Advances in Bioscience and Biotechnology
Vol.4 No.4A(2013), Article ID:30658,11 pages DOI:10.4236/abb.2013.44A013
Immune system as a new therapeutic target for antibiotics*
![]()
Early Arthritis Clinic, Institute of Rheumatology, Warsaw, Poland
Email: kwiatkowskabrygida@gmail.com
Copyright © 2013 Brygida Kwiatkowska et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 20th, 2013; revised March 14th, 2013; accepted April 26th, 2013
Keywords: Immunomodulatory; Macrolides; Tetracyclines; Sulphonamides
ABSTRACT
Since the discovery of penicillin by Fleming in 1928, the knowledge of the antibiotics’ spectrum and mechanism of action has been steadily increasing. Antibiotics became an effective tool in the fight against many pathogens, changing the prognosis of outcome for many serious diseases. It is already known that antibiotics not only have the antibacterial activity, but many of them—e.g. macrolides, sulphonamides and tetracyclines—have immunomodulating effect, affecting functions of lymphocytes, macrophages and costimulatory molecules, macrophage migration and phagocytosis, as well as proinflammatory cytokine secretion. The expanding knowledge of the effects of antibiotics on the immune system has brought with it new applications of antibiotics in organ transplantation, invasive cardiology and treatment of autoimmune diseases such as rheumatoid arthritis or asthma.
1. INTRODUCTION
The name antibiotics is derived from the Greek language: anti-against and bios life. The activity of B lactams is based on the bacterial cell death, for example, by interfering with the synthesis or increasing the permeability of the cell wall, synthesis of nucleic acids and proteins (bactericidal) or metabolism (bacteriostatic). The choice of antibiotic is guided by their selective toxicity to bacteria in the absence of specific toxicity to cells of the human body. Folk medicine intuitively applied the mixtures, in which antibiotics like tetracycline could be found and which Nubians consumed with the beer produced by bacteria of the genus Streptomyces or garlic containing allicin inhibiting the growth of microorganisms. Already in the nineteenth century the impact on bacteria by mold and bacterial growth inhibition was observed in the presence of others (“the life forces another life” Louis Pasteur). The discovery of penicillin by Flaming in 1928 and its industrial-scale synthesis in 1940 (Howard Florey and Ernst Chain) caused a real revolution in the treatment of infections. Today, it is already known that antibiotics possess not only antibacterial properties, but also many of them have immunomodulating effects. In some cases, the side effects have also been used in therapy, such as the effect on gastrointestinal motility of macrolides or tetracycline to (ADH) [1-3]. Raising awareness about the immunomodulatory effects of antibiotics helped to apply any derivatives thereof in the organ transplant and interventional cardiology (e.g. rapamycin-eluting stents). In discussing the various groups of antibiotics we emphasize their outside bacterial action and draw attention to the effects on the immune system (Table 1).
2. Β LACTAMS (FIGURE 1)
According to the chronology of the discoveries an antibiotic first used in modern medicine was penicillin and its derivatives. They all act by inhibiting the antibacterial enzymes involved in the biosynthesis of bacterial peptidoglycan and inhibiting penicillin binding proteins (PBP, penicillin binding protein), resulting in the activation of autolytic enzymes and finally providing to lysis bacteria. This group includes natural and semi-synthetic penicillins, cephalosporins, cephamycins, carbapenems and monobactams. What is characteristic for this group is the b lactam binder that breaks under the influence of b-lactamase produced by some bacteria. Beta-lactamase inhibitors, such as clavulanic acid and tazobactam are also classified as beta-lactam antibiotics. In addition to antimicrobial activity b lactams have influence on the inhibition of platelet function, may induce dysulfiric reac
Table 1. Classification presented antibiotics based on general mechanism of action.
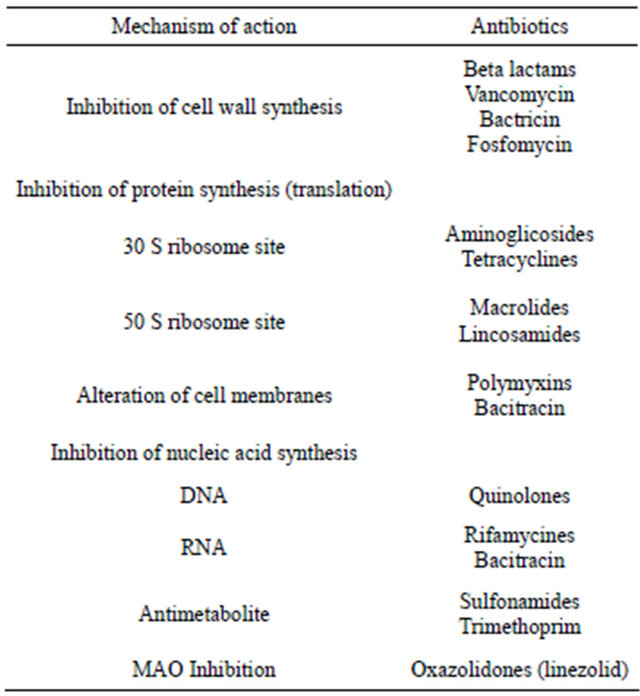
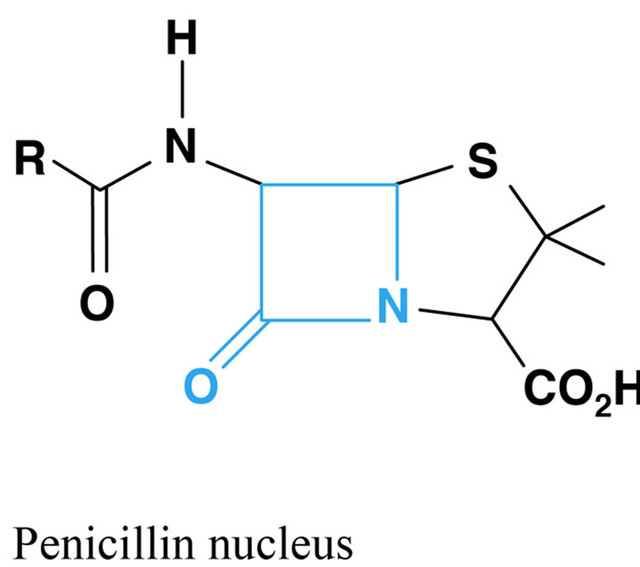
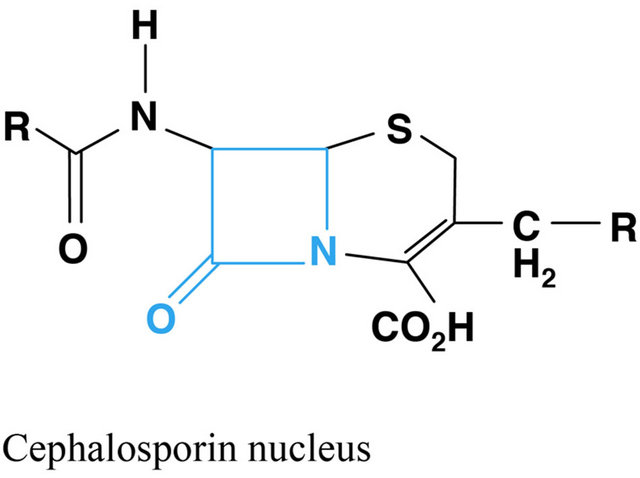
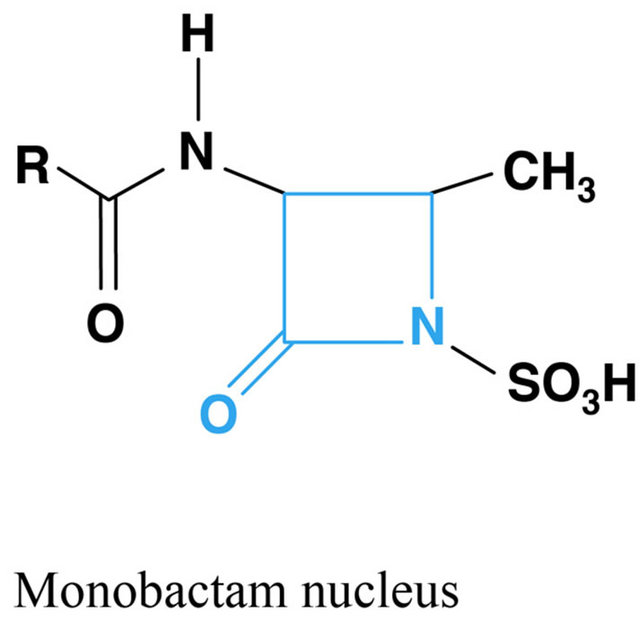

Figure 1. Chemical structure of beta lactams antibiotics.
tions and osmotic diuresis. It was also found that they influence human interferon gamma (IF γ)—but each of them have different influence on IF γ. Clavulanic acid, cefotaxime, phenoxymethylpenicyllin (V-cillin) and also ceftriaxone have the strongest inhibiting action on IF γ. Penicillin G and ampicillin act to a lesser extent, meropenem and aztreonam are the weakest and no such characteristic is found in d-penicyllamine. By inhibitory effects on IFγ b lactams have some impact on the increased production of IL 4 and thus promoting IgE synthesis by Th2 [8.9]. This dependence can be explained by the development of allergic reaction after using these drugs. IF γ, which belongs to class II interferons, is secreted by T cells (particularly Th1 and cytotoxic T lymph) and activates NK lymphocytes, increase antigen presentation and lysosomal activity of macrophages. There was no action of b lactams on other cytokines, however, by the impact of some of them on IF gamma they may have influence on the secretion of TNF or IL-1 [4]. Among b lactams cefaclor has the characteristics to promote phagocytosis by increasing chemotaxis and induction of the proinflammatory response of type 1 [5]. Ceftriaxone has an effect on glutamate transporter (GLT1), which is associated with some neurological disorders, including Amyotrophic Lateral Sclerosis (ALS), also epilepsy and stroke. It is thought that GLT1 protects against toxicity of glutamine released into the synaptic cleft. B lactams can enhance the activity of GLT1, as seems to be no increase in its synthesis. In a study of transgenic mice with familial ALS Ceftriaxone administration for 5 weeks resulted in less loss of neurons and muscle strength compared with those receiving placebo (sialine treated) [6]. It has been shown that cephalosporin analog Cefodyzime has the effect on phagocytic activity of granulocytes and oxygen-dependent bactericidal activity of neutrophils. Cefodyzime applied in in vitro studies stimulated proliferation of T and B lymphocytes [7].
3. MACROLIDES (FIGURE 2)
A family of different antibiotics produced by Streptomyces and some bacteria such as Arthrobacter sp containing one or more deoxy sugars (cladinose and desosamine). Their construction is based on the macrolacton ring which determines the different properties of macrolides. Lactone ring usually consist of 14 - 15 or 16- membered rings containing carbon. To macrolides which have 14-membered ring include: erythromycin, clarithromycin, dirithromycin, oleandomycin, roxithromycin, the 16-membered ring: josamycib, midecamycin, micamycin, spiromycin. Azithromycin is an azalide with 15-membered ring.
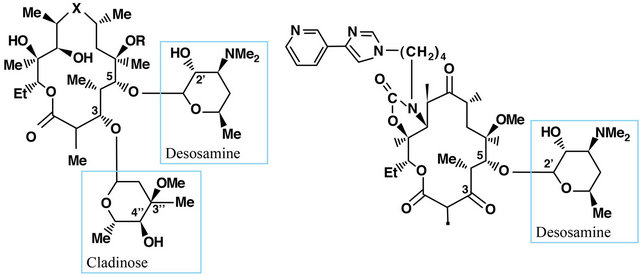
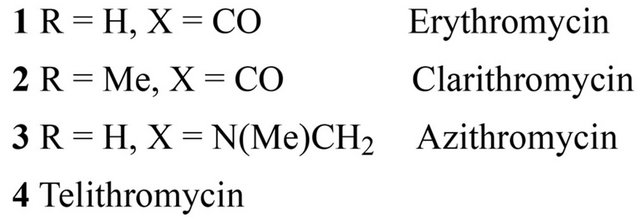
Figure 2. Chemical structure of 14-membered macrolides antibiotics.
Ketolides are also 14-membered ring as telithromycin and cetromycin. The mechanism of macrolide antibacterial action involves binding to the 50 S ribosome subunit, resulting in inhibition of protein synthesis at the ribosomal level [8,9]. Both macrolides and ketolides bind to the domain V of 23 S ribosomal RNA (rRNA), which is part of the 50 S subunit of the bacterial ribosome. Only 14- and 15-membered macrolides have the effect on cytokine secretion and pro-inflammatory effect and thus their antibacterial activity is irrespective of the inflammatory processes [10]. It has been proven that macrolides inhibit the production and secretion of IL-1β and TNF-α in monocytes [11], and IL-1β, IL-6, TNF-α and GM-CSF in mast cells [12], and IL-8 protein epithelial neutrophil activating (ENA-78), macrophage inflammatory protein (MIP-1) in macrophages and leukocytes [13]. It was also shown that clarithromycin inhibits the production of IL-6 and IL-1β by fibroblast-like cells of the synovial membrane [14]. Erythromycin and clarithromycin decrease the expression of IL-8 on mRNA level in bronchial epithelial cells of patients with chronic inflammatory airway disease. Erythromycin also affects the migration of neutrophils, cell proliferation [15] and differentiation of monocytes. Erythromycin and roxitromycin exhibit antioxidant properties and prevent the activation of NF-κB. They also inhibit the release of eosinophil-chemotactins, cytokines RANTES and eotaxin in lung fibroblasts [16]. Immunomodulatory effect of macrolides has been documented in the treatment of rheumatoid arthritis (RA), still’s disease in adults and inflammatory bowel diseases as well as in adjuvant arthritis. In the case of RA the efficacy of roxithromycin and clarithromycin was demonstrated. The use of roxithromycin (300 mg 1 time per day for 3 months) in patients with early rheumatoid arthritis (eRA), with no other disease-modifying therapy (DMARD) showed statistically significant higher efficacy compared to placebo [17]. This study involved a small group of patients (16 treated with roxithromycin and 15 in the placebo arm), so the results require confirmation in a larger population of patients. Roxithromycin has inhibitory effects on angiogenesis through the vascular entothelial growth factor (VEGF)-induced TNF-alpha [18]. Similarly good results in patients with early RA and DMARD treatment were achieved without the use of clarithromycin (500 mg 1 time daily for 6 months) in the group of 81 patients. In the clarithromycin-treated group compared to placebo ACR 20.50 and 70 (percentage of improvement in tender or swollen joint counts) vs. reached turn 59. 33%, 34% vs. 10% and 20% vs. 3 and these differences were statistically significant. [19] Thus, antibiotic macrolides may become a new therapeutic option in the treatment of early RA. In Still’s disease onset in adults clarithromycin has proved to be highly effective (500 mg 2 times daily) in patients who do not respond to standard treatment of the disease, i.e. glucocorticoids combined with methotrexate. In all patients a reduction occurred until the normalization of TNF-α and IL-6 [20,21]. In vivo studies demonstrated the efficacy of azithromycin and erythromycin for the treatment of adjuvant arthritis. It has been shown that these antibiotics reduce the activity of circulating lysosomal enzymes [22]. It has also been demonstrated that azithromycin derivative CSY0073 has no antibacterial activity but had the immunoregulatory effect in experimental Inflammatory Bowel Disease as in rheumatoid arthritis patients [23]. Pimecrolimus and tacrolimus can be named mong novel macrolide derivatives without direct antibacterial activity. They act in the cytoplasm of the target cell with a specific receptor protein called macrophilin-12, referred to as tacrolimus binding protein-FKBP (FK506 binding-protein). Tacrolimus/pimecrolimus-macrofilin-12 blocks calcineurin complex. Tacrolimus is an immunosuppressive drug the activity of which is similar to that of cyclosporine. Calcineurin inhibition leads to the lack of expression of the genes of many mediators of inflammation. Another drug of macrolide construction if sirolimus (rapamycin), acting in a complex with cytosolic protein FK-BP 12 and causing inhibition of TOR (target of rapamycin), and thus inhibiting the intracellular signal pathway [24]. It acts on T cells and influences angiogenesis by reducing the production of VEGF. The new derivatives of macrolides are used in the treatment of psoriasis and other skin diseases, but also in transplantation, interventional cardiology (stents coated) [24]. The study on the effect of macrolides (rapamycin) on the phenomenon of apoptosis may open the possibilities to treatment of such diseases as progeria [25]. Therefore, research is carried out on new drugs with the structure of 14 member lactone ring, which are devoid of antibacterial properties while maintaining immunomodulatory effect. Immunoregulatory properties of macrolides undoubtedly require a separate monograph.
4. RIFAMPICINE (FIGURE 3)
Macrocyclic antibiotic produced by a strain of Streptomyces meditarranei and acts by primarily inhibition of production nucleic acids, therefore has antibacterial and tuberculostatic activity. In many countries rifampicine is reserved for the treatment of tuberculosis. It affects the suppression of T-cell activity. Calleja et al. drew the attention to the possibility of rifampicine activating the human glucocorticoid receptor, however other works have not confirmed such opportunities of rifampicin [26,27].
5. TETRACYCLINES (FIGURE 4)
Tetracyclines are a group of bacteriostatic antibiotics that inhibit bacterial growth by binding to the bacterial 30 S
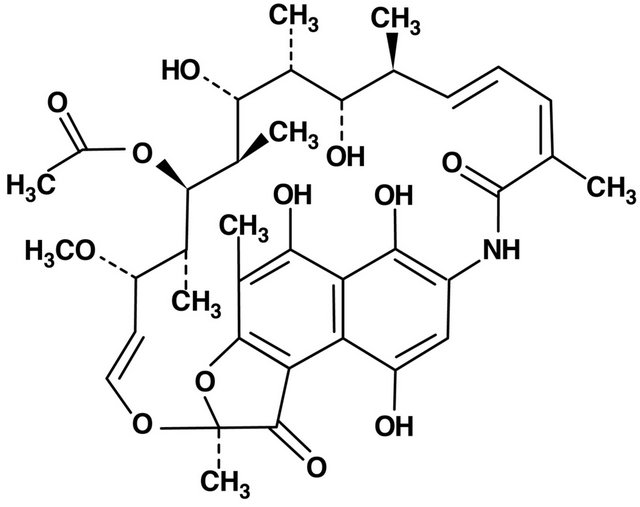
Figure 3. Rifamycin structure.
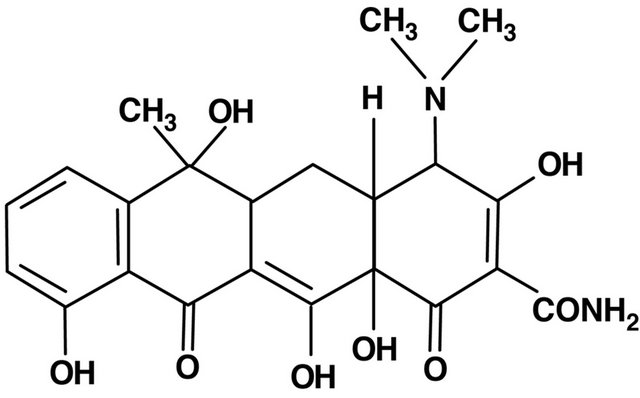
Figure 4. Tetracycline structure.
ribosomal subunit. Currently, next to the oldest group of natural tetracyclines discovered in 1948, the second-generation drugs can be distinguished-semi-synthetic: doxycycline and minocycline, and third-generation, chemically modified, which includes tigecycline discovered in the 90’s. They are broad-spectrum antibiotic, including gram positive, gram negative bacteria, chlamydia, rickettsia and spirochetes [2,28]. Golub et al. in 1983 found that the apart from antimicrobial properties, tetracyclines are able to inhibit the activity of metalloproteinases [29]. Thus, novel applications of this group of drugs in various diseases, using the anti-inflammatory, immunomodulatory properties and their and effect on cell proliferation, angiogenesis and apoptosis [28]. Anti-inflammatory effects of tetracyclines based on the inhibition of neutronphil migration and chemotaxis, inhibiting the activity of a group of enzymes called matrix metalloproteinases (MMPs), numbered from 1 to 28. These include, among others, collagenase (MMP-1, MMP-8, MMP-13), gelatinases (MMP-2, MMP-9), stromelysin (MMP-3, 10), metaloelastase (MMP-12) involved in the metabolism of connective tissue and in the process of cell formation. Metalloproteinase activity is inhibited due to the ability of tetracyclines to chelate metal ions. Block Zn2+, which is necessary to maintain biological activity, causes a decrease in enzyme activity metaloproteines, which can be used in the treatment of connective tissue diseases, coronary heart disease and cancer [1,2,28,29]. Tetracycline were used for the first time in the treatment of rheumatoid arthritis in the 50’s. It was justified by the theory that the disease is caused by organisms such as Mycoplasma. Subsequent studies have shown that hardly and rather their anti-inflammatory and immunomodulatory effects than antibacterial effects had influence on the course of rheumatoid arthritis. Immunomodulating effects of tetracyclines, consisting in the inhibition of Tcell activity and a decrease in levels of proinflammatory cytokines IL-2, TNF, IL-1 [2,29,30], in combination with anti-inflammatory effect, which inhibits the activity of matrix metalloproteinases, and also collagenases in the synovium [30-32] allows for its use in patients with rheumatoid arthritis. A randomized study conducted using the tetracycline in the treatment of RA, as compared with placebo or disease-modifying drug, showed that medications, especially minocycline, significantly reduce the activity of RA [33-36]. Currently, rheumatologists put special emphasis on early treatment and good control of the disease. A randomized study using Minocycline in patients with rheumatoid arthritis, HIV positive, suffering from <1 year showed significant improvement compared to placebo and group treated with hydroxychlorochine [34-37]. It was demonstrated on animals (dogs) with osteoarthritis (OA) of the knee the increase of collagenase activity in cartilage extracts and positive effect on dogs treated with doxycycline indicates the potential use of this class of drugs for the treatment of OA [38,39]. In spite of improved treatments, cancer is the second, after cardiovascular disorders leading cause of death in many countries. As many as 90% of deaths in the course of cancer are caused by a previously treated metastatic cancer. Over the past 20 years, it turned out that the metalloproteinases, particularly gelatinase A (MMP-2) and B (MMP-9), play a key role in the early stage of development and the growth of metastatic tumors—this concerns mainly prostate, breast, and lungs [38,40]. Based on pre-clinical studies of models of prostate cancer in animals, it was found that chemically modified tetracycline (CMT) with antibacterial properties is effectively inhibiting the development of bone metastases and metastatic tumor growth [39]. Using prostate cancer cell lines showed that among the eight chemically modified tetracyclines (CMTS), CMT-3 is the most specific inhibitor of the proliferation of tumor cells [40,41]. These observations have initiated pre-clinical studies in patients with advanced stages of breast, prostate and lung cancer as well as osteosarcoma [39,40]. Gelatinases (MMP-2 and -9) are also involved in the development of cardiovascular diseases such as atherosclerosis, hypertension, heart failure and ischemic heart disease [41-43]. Randomized studies have shown that the use of low doses of doxycycline in patients with ischemic heart disease significantly reduce CRP, interleukin-6 and MMP-9 in the course of 6 months of treatment. The high activity of MMPs has also been shown in patients with inflammatory diseases such as heart and Kawasaki disease and viral myocarditis. Metalloproteinase activity also increased in patients with sepsis, that is why different models of septic shock doxycycline were tested as a potential therapeutic agent [43,44]. Disorders of apoptosis, the programmed cell death, leading to disturbances in homeostasis, underlie cancer and neurodegenerative disorders. The apoptotic process is influenced by inter proteolytic enzymes-caspases—(cysteinyl aspartate specific proteinases). It has been shown in experimental trials that Caspase-1, also known as interleukin-1β, is an important cytokine that participates in the process of neuronal cell death after experimental brain injury in mice [45]. Minocycline inhibiting Caspase-1 may protect nerve cells from death, as shown in experimental samples after brain injury [46]. The neuroprotective and anti-inflammatory effect, especially of minocycline, may also result from its inhibition of microglial activation, as shown by experimental studies in rats [47]. In animal models neuroprotective and immunomodulating effect of minocycline are also observed in brain neurodegenerative diseases such as multiple sclerosis [5,48], Huntington’s disease, amyotrophic lateral sclerosis (ALS) [5]. Analgesic effect of minocycline is noteworthy, as it has been shown that it inhibits both nociceptive pain and neurogenic inflammation [49]. Tetracyclines, especially doxycycline and minocycline are effective in the treatment of sarcoidal granuloma in the skin and eye muscles as thanks to the inhibittion of protein kinase C the formation of granulomas does not occur [50,51]. After 13 months of treatment with minocycline in a dose of 200 mg/d obtained regression of formations of sarcoidal granulomas in limb muscle was obtained as well as a decrease in angiotensin-converting enzyme (ACE), interleukin 12, and interferon-induced protein 10 (IP-10, intenferon-inducible protein-10) [51]. Both IL-12 and IP-10 produced in inflammatory sites, are produced by macrophages of granulomas which suggests that minocycline inhibits their growth in the mechanism of anti-inflammatory and not anti-bacterial. This mechanism may also confirm the observed relapse of changes after discontinuation of treatment [44,50]. For many years, tetracycline was used in dermatology for the treatment of acne and acne rosaceaespecially during inflammation thanks to antibacterial effect against Propionibacterium acnes. Currently, an important role is attributed to the mechanisms of anti-inflammatory cytokines and the inhibition of MMP-9. However, the efficacy of treatment of rosacea with tetracycline is associated mainly to the inhibition of angiogenesis [28]. 12- month uncontrolled study of 11 patients with minocycline therapy for early generalized scleroderma showed a good response in the skin lesions in 4 patients [52]. Observation by Singer and Rotenberg polyuria in patients treated with demeclocyclin for skin diseases contributed to attempts to use tetracyclines for patients with SIADH (inappropriate secretion of vasopressin), resulting in a low molarity plasma and hyponatraemia [53, 54].
6. QUINOLONES (FIGURE 5)
Quinolones—by inhibiting gyrase and topoisomerase IV they inhibit the replication of bacterial DNA. The first quinolone with antibacterial activity was nalidixic acid synthesized in 1962. It is a representative of the first generation quinolones, which include oxolinic and pipemidinic acid as well as cinoxacin. This group is used for the treatment of urinary tract infections with Gramnegative organisms. The introduction of fluorine into the molecule of quinolone significantly changed its properties. In 1980 norfloxacin—the first fluorinated quinolone was allowed to be used in treatment. Since then the era of second-generation fluoroquinolones began—such as ofloxacin, ciprofloxacin, pefloksacin, temafloksacin, third generation—levofloxacin, grepafloxacin gatifloksacin, sparfloxacin and fourth generation of moxifloxacin and trovafloxacin. New groups have improved in comparison with not fluorinated quinolone activity against Grampositive and Gram-negative bacteria. It was demonstrated that fluoroquinolones have modulating effect on cytokine production by monocytes. Kahn et al. [55] have demonstrated that trovafloxacin at therapeutic doses suppress the production of cytokines (IL-1α, IL-1β, IL-6, IL-10, granulocyte-macrophage colony stimulating factor (GM-CSF) and tumor necrosis factor alpha (TNF-α)) secreted by lipopolysaccharide (LPS)-stimulated monocytes or pansorbin. Immunosuppressive effect depends on the concentration of cAMP in monocytes. Fluoroquinolones induce the formation of prostaglandin E2 in the presence of monocytes, independently of interleukin 18 by strengthening cyclooxygenase-2 (COX-2) expression. In this way, the increase of intracellular cAMP, which results in inhibition of TNF production, enhances the production of IL-6 but does not affect the production of IL-1α and IL-lβ. Pefloxacin and ciprofloxacin at doses <100 mg/L reduces the production of IL-1, ciprofloxacin and ofloxacin at concentrations >25 mg/L reduces the synthesis of TNF-α in monocytes stimulated with LPS.
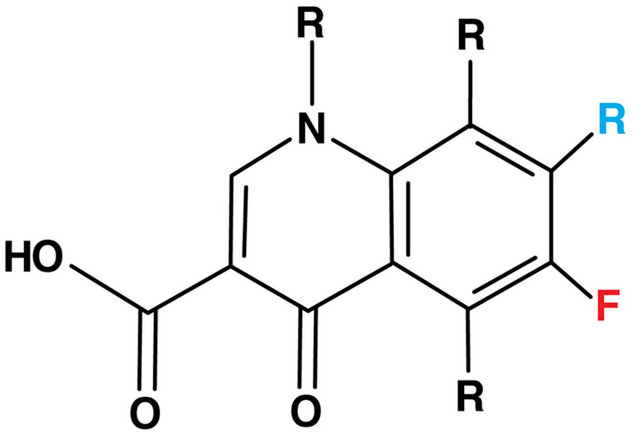
Figure 5. The basic structure of all quinolones.
Grepafloxacin also inhibits the production of IL-8 in cells stimulated by TNF-α, because it uses a level of transcription of the gene so that it inhibits the expression of mRNA of IL-1α, IL-1β, TNF-α, IL-6 and IL-8 [55]. Immunomodulating effect of ciprofloxacin has been shown in work of Lahat et al. performed on mice with induced inflammatory bowel disease (TNBS colitis)—it was demonstrated that the inhibition of CIP significantly beyond antimicrobal inflammatory effect, decreased the levels of TNF and IL 1β and had an impact on the level of IL 8 by inhibiting the activity of neutrophils. It also reduced the level of NF-κB, the central transcription factor of macrophages and lymphocytes [56]. Moxifloxacin had an impact on monocytes stimulated by zymogen A S. aureus—in the first phase it increased the release of NO and hydrogen peroxidate, in the next (after 4 pm) it influenced the release of lysosomal enzymes, lipid peroxydaction and inhibited the release of pro-inflammatory cytokines.Such action not only raised the efficiency of antimicrobial effect,but also led to the extinction inflammatory response [57]. In therapy for relapsing remitting multiple sclerosis-(RRMS) immunomodulating effect of laquinimod, whichis a new Quinoline-3-carboxamide was observed in animal models. Laquinimod shows immunomodulatory effects, probably through Th1/Th2 shift, but does not lead to immunosuppression. It has also been demonstrated in animal models of multiple sclerosis and autoimmune encephalomyelitis (EAE), including inhibition by LAQ Th1 and Th17 and regulatory T cells increase (Treg), and a reduction in the activity of inflammation in the CNS [58].
7. LINCOSAMIDES (FIGURE 6)
Their mechanism of action is based on a combination of the bacterial 50 S ribosomal subunit as macrolides and inhibition of protein synthesis in the bacterial cell. They exhibit a bacteriostatic effect. In particular, clindamycin is used in combination with penicillin for the treatment of severe infections of Streptococcus pyogenes. It is believed that clindamycin inhibits the synthesis of bacterial toxins, but also has an effect on the inhibition of bacterial protein M. The use of clindamycin in combination therapy increases the possibility of bacterial phagocytosis and hence the effectiveness of antibiotic [59].
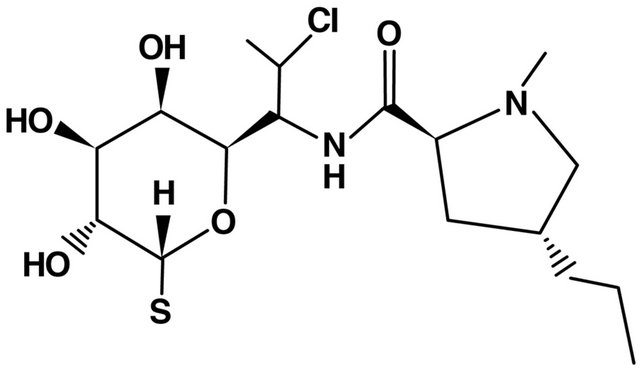
Figure 6. Lincosamides structure.
8. OXAZOLIDONES (FIGURE 7)
Linezolid (LNZ) is a new drug registered for the treatment of gram-positive infections also resistant to vancomycin therapy. It is uses also in the treatment of multidrug-resistanttuberculosis. LNZ is an inhibitor of monoamnino oxidase (MAO) and it exhibits potential adrenergic action. Simultaeous application of both serotonergic and LNZ preparations can lead to serotonin syndrome. LZN effect on the inhibition of IF γ and TNF α secretion as also observed [60,61]. Among others, it has also been shown that linezolid, clindamycin, trimethoprim/co-trimoxazole have an influence on the inhibition of the production by PBMC (peripheral blood mononuclear cells) stimulated by S. aureus toxine-pro-inflammatory cytokines such as TNF-α, IL6, IL1, IL8. In Pichereau et al. paper also highlights the new drug from the group of glicylcyklines-tigecycline and its effect on the inhibition of the inflammatory response in severe infections [62].
9. AMINOGLYCOSIDES (FIGURE 8)
Antibiotics blocking bacterial protein synthesis by binding to the 30 S ribosomal subunit. Their bactericidal properties work only on gram-negative aerobic bacteria. Due to its toxicity (nephro and ototoxicity) their wider use is limited to serious infections. They are known for their effect on neuromuscular muscle—it is a serious side effect of this class of drugs. In animal studies the impact of aminoglycosides on presynaptic release of acetylcholine (Ach) and internalization of calcium in the presynaptic part of the axone was demonstrated. They can also weaken the postsynaptic receptor response to Ach. There are works demonstrating the impact of aminoglycosides, gentamicin in particular, on the regulation of CFTR gene mutations which may have clinical importance for the
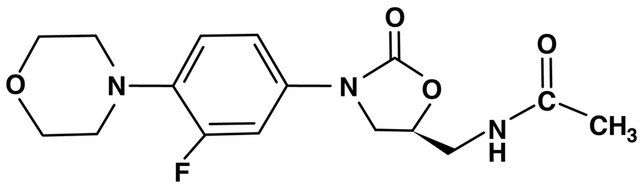
Figure 7. Linezolid (oxazolidones) structure.
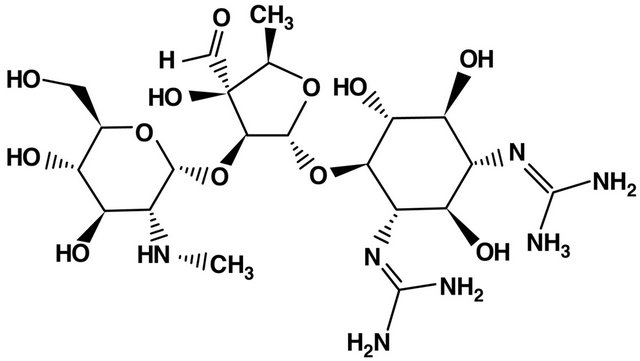
Figure 8. Streptomycin structure.
treatment of patients with cystic fibrosis (CF) [1,2]. Research has also shown that aminoglycosides have an influence on the chemotaxis of polymorphonuclear leukocytes, but there is no clarity as to their anti-inflammatory activity.
10. SULFONES AND SULPHONAMIDES (FIGURE 9)
Chemotherapeutic agents structurally related to p-aminobenzoic acid needed for bacterial synthesis of folic acid, and in this way inhibitor of bacterial synthesis of folic acid. Some of their activities, beyond antimicrobial properties of sulfonamides, have been used in medicine as a hypoglycemic effect (sulfonylureas-sulfonyloureas) in the treatment of diabetes and inhibition of carbonic anhydrase in the acetolazamid. The effect of the diuretic is used in the treatment of glaucoma. It is known that sulfones block neutrophil functions, such as chemotaxis and oxidant production, the impact on myeloperoxidase (MPO) and the formation of the inactive form of lower prostaglandin E2 synthesis in neutrophils. Sulfonamides and their derivatives also cause inhibition of phagocytesis of phagocytic cells. It is suggested that the inhibition of nuclear factor NF-κB, sulfonamides, such asfor example sulfasalazine, have anti-inflammatory effect. Hence, they are used in the treatment of autoimmune diseases such as RA and Crohn’s disease [2,5].
11. BENZYLPYRIMIDINES
Benzylpyrimidines also act by inhibiting the synthesis of folic acid. They inhibit dihydrofolic acid reductase. Among these compounds trimehtoprim (TMT) is widely used in the treatment of infectious diseases, in combination with sulfomethoxazole-synergism occurs through inhibition of purine to the bacterial cells. TMT also inhibits neutrophil chemotaxis, reduces the formation of free radicals and impair the function of the cytoplasmic membrane. In combination with sulfametoxazole it was also used in the treatment of non-infectious diseases such as ANCA depends granulomatosis (Wegener’s granulomatosis), particularly for the disease limited to the upper aerodigestive tract [63].
12. PEPTIDE ANTIBIOTICS
Peptide antibiotics have diverse mechanisms of action of
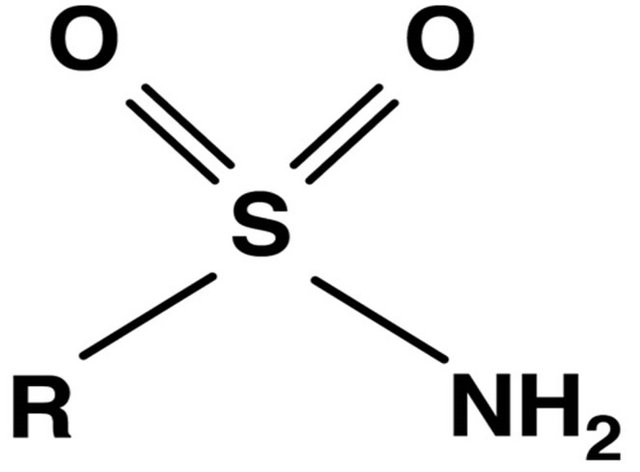
Figure 9. Sulfonamide group structure.
the cytoplasmic membrane dysfunction as polymyxin, inhibition of cell wall synthesis (bacitracin, glycopeptides, lipoglicopeptides) or inhibition of protein synthesis as streptogramins, well known polymyxin B compound is affecting the protein kinase C (inhibition), which stimulates monocytes, synthesis of cytokines such as IL 1, IL 6, GM CSF, stimulates the production of proteins of the complement system. Bacitracin in vitro inhibits the phagocytic activity of leukocytes and macrophages [64]. Used in severe infections, vancomycin and teicoplanin at high concentrations (toxic to the human body) reduce the activity of polymorphonuclear leukocytes and increase the synthesis of proinflammatory cytokines by monocytes.
13. FUSIDIC ACID
Chemotherapeutic inhibiting bacterial translation process. In studies on mice it was found that fusidic acid has effect on the inhibition of TNF and INFγ release, and inhibition of enterocyte damage by bacterial LPS and staphylococci toxin A. The impact of the fusidic acid on the reduction of the polymorphonuclear leukocytes activity is also possible. The properties of fusidic acid are also compared with the action on the immune system of cyclosporin A [65]. In an experimental model of acute pancreatitis (AP) Osman et al. demonstrated the effect of fusidic acid on the decrease of TNF and IL-8 concentration and related reduction in mortality and the occurrence of ascites in AP [66].
14. FOSFOMYCIN
(1-cis-1,2-epoxypropylphosphoric acid) inhibits bacterial cell wall synthesis by acting on the enzyme phosphotransferase phenyl pyruvate phosphate. In vitro studies have confirmed its effect on the activity of T and B lymphocytes and reduced secretion of inflammatory mediators such as histamine. In the phagocytes it reduces TNF α and IL-1, IL as well as increases the concentration of IL 6 [67]. Different effects on IL 6 described by Zeitlinger et al., suggest that the immunostimulatory effect of fosfomycin is rather based on the impact of fosfomycin on the reduction of trancription than on proinflammatory cytokine release [68]. Fosfomycin has an effect on the immune system, however, it requires further investigations.
15. CONCLUSIONS
In clinical practice antibiotics are increasingly used because of their other than simply antibacterial actions. Yet, at present, only particular of the described antibiotic classes find such application.
New macrolides and their derivatives are used in interventional cardiology for coating stents (rapamycin) and their ability to block calcineurin allowed for their use in transplantation (tacrolimus, pimecrolimus). The use of macrolides in the treatment of RA and inflammatory bowel disease, although proved effective in studies on humans, needs further clinical tests and statistical data before these conditions become indications for macrolide use.
The second class of antibiotics with clinically documented extraantibiotic properties-tetracyclines-also finds presently limited use in clinical practice: doxycycline and minocycline are indicated for use in dermatology to treat acne, due to their other than strictly antimicrobial activity. Other tetracyclines, minocycline in particular with its use in RA, need further clinical testing before their extraanitbiotic properties find wider clinical use.
Finally, the sulfonamides and also their derivatives find clinical use in the treatment of various diseases such as: RA (SSZ), diabetes (sulfonylureas) and glaucoma (acetozalamide) due to their non-antibiotic actions.
As for the other antibiotic classes, the data from animal models concerning their immunological properties is contradictory and despite steadily widened knowledge of the effects of antibiotics on the immune system, it does not allow for their wide non-antibiotic application.
Limitations arise both from their side effects including: the risk of allergic reactions (b lactams, sulfonamides), the potential toxicity (e.g. nephroand ototoxicity of aminoglycosides), influence on cardiovascular system (e.g. quinolones) or on neuromuscular conduction (aminoglicosides, tertracyclines, clindamycin). The situation is further complicated by contraindications for use in children (e.g. tetracyclines or quinolones) and restricted applicability during pregnancy (only few antibiotic classes can be used during pregnancy e.g. macrolides, β lactams without clavulanic acid). Furthermore long term use of many antibiotics bears a risk of development of antibiotic resistance. Hence, the search for new compounds pharmacologically belongs to antibiotic classes, yet having no antibiotic properties, while presenting influence on the immune system.
Summarizing conclusions—the knowledge of the effects of antibiotics on the: immune response, the synthesis and secretion of pro-inflammatory cytokines allows us to see the drugs known for years in a new light. This includes finding for them new applications beyond the infection treatment. Yet, undoubtedly, this line of drug development needs further research.
REFERENCES
- Pasquale, T.R. and Tan, J.S. (2005) Nonantimicrobial effects of antibacterial agents. Clinical Infectious Diseases, 40, 127-135.
- Guz, K. and Bugla-Płaskońska, G. (2007) The immunomodulatory and anti-inflammatory properties of diferent antimicrobial agents. Postępy Higieny i Medycyny Doświadczalnej, 61, 828-837.
- Kwiatkowska, B. and Maślińska, M. (2012) Macrolide therapy in chronic inflammatory diseases. Mediators of Inflammation, 2012, Article ID: 636157. doi:10.1155/2012/636157
- Brooks, B.M., Flanagan, B.F., Thomas, A.L. and Coleman, J.W. (2001) Penicillin conjugates to interferon-gamma and reduces its activity: A novel drug-cytokine interaction. Biochemical and Biophysical Research Communications, 288, 1175-1181.
- Mangano, K., Quattrocchi, C., Scalia, G., Speciale, A., Nicoletti, G. and Di Marco, R. (2006) Immunomodulatory properties of cefaclor: In vivo effect on cytokine release and lymphoproliferative response in rats. Journal of Chemotherapy, 18, 641-647.
- Tauber, S.C. and Nau, R. (2008) Immunomodulatory properties of antibiotics. Current Molecular Pharmacology, 1, 68-79.
- Wenisch, C., Parschalk, B., Hasenhündl, M., Wiesinger, E. and Graninger, W. (1995) Effect of cefodizime and ceftriaxone on phagocytic function in patients with severe infections. Antimicrobial Agents and Chemotherapy, 39, 672-676. doi:10.1128/AAC.39.3.672
- Alekshun, M.N. (2005) New advances In antibiotic development and discovery. Expert Opinion on Investigational Drugs, 14, 117-134. doi:10.1517/13543784.14.2.117
- Wilson, D.N. (2009) The A-Z of bacterial translation inhibitors. Critical Reviews in Biochemistry and Molecular Biology, 44, 393-433. doi:10.3109/10409230903307311
- Kanoh, S. and Rubin, B.K. (2010) Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clinical Microbiology Reviews, 23, 590-615. doi:10.1128/CMR.00078-09
- Suzaki, H., Asano, K., Ohki, S., et al. (1999) Supressive activity of a macrolide antibiotic, roxitromycin, on proinflammatory cytokine production in vitro and in vivo. Mediators of Inflammation, 8, 199-204. doi:10.1080/09629359990351
- Shimane, T., Asano, K., Suzuki, M., et al. (2001) Influence of a macrolide antibiotic, roxitromycin, on mast cell growth and activation in vitro. Mediators of Inflammation, 10, 323- 332. doi:10.1080/09629350120102343
- Schultz, M.J., Speelman, P., Hack, C.R., et al. (2000) Intravenous infusion of erythromycin inhibits CXC chemokine production, but augments neutrophil degranulation in whole blood stimulated with Streptococcus pneumoniae. Journal of Antimicrobial Chemotherapy, 46, 235- 240. doi:10.1093/jac/46.2.235
- Matsuoka, N., Eguchi, K., Kawakami, A., et al. (1996) Inhibitory effect of clarithromycin on costimulatory molecule expression and cytokine production by synovial fibroblast-like cells. Clinical & Experimental Immunology, 104, 501-508. doi:10.1046/j.1365-2249.1996.46752.x
- Keicho, N., Kudoh, S., Yotsumo, H., et al. (1993) Antilymphocytic activity of erythromycin distinct from that of FK506 or cyclosporine A. The Journal of Antibiotics, 46, 1406-1413. doi:10.7164/antibiotics.46.1406
- Sato, E., Nelson, D.K., Koyama, J., et al. (2000) Erythromycin modulates eosinophil chemotactic cytokine production by lung fibroblasts in vitro. Antimicrobial Agents and Chemotherapy, 45, 401-406. doi:10.1128/AAC.8.2.401-406.2001
- Ogrendik, M. (2009) Efficacy of roxitromycin in adult patients with rheumatoid arthritis who had not received disease-modifying antirheumatic drugs: A 3-month, randomized, double-blind, placebo-controlled trial. Clinical Therapeutics, 31, 1754-1764. doi:10.1016/j.clinthera.2009.08.014
- Oyama, K., Sakuta, T., Matsushita, K., Maruyama, I., Nagaoka, S. and Torii, M. (2000) Effects of roxithromycin on tumor necrosis factor-alpha-induced vascular endothelial growth factor expression in human periodontal ligament cells in culture. Journal of Periodontology, 71, 1546- 1553doi:10.1902/jop.2000.71.10.1546
- Ogrendik, M. (2007) Effects of clarithromycin in patients with active rheumatoid arthritis. Current Medical Research and Opinion, 23, 515-522. doi:10.1185/030079906X167642
- Sawiola, G., Benucci, M., Abdi-Ali, L., Baiardi, P., Manferdi, M., Bucci, M. and Cirino, G. (2010) Clarithromycin in adult-onset Still’s disease: A study of 6 cases. Rheumatology International, 30, 555-560. doi:10.1007/s00296-009-1277-9
- Thanou-Stavraki, A., Aberle, T., Aksentijevich, I., Bane, B.L. and Harley, J.B. (2011) Clarithromycin in adult-onset still’s disease: A potentially useful therapeutic. Journal of Clinical Rheumatology, 17, 373-376. doi:10.1097/RHU.0b013e3182320680
- Carević, O. and Djokić, S. (1988) Comparative studies on the effects of erythromycin A and azithromycin upon extracellular release of lysosomal enzymes in inflammatory processes. Agents and Actions, 25, 124-131. doi:10.1007/BF01969103
- Mencarelli, A., Distrutti, E., Renga, B., et al. (2011) Development of non-anticiotic macrolide that corrects inflammation-driven immune dysfunction in models of inflammatory bowel diseases and arthritis. European Journal of Pharmacology, 665, 29-39.
- Eisen, H.J., Tuzcu, E.M., Dorent, R., et al. (2003) Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. The New England Journal of Medicine, 349, 847-858.
- Blagosklonny, M.V. (2011) Progeria, rapamycin and normal aging: Recent breakthrough. Aging, 3, 685-691.
- Calleja, C., Pascussi, J.M., Mani, J.C., Maurel, P. and Vilarem, M.J. (1998) The antibiotic rifampicin is a nonsteroidal ligand and activator of the human glucocorticoid receptor. Nature Medicine, 4, 92-96. doi:10.1038/nm0198-092
- Herr, A.S., Wochnik, G.M., Rosenhagen, M.C., Holsboer, F. and Rein, T. (2000) Rifampicin is not an activator of glucocorticoid receptor. Molecular Pharmacology, 57, 732- 737.
- Sapadin, A.N. and Fleischmajer, R. (2006) Tetracyclines: Nonantibiotic properties and their clinical implications. Journal of the American Academy of Dermatology, 54, 258-265. doi:10.1016/j.jaad.2005.10.004
- Golub, L.M., Lee, H.M., Lehrer, G., Nemiroff, A., McNamara, T.F., Kaplan, R., et al. (1983) Minocycline reduses gingival collagenolytic activity during diabetes. Preliminary observations and a proposed new mechanism of action. Journal of Periodontal Research, 18, 516-526. doi:10.1111/j.1600-0765.1983.tb00388.x
- Kloppenburg, M., Verweij, C.L., Miltenburg, A.M.M., Verhoeven, A.J., Daha, M.R., Dijkmans, B.A.C. and Breedveld, F.C. (1995) The influence of tetracyclines on T cell activation. Clinical & Experimental Immunology, 102, 635-641. doi:10.1111/j.1365-2249.1995.tb03864.x
- Greenwald, R.A., Golub, L.M., Lavities, B., Ramamurthy, N.S., Gruber, B., Laskin, R.S. and McNamara, T.F. (1987) Tetracyclines inhibit human synovial collagenase in Vivo and in Vitro. The Journal of Rheumatology, 14, 28-32.
- Schnabel, L.V., Papich, M.G., Watts, A.E. and Fortier L.A. (2010) Orally administred doxycycline accumulates in synovial fluid compared to plasma. Equine Veterinary Journal, 42, 208-210. doi:10.2746/042516409X478514
- Kloppenburg, M., Breedveld, F.C., Terwiel, J.Ph., Mallee, C. and Dijkmans, B.A.C. (1994) Minocycline in active rheumatoid arthritis. A double-blind, plcebo-controlled trial. Arthritis & Rheumatism, 37, 629-636. doi:10.1002/art.1780370505
- O’Dell, J.R., Haire, C.E., Palmer, W., Drymalski, W., Wees, S., Blakely, K., Churchill, M., Eckhoff, P.J., Weaver, A., Doud, D., Erikson, N., Diez, F., Olson, R., Maloley, P., Klassen, L.W. and Moore, G. (1997) Reatment of early rheumatoid arthritis with minocycline or placebo. Result of a randomized, double blind, placebo-controlled trial. Arthritis & Rheumatism, 40, 842-848. doi:10.1002/art.1780400510
- Stone, M., Fortin, P.R., Pacheco-Tena, C. and Inman, R.D. (2003) Should tetracycline treatment be used more extensively for rheumatoid arthritis? Metaanalysis demonstrates clinical benefit with reduction in disease activity. The Journal of Rheumatology, 30, 2112-2122.
- O’Dell, J.R., Blakely, K.W., Mallek, J.A., Eckhoff, P.J., Leff, R.D., Sems, K.M., Fernandez, A.M., Palmer, W.R., Klassen, L.W., Paulsen, G.A., Hair, C.E. and Moore G.F. (2001) Treatment of early seropositive rheumatoid arthritis: A two-year, double-blind comparison of minocycline and hydroxychloroquine. Arthritis & Rheumatism, 44, 2235-2241. doi:10.1002/1529-0131(200110)44:10<2235::AID-ART385>3.0.CO;2-A
- Laasila, K., Laasonen, L. and Leirisalo-Repo, M. (2003) Antibiotic treatment and long term prognosis of reactive arthritis. Annals of the Rheumatic Diseases, 62, 655-658. doi:10.1136/ard.62.7.655
- Yu, L.P., Smith, G.N., Brandt, K.D., Myers, S.L., O’Connor, B.L. and Brandt, D.A. (1992) Reduction of the severity of cannine osteoartthritis by prophylactic treatment with oral doxycycline. Arthritis & Rheumatism, 35, 1150- 1159. doi:10.1002/art.1780351007
- Lokeshwar, B.L. (2001) Chemically modified non antimicrobial tetracyclines are multifunctional drugs against advanced cancers. Pharmacolgical Research, 63, 146- 150. doi:10.1016/j.phrs.2010.11.003
- Lokeshwar, B.L., Houston-Clark, H.L., Selzer, M.G., Block, N.L. and Golub, L.M. (1998) Potential application of a chemically modified non antimicrobial tetracycline (CMT- 3) against metastatic prostate cancer. Advances in Dental Research, 12, 97-102.
- Castro, M.M., Rizzi, E., Figueiredo-Lopes, L., Fernandes, K., Bendhack, L.M., Pitol, D.L., et al. (2008) Aetalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis, 198, 320-331. doi:10.1016/j.atherosclerosis.2007.10.011
- Creemers, E.E., Cleutjens, J.P., Smith, J.F. and Daemen, M.J. (2001) Matrix metalloproteinase inhibition after myocardial infarction: A new approach to prevent heart failure? Circulation Research, 89, 201-210. doi:10.1161/hh1501.094396
- Castro, M.M., Kandasamy, A.D., Youssef, N. and Schulz, R. (2011) Matrix metalloproteinase inhibitor properties of tetracyclines: Therapeutic potential in cardiovascular diseases. Pharmacologycal Research, 64, 551-560. doi:10.1016/j.phrs.2011.05.005
- Cena, J.J., lalu, M.M., Cho, W.J., Chow, A.K., Bagdan, M.L., Daniel, E.E., et al. (2009) Inhibition of matrix metalloproteinase activity in vivo protects against vascular hyporeactivity in endotoxemia. American Journal of Physiology-Heart and Circulatory Physiology, 298, 45- 51. doi:10.1152/ajpheart.00273.2009
- Li, M., Ona, V.O., Chen, M., Kaul, M., Tenneti, L., Zhang, X., et al. (2009) Functional role and therapeutic implications of neuronal caspase-1 and-3 in a mouse model of traumatic spinal cord injury. Neuroscience, 99, 333-342. doi:10.1016/S0306-4522(00)00173-1
- Sanchez, M.R.O., Ona, V.O, Li, M. and Friedlander, R.M. (2001) Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage and neurogical dysfunction. Neurosurgery, 48, 1393-1401.
- Lai, A.Y. and Todd, K.G. (2006) Hipoxia-activated microglial mediators of neuronal survival are differentially regulated by tetracyclines. Glia, 53, 809-816. doi:10.1002/glia.20335
- Brundula, V., Rewcastle, N.B., Metz, L.M., Bernard, C.C. and Yong, V.W. (2002) Targeting leucocyte MMPs and transmigration: Minocycline as a potential therapy for multiple sclerosis. Brain, 125, 1297-1308. doi:10.1093/brain/awf133
- Bastos, L.F.S., De Oliveira, A.C.P., Watkins, L.R., Moraes, M.F.D. and Coelho, M.M. (2012) Tetracyclines and pain. Naunyn-Schmiedeberg’s Archives of Pharmacology, 385, 225-241. doi:10.1007/s00210-012-0727-1
- Miyazaki, E., Ando, M., Fukami, T., Nureki, S., Eishi, E., Kumamoto, T. (2008) Minocycline for the treatment of sarcoidosis: In the mechanism of action immunomodulating or antimicrobial effect? Clinical Rheumatology, 27, 1195-1197. doi:10.1007/s10067-008-0903-3
- Agostini, C., Cassatella, M., Zembello, R., et al. (1998) Involment if the IP-10 chemokine in sarcoid granulomatous reaction. Journal of Immunology, 161, 6413-6420.
- Le, C.H., Morales, A. and Trentham, D.E. (1998) Minocycline in early diffuse scleroderma. Lancet, 28, 1755- 1756.
- Singer, I. and Rosenberg, D. (1973) Demociline induced nephrogenic diabetes insipidus: In vivo and in vitro studies. Annals of Internal Medicine, 79, 679-683. doi:10.7326/0003-4819-79-5-679
- Cherrill, D.A., Stote, R.M., Birge, J.R. and Singe, I. (1975) Demeclocycline treatment in the syndrome of inappropriate antidiuretic hormone secretion. Annals of Internal Medicine, 83, 654-656. doi:10.7326/0003-4819-83-5-654
- Kahn, A.A., Slifer, T.R. and Remington, J.S. (1998) Effect of Trovafloxacin on production of cytokinez by human monocytes Antimicrob. Agents Chemother, 42, 1713- 1717.
- Lahat, G., Halperin, D., Barazovsky, E., Shalit, I. and Rabau, M. (2007) Immunomodulatory effects of ciprofloxacin in TNBS-induced colitis in mice. Inflammatory Bowel Diseases, 13, 557-565.
- Hall, I.H., Schwab, U.E., Ward, E.S. and Ives, T.J. (2003) Effects of moxifloxacin in zymogen A or S. aureus stimulated human THP-1monocytes on the inflammatory process and the spread of infection. Life Sciences, 73, 2675-2685. doi:10.1016/S0024-3205(03)00611-8
- Fernández, O. (2011) Oral laquinimod treatment in multiple sclerosis. Neurologia, 26, 111-117.
- Gemmell, C.G., Peterson, Ph.K., Schmeling, D. and Kim, Y. (1981) Potentiation of opsonization and phagocytosis of Streptococcus pyogenes following growth in the presence of clindamycin. The Journal of Clinical Investigation, 67, 1249-1256.
- [61] Takahashi, G., Sato, N., Yaegashi, Y., et al. (2010) Effect of linezolid on cytokine production capacity and plasma endotoxin levels in response to lipopolysaccharide stimulation of whole blood. Journal of Infection and Chemotherapy, 16, 94-99.
- [62] Garcia-Roca, P., Mancilla-Ramirez, J., Santos-Segura, A., Fernández-Avilés, M. and Calderon-Jaimes. E. (2006) Linezolid diminishes inflammatory cytokine production from human peripheral blood mononuclear cells. Archives of Medical Research, 37, 31-35.
- [63] Pichereau, S., Moran, J.M.M., Hayney, M.S., Shukla, S.K., Sakoulas, G. and Rose W.E. (2012) Concentrationdependent effects of antimicrobials on Staphylococcus aureus toxin-mediated cytokine production from peripheral blood mononuclear cells. Journal of Antimicrobial Chemotherapy, 67, 123-129.
- [64] Müller, S., Eljack, S. and DelGaudio, J.M. (2011) Wegener’s Granulaomatosis. Head and Neck Pathology, 5, 268-272.
- [65] Brom, C., Brom, J. and König, W. (1992) Neomycin induces stimulatory and inhibitory effects on leukotriene generation, guanine triphospatase activity, and actin polymeryzation within human neutrophils. Immunology, 75, 150-156.
- [66] Bendtzen, K., Diamant, M. and Faber, V. (1990) Fusidic acid, an immunosuppressive drug with functions similar to cyclosporin A. Cytokine, 2, 423-429.
- [67] Osman, M.O., El-Sefi, T., Lausten, S.B., Jacobsen, N.O.G., Larsen, C.G. and Jensen, S.I. (1998) Sodium fusidate and the cytokine response in an experimental model of acute pancreatitis. British Journal of Surgery, 85, 1487-1492.
- [68] Morikawa, K., Oseko, F.I., Morikawa, S. and Sawada, M. (1993) Immunosuppressive activity of fosfomycin on human T-lymphocyte function in Vitro. Antimicrobial Agents and Chemotherapy, 37, 2684-2687.
- [69] Zeitlinger, M., Marsik, C., Steiner, I., Sauermann, R., Seir, K., Jilma, B., Wagner, O. and Joukhadar, C. (2007) Immunomodulatory effects of fosfomycin in an endotoxin model in human blood. The Journal of Antimicrobial Chemotherapy, 59, 219-223.
NOTES
*All authors have nothing to disclose. All authors have no commercial or financial interest in the products or companies described in this paper.

