Materials Sciences and Applications
Vol.06 No.11(2015), Article ID:61516,10 pages
10.4236/msa.2015.611105
Corrosion Protection of Iron Alloy Using Peganum harmala Extract as Inhibitor in Acidic Solution
Khulood Abid Al-Saadie1*, Hayfaa Ameer Abas1, Haider Abdulkareem Yousif Almashhdani2
1Department of Chemistry, College of Science, Baghdad University, Baghdad, Iraq
2Department of Dentistry, Al-Rasheed University College, Baghdad, Iraq

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 18 October 2015; accepted 24 November 2015; published 27 November 2015

ABSTRACT
This work describes the inhibition effect of different concentration of an aqueous extracted of Peganum harmala seed on the corrosion of carbon steel in 1 M H2SO4 solution using potentiostatic polarization techniques at the temperature range (298 - 328) K. The result showed that the inhibition efficiency increased with increasing of extract inhibitor concentration and temperature. The adsorption of inhibitor on carbon steel surface was fit into Langmuir adsorption isotherm. Kinetic and thermodynamic parameters governing the corrosion and the adsorption process were calculated and discussed.
Keywords:
Corrosion, Carbon Steel, Seed, Peganum harmala, Acidic Solution

1. Introduction
Corrosion is recognized as the number one life limiting plant degradation mechanism [1] and exhibited by most engineering materials in nearly all environments [2] . There are numerous methods for controlling the corrosion of metals but the use of inhibitors is still one of the best methods of protecting metals against corrosion. Many organic compounds are used as corrosion inhibitors for metals in acidic environments [3] . Corrosion inhibitors are substance that when adsorbed onto the metal-solution interface, blocks the corrosive material from coming into contact with metal. A number of heterocyclic compounds containing nitrogen, oxygen and sulphur either in the aromatic or in long chain carbon system have been reported to be effective inhibitors [4] . Extracts of various parts of the plant have been used as corrosion inhibitors [5] the yield of these compounds as well as their corrosion inhibition abilities varies widely depending on the part of the plant and its location.
The extracts from the leaves, seeds heartwood, bark root and fruit of plant have been reported to inhibit metallic corrosion in acidic media [6] . The plant extracts become important as eco-friendly, economical, readily available and renewable sources of effective corrosion inhibitors [7] . The seeds of Peganum harmala (Syrian rue, harmal), is a herbaceous perennial of the family Zygophyllaceae native to countries around the Mediterranean sea, the Middle East, India, Pakistan, South Australia, and Western United States, [8] . Peganum harmala is found growing wild in the middle and northern part of Iraq. The plant is rich in alkaloids and contains up to 4% total alkaloids [9] . The chemical structures of these compounds are as shown in Figure 1. Seeds and roots contain the highest levels of alkaloids with low levels in stems and leaves, and absent in flowers. Harmine and harmaline accumulate in dry seeds at 4.3% and 5.6% (w/w), respectively, and 0.6% of harmalol [10] .
Carbon steel is one of the major construction materials which are extensively used on chemical and allied industries for the handling of acid, alkali and salt solutions [11] and widely used in most of the chemical industries due to its low cost and easy availability for fabrication of various reaction vessels. It suffers from severe corrosion in aggressive environment [12] . Therefore, the need arises to examine their corrosion behavior so as to reduce or stop their corrosion [13] . Acid has been used for drilling operation, pickling bath and in de-scaling process [14] . Sulfuric acid is more produced than any other chemical in the world. It is used directly or indirectly in nearly all industries and is a vital commodity in our national economy [15] .
2. Materials and Methods
For all the studies, the specimens took of sheet carbon steel with following composition.
The sheet of carbon steel (C.S.) was mechanically pressed cut into circular with 2.5 cm diameter, used as a flat working electrode and the exposed measuring surface was 1 cm2, each coupon were polished to mirror finish with grade emery polishing papers washed with distilled water, degreased with acetone and dried in air , saved in a desiccators. Double distilled water was used for the preparation of all reagent used in this study. The seeds of Peganum harmala for this investigation collected from local market. An aqueous extract was prepared by grinding different weight (0.5, 1, 2, 4) grams of dry seeds with 1000 ml of distilled hot water, continuous extraction three time, filtering and used freshly. Electrochemical measurement were carried out in glass cell with a capacity of 1000 ml, a platinum electrode and silver-silver chloride electrode were used as a counter electrode and reference electrode respectively, the carbon steel(working electrode) sheet was placed in the test solution (uninhibited and inhibited solution) for 15 minutes before electrochemical measurement. Polarization studies were conducted in an electrochemical measurement unit [model 200 (2007) Germany]. The Tafel polarization measurement was made for a potential range (−200 to +200) mV with respect to open circuit potential.
3. Results and Discussion
3.1. The Corrosion Process
Electrochemical corrosion parameters such as corrosion potential (Ecorr), cathodic and anodic Tafel slopes (bc, ba)
Figure 1. The chemical structure of β-carboline alkaloids (harmine, harmaline, and harmalol isolated from Peganum harmala seeds.
and corrosion current density (Icorr) obtained by extrapolation of anodic and cathodic regions of the Tafel lines, are given in Table 1. The date of the table shows that the corrosion current density (which is directly proportion with the rate constant (k) for corrosion process), increased with increasing the temperature in absence and presence of different Peganum harmala extraction (p.h.e.) concentration. The corrosion potential (Ecorr) values is slightly shifted toward both positive and negative in the presence of different Peganum harmala extraction concentration, indicating that the inhibitor acted as a mixed type inhibitor [16] . The way in which a metal changes its potential upon immersion in solutions indicates the nature of reaction taking place at its surface. A shift in potential towards more positive values denotes film formation and thickening, a shift in the negative direction signifies film destruction and the exposure of more of the bare metal to the aggressive solution. The results obtained were made to be used to discuss the mechanism of oxide film growth or corrosion of the metal in solutions [17] . Figure 2 shows the Tafel polarization curves for the corrosion of C.S. in 1M-H2SO4 solution in different p.h.e. Concentrations at 25˚C. Similar results were obtained at the other temperature involved in study.
Table 1. Electrochemical corrosion parameters for carbon steel in 1 M H2SO4 solution containing different concentrations of P.h.e. at temperature range (298 - 328)K.
Figure 2. Potentiostatic polarization curves for carbon steel in 1 M H2SO4 without and with different P.h.e. concentrations at 298.
The results show that the C.S. corrosion rate decreases with increase P.h.e. concentration but increases with increase in temperature.
The inhibition efficiency IE% was calculated using the following equation [18]
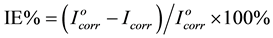 (1)
(1)
where
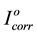 and Icorr are the corrosion current densities in absence and presence of P.h.e. respectively. The values of the inhibition efficiencies (IE), increases with increase temperature from (298 to 328) K as shown in Figure 3. This can be attributed to the increase in the protective inhibitive film that formed on carbon steel surface at higher temperatures.
and Icorr are the corrosion current densities in absence and presence of P.h.e. respectively. The values of the inhibition efficiencies (IE), increases with increase temperature from (298 to 328) K as shown in Figure 3. This can be attributed to the increase in the protective inhibitive film that formed on carbon steel surface at higher temperatures.
The influence of temperature on the kinetic process of corrosion in free acid and the presence of adsorbed inhibitor leads to get more information on the electrochemical behavior of metallic materials in aggressive media [19] . The corrosion reaction of C.S. in sulphuric acidic media depends on temperature, the dependence of the rate constant k of corrosion process on the temperature T is the expressed by the Arrhenius law [20] how predicts that the corrosion rate increases with the temperature and Ea and A may vary with temperature.
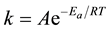 (2)
(2)
The linear regression plots between logk and 1/T are presented in Figure 4. The calculated activation energies (Ea) and pre-exponential factors (A) at different concentrations of the inhibitor are listed in Table 2.
Figure 3. Variation of inhibition efficiency with temperature at various concentrations.
Figure 4. Plot of logk versus 1/T for the corrosion of carbon steel in 1 M H2SO4 containing various concentration of p.h.e.
Table 2. Corrosion kinetics parameters of C.S. in 1 M H2SO4 solution containing various concentration of P.h.e.
The calculated values of activation energies ranged from 91.33 to 40.01 kJ∙mol−1 in the presence and absence of inhibitor respectively. From the results it is apparent that the activation energies for the corrosion of carbon steel in the presence of Peganum harmala extract decreased with increase in the inhibitor concentration. The decrease of Ea values with increasing concentrations of inhibitor is attributed to an appreciable increase in the degree of adsorption on the inhibitor on metal surface with corresponding decrease in reaction rate [21] and this type of inhibitor diminished corrosion at ordinary temperature but at elevated at higher temperature it can Suggest that the energy barrier of corrosion reaction decreases with the inhibition concentration the lower values of Ea than the threshold value of 80 KJ∙mol?1 required for chemical adsorption indicated that the adsorption of P.h.e. on carbon steel surface is physical adsorption [22] .
Other kinetic date (enthalpy and entropy of activation) are accessible using the alternative formulation of Arrhenius equation [23] .
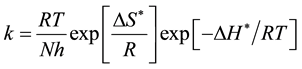 (3)
(3)
where h, is plank’s constant N, is Avogadro number ΔS* and ΔH* are the entropy and enthalpy of activation complex respectively. Figure 5 shows plots of log(k/T) vs. the reciprocal of temperature T show straight lines with a slope equal to (−ΔH*/2.303R) and an intercept of (LogR/Nh + ΔS*/2.303R) the values of ΔH* and ΔS*are also presented in Table 2. ΔH* values in the presence of inhibitor ranged from −71.53 to −37.08 kJ/mol (mean = 46.15) indicating that the processin presnt of Peganum harmala extract on the carbon steel surface is endothermic. A negative values of ΔS* ranged from 7.29 - (−111.0) kJ∙mol−1 (mean = −77.82) indicate that the activated complex may be the rate-determining step and represents association rather than dissociation meaning that a decrease in Disordering takes place on going from reactants to the activated complex [24] .
3.2. Adsorption Process
In order to understand the mechanism of corrosion inhibition, the adsorption behavior of the adsorbate on the carbon steel surface must be known. The degree of surface coverage (θ) for different concentrations of inhibitor was evaluated from potentiostatic polarization measurements data by using the flowing Equation (16).
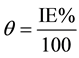 (4)
(4)
where IE(%) is the percentage inhibition efficiency as calculated using Equation (1). The data was applied to various isotherms including Langmuir, Temkinand and Frumkin isotherms. It was found that the data best fits with Langmuir adsorption isotherm and can be represented by using the flowing relation [20] .
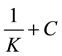 (5)
(5)
where K is the adsorption/desorption equilibrium constant, C is the inhibitor concentration in the solution, and (θ) is the surface coverage. The plot of C/θ vs. C. gave a straight line with an intercept of 1/K. The results obtained isotherm is presented in Table 3, it shows that the R2 values are very close unity, indicating the obey Langmuir adsorption isotherm. Figure 6 shows the Langmuir adsorption isotherms for the adsorption of p.h.e. on the carbon steel surface the values of K denotes the strength between the adsorbate and adsorbent. Large values of K imply more efficient adsorption and hence better protection efficiency [25] .
Thermodynamic function for the adsorption process (ΔHads, ΔSads, ΔGads) can be calculated by using the known formulas [26] .
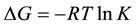 (6)
(6)
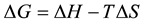 (7)
(7)
Figure 7 show a linear relationship between lnK and 1/T with the slop represented (−ΔHads/R) and the intercept represents (ΔSads/R). The thermodynamic function calculated are listed in Table 3.
Figure 5. Plot of log(k/T) versus 1/T for the corrosion of carbon steel in 1M H2SO4 containing various concentration of P.h.e.
Figure 6. Langmuir isotherm plot for the adsorption of p.h.e. on the surface of C.S.
Figure 7. lnK verses 1/T for adsorption of P.h.e. on C.S. in 1 M H2SO4.
Generally, values of ΔGads around −20 kJ∙mol−1 or lower are consistent with the electrostatic interaction between the charged molecules and the charged metal (physisorption); those lower −20 kJ∙mol−1, or higher involve charge sharing or charge transfer from organic molecules to the metal surface to form a coordinate type of bond (chemisorption) [27] . The positive values of ΔSads suggested that the adsorption is coupled with an increase of the system disorder due to the adsorption of inhibitor on the carbon steel surface. The positive value of enthalpy (ΔHads) of adsorption indicates that the adsorption of the inhibitor molecule is an endothermic process.
3.3. Fourier Transforms Infrared (FT-IR) Spectroscopy
Fourier Transformation Infrared Spectra for P.h. e. seed and the corrosion product are represented as shown in (Figure 8 and Figure 9) respectively. A strong a stretching O-H band at 3415 cm−1, N-H Strchingat 3247, 90 cm−1, C = N stretching in 1276.72 cm−1, C=C stretching at 1641 cm−1 and N-H bending at 1631.67 cm−1.
It is clear from comparison between FT-IR spectra for isolated Peganum harmala seed extract and it film on the metal surface, that the absorbance frequency of all functional groups have been shifted to less value due to the force of binding between the interface of metal surface and the inhibitor, Table 4. Also the figures appear that the vibration of all functional group that affected by inhibition process have a less intensity in transition bands.
Table 3. The thermodynamic function of the adsorption process.
Table 4. Wave number of FT-IR adsorption for pure P.h.e. and P.h.e. as corrosion inhibitor.
Figure 8. FT-IR spectra of pure Peganum harmala seed.
3.4. Atomic Force Microscope (AFM)
AFM analysis can be used to prove that adsorption of inhibitor was happen on C.S. surface by follow the different in particle size distribution between the free metal surface represented in Figure 10 and for inhibitor crystals extracted Figure 11 and the distribution of particle for the corrode metal in the presence of P.h.e. inhibitor Figure 12. It is clear that the inhibitor covered C.S. with a protected layer with average diameter of 108.93 nm with highest volume percent reach to 20% reflected to particles diameter 90 nm of inhibitor. The distribution of particles size of free metal was with average diameter 101.53 nm and for extracted inhibitor atom is 110 nm.
4. Conclusion
From the result, it seems that the addition of an aqueous extract of p.h.e. shifted the corrosion potential Ecorr to less negative potential, lowered current density (corrosion rate) and increased the inhibition efficiency %IE to attain 96.01%. This behavior reflects that the Peganum harmala extracts act as good corrosion inhibitor which
Figure 9. FT-IR spectra of P.h.e. film formed on the carbon steel surface after immersion in 1M H2SO4 solution.
Figure 10. 3D views and the distribution of particle for the AFM images of carbon steel specimen.
Figure 11. 3D views and the distribution of particle for the AFM images of P.h.e.
Figure 12. 3D views and the distribution of particle for the AFM images of P.h.e. Layers constructed on carbon steel specimen.
controlling the corrosion of carbon steel in aqueous solution of 1 M H2SO4. The adsorption behavior is investigated as a Langmuir adsorption isotherm and the process is attributable unequivocally to physical adsorption.
Cite this paper
Khulood AbidAl-Saadie,Hayfaa AmeerAbas,Haider Abdulkareem YousifAlmashhdani, (2015) Corrosion Protection of Iron Alloy Using Peganum harmala Extract as Inhibitor in Acidic Solution. Materials Sciences and Applications,06,1061-1070. doi: 10.4236/msa.2015.611105
References
- 1. Adamu, M., Ejibunu Umoru, L. and Oluremi Ige, O. (2014) Effect of Calcium Nitrate and Sodium Nitrite on the Rebar Corrosion of Medium Carbon Steel in Seawater and Cassava Fluid. Journal of Minerals and Materials Characterization and Engineering, 2, 223-229.
http://dx.doi.org/10.4236/jmmce.2014.23027 - 2. Adamu, M., Ejibunu Umoru, L. and Oluremi Ige, O. (2014) Effect of Toluene and Dioctylphthalate on the Rebar Corrosion of Medium Carbon Steel in Seawater and Cassava Fluid. Journal of Minerals and Materials Characterization and Engineering, 2, 1-7.
http://dx.doi.org/10.4236/jmmce.2014.21001 - 3. Tagbo Nwabanne, J. and Nwoye Okafor, V. (2012) Adsorption and Thermodynamics Study of the Inhibition of Corrosion of Mild Steel in H2SO4 Medium Using Vernonia amygdalina. Journal of Minerals and Materials Characterization and Engineering, 11, 885-890.
http://dx.doi.org/10.4236/jmmce.2012.119083 - 4. Kathirve, K., Thirumalairaj, B. and Jaganathan, M. (2014) Quantum Chemical Studies on the Corrosion Inhibition of Mild Steel by Piperidin-4-One Derivatives in 1 M H3PO4. Open Journal of Metal, 4, 73-85.
- 5. Okafor, P., Ikpi, M., Uwah, I., Ebenso, E., Ekpe, U. and Umoren, S. (2008) Inhibitory Action of Phyllanthus amarus Extracts on the Corrosion of Mild Steel in Acidic Media. Corrosion Science, 50, 2310-2317.
http://dx.doi.org/10.1016/j.corsci.2008.05.009 - 6. Nwankwo, M., Offor, P., Neife, S., Oshionwu, L. and Ndubuisi, E. (2014) Amaranthus cordatus as a Green Corrosion Inhibitor for Mild Steel in H2SO4 and NaCl. Journal of Minerals and Materials Characterization and Engineering, 2, 194-199.
http://dx.doi.org/10.4236/jmmce.2014.23024 - 7. Oki, M., Charles, E., Alaka, C. and Kayode Oki, T. (2011) Corrosion Inhibition of Mild Steel in Hydrochloric Acid by Tannins Rom Rhizophora Racemosa. Journal of Materials Sciences and Applications, 2, 592-595.
- 8. Mustapha, F., Akino, Z., Abdellatif, J., Zafar, S.H. and Badiaa, L. (2013) Cytotoxicity of Alkaloids Isolated from Peganum harmala Seeds. Pakistan Journal of Pharmaceutical Science, 26, 699-706.
- 9. Minan, Y. (2010) Antimicrobial Effects of Aqueous and Alcoholic Extract of Peganum harmala L. Seeds on Two Types of Salivary Isolated Microorganisms in Al-Ramadi City. Journal of King Abdulaziz University—Medical Sciences, 17, 3-17.
- 10. Asgarpanah, J. and Ramezanloo Chemistry, F. (2012) Chemistry, Pharmacology and Medicinal Properties of Peganum harmala L. African Journal of Pharmacy and Pharmacology, 6, 1573-1580.
- 11. Noor, A. and Aisha, H. (2008) Corrosion Behavior of Mild Steel in Hydrochloric Acid Solutions. International Journal of Electrochemical Science, 3, 806-818.
- 12. Ogundare, O., Momoh, I., Akinribide, O., Adetunji, A., Borode, J. and Olusunle, S. (2012) Comparative Study of Corrosion Sensitivity of Selected Ferrous Metals in Crude Oil. Journal of Minerals & Materials Characterization & Engineering, 11, 559-568.
http://dx.doi.org/10.4236/jmmce.2012.116040 - 13. Matheswaran, P. and Ramasamy, A.K. (2010) A Study of Mild Steel Corrosion Using Adhatoda Vasica (AV) Extract as Inhibitor in Different Acid Medium. E-Journal of Chemistry, 7, 1284-1289.
http://dx.doi.org/10.1155/2010/546360 - 14. Matheswarn, P. and Ramasamy, A. (2010) A Study of Mild Steel Corrosion Using Adhatoda Vasica (AV) Extract as Inhibitor in Different Acid Medium. E-Journal of Chemistry, 7, 3-17.
- 15. Fontana, G. (1986) Corrosion Engineering. Third Edition, McGraw-Hill, New York, 225.
- 16. Cang, H., Fei, Z., Shao, J., Shi, W. and Xu, Q. (2013) Corrosion Inhibition of Mild Steel by Aloes Extract in HCl Solution Medium. International Journal of Electrochemical Science, 8, 720-734.
- 17. El Desouky, H. and Hisham, A. (2014) Effect of Chloride Concentration on the Corrosion Rate of Maraging Steel. Open Journal of Physical Chemistry, 4, 147-165.
http://dx.doi.org/10.4236/ojpc.2014.44018 - 18. Zeng, D. and Qin, W. (2012) Study on a Novel Composite Eco-Friendly Corrosion and Scale Inhibitor for Steel Surface in Simulated Cooling Water. Journal of Surface Engineered Materials and Advanced Technology, 2, 137-141.
http://dx.doi.org/10.4236/jsemat.2012.23022 - 19. Murgulescu, I. and Radovici, O. (1961) Corrosion Resistance and Polarization Characteristics of Al-Zn Alloys. 1st International Congress on Metallic Corrosion, London, 10-15 April 1961, 202-205.
- 20. Prabhu, D. and Rao, P. (2013) Garcinia indica as an Environmentally Safe Corrosion Inhibitor for Aluminium in 0.5 M Phosphoric Acid. International Journal of Corrosion, 2013, Article ID: 945143.
http://dx.doi.org/10.1155/2013/945143 - 21. Nnabuk, O., Eno, E. and Ebenso, E. (2010) Adsorption, Synergistic Inhibitive Effect and Quantum Chemical Studies of Ampicillin (AMP) and Halides for the Corrosion of Mild Steel in H2SO4. Journal of Applied Electrochemistry, 40, 445-456.
http://dx.doi.org/10.1007/s10800-009-0015-z - 22. Tagbo, J. and Okafor, V. (2012) Adsorption and Thermodynamics Study of the Inhibition of Corrosion of Mild Steel in H2SO4 Medium Using Vernonia amygdalina. Journal of Minerals and Materials Characterization and Engineering, 11, 885-890.
http://dx.doi.org/10.4236/jmmce.2012.119083 - 23. Dahmani, M., Et-Touhami, A., Al-Deyab, S.S., Hammouti, B. and Bouyanzer, A. (2010) Corrosion Inhibition of C38 Steel in 1 M HCl: A Comparative Study of Black Pepper Extract and Its Isolated Piperine. International Journal of Electrochemical Science, 5, 1060-1069.
- 24. Zarrouk, A., Dafali, A., Hammouti, B., Zarrok, H., Boukhris, S. and Zertoubi, M. (2010) Synthesis, Characterization and Comparative Study of Functionalized Quinoxaline Derivatives towards Corrosion of Copper in Nitric Acid Medium. International Journal of Electrochemical Science, 5, 46-55.
- 25. Khamis, A., Mahmoud, M., Mohamed, I. and El-Anadouli, B. (2014) Inhibitory Action of Quaternary Ammonium Bromide on Mild Steel and Synergistic Effect with Other Halide Ions in 0.5 M H2SO4. Journal of Advanced Research, 5, 637-646.
http://dx.doi.org/10.1016/j.jare.2013.09.003 - 26. Paunovic, M. and Schlesinger, M. (2006) Fundamentals of Electrochemical Deposition. Second Edition, John Wiley & Sons, Inc., Toronto, 81.
http://dx.doi.org/10.1002/0470009403 - 27. Cang, H., Fei, Z., Shi, W. and Xu, Q. (2012) Experimental and Theoretical Study for Corrosion Inhibition of Mild Steel by L-Cysteine. International Journal of Electrochemical Science, 7, 10121-10131.
NOTES
*Corresponding author.


















