and O’Connor, J.F.B. (1998) Alcoholic liver disease: Proposed recommendations for the American College of Gastroenterology. The American Journal of Gastroenterology, 93, 2022-2036. doi:10.1111/j.1572-0241.1998.00587.x
- Maltoff, D.S., Selinger, M.J. and Kaplan, M.M. (1980) Hepatic transaminase activity in alocholic liver disease. Gastroenterology, 78, 1389-1392.
- Diehl, A.M., Potter, J., Boitnott, J., Van Duyn, M.A., Herlong, H.F. and Mezey, E. (1984) Relationship between pyridoxal 5-Phosphate deficiency and aminotransferase levels in alcoholic hepatitis. Gastroenterology, 86, 632-636.
- Nalpas, B., Vassault, A., Le Guillou, A., Lesgourgues, B., Ferry, N., Lacour, B. and Berthelot, P. (1984) Serum activity of mitochondrial aspartate aminotransferase: A sensitive marker of alcoholism with or without alcoholic hepatitis. Hepatology, 4, 893-896. doi:10.1002/hep.1840040517
- Sorbi, D., Boynton, J. and Lindor, K.D. (1999) The ratio of aspartate aminotransferase to alanine aminotransferase: Potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. The American Journal of Gastroenterology, 94, 1018-1022. doi:10.1111/j.1572-0241.1999.01006.x
- Anderson, F. H., Lecheng, Z., Rock, N. R. and Yoshida, E. M. (2000) An assessment of the clinical utility of serum ALT and AST in chronic hepatitis C. Hepatology Research, 18, 63-71. doi:10.1016/S1386-6346(99)00085-6
- Assy, N. and Minuk, G.Y. (2000) Serum aspartate but not alanine aminotransferase levels help to predict the histological features of chronic hepatitis C viral infections in adults. The American Journal of Gastroenterology, 95, 1545-1550. doi:10.1111/j.1572-0241.2000.02027.x
- Pohl, A., Behling, C., Oliver, D., Kilani, M., Monson, P. and Hassanein, T. (2001) Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. The American Journal of Gastroenterology, 96, 3142-3146. doi:10.1111/j.1572-0241.2001.05268.x
- Giannini, E., Risso, D. and Testa, R. (2001) Transportability and reproducibility of the AST/ALT ratio in chronic hepatitis C patients. The American Journal of Gastroenterology, 96, 918-919. doi:10.1111/j.1572-0241.2001.03646.x
- Giannini, E., Risso, D., Botta, F., Chiarbonello, B., Fasoli, A., Malfatti, F., Romagnoli, P., Testa, E., Ceppa, P. and Testa, R. (2003) Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Archives of Internal Medicine, 163, 218-224. doi:10.1001/archinte.163.2.218
- Pinto, H.C., Baptista, A., Camilo, M.E., Valante, A., Saragoca, A. and De-Moura, M.C. (1996) Nonalcoholic steatohepatitis. Clinicopathological comparison with alcoholic hepatitis in ambulatory and hospitalized patients. Digestive Diseases and Sciences, 41, 172-179. doi:10.1007/BF02208601
- Bacon, B.R., Farahvash, M.J., Janney, C.G. and Neuschwander-Tetri, B.A. (1994) Nonalcoholic steatohepatitis: An expanded clinical entity. Gastroenterology, 107, 1103- 1109.
- Matteoni, C.A., Younossi, Z. M., Gramlich, T.L., et al. (1999) Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology, 116, 1413-1419. doi:10.1016/S0016-5085(99)70506-8
- Schiele, F., Henny, J., Hitz, J., et al. (1983) Total bone and liver alkaline phosphatase in plasma: Biological variations and reference limits. Journal of Clinical Chemistry, 29, 634-641.

