Advances in Bioscience and Biotechnology
Vol.4 No.1(2013), Article ID:27261,4 pages DOI:10.4236/abb.2013.41013
Development and characterization of polymeric nanoparticles as Barbatimão (Stryphnodendron obovatum) standardized fraction carrier
![]()
Department of Pharmaceutical Products, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil
Email: *roc2006@farmacia.ufmg.br
Received 19 November 2012; revised 25 December 2012; accepted 6 January 2013
Keywords: Antioxidants; Barbatimão; Chitosan; Drug Delivery Systems; Nanoparticles
ABSTRACT
Recent studies have shown that Barbatimão fractions are promising for dermatological diseases treatment, due to tannins content and its strong antioxidant activity. In this study, chitosan was used as polymeric matrix for the development of nanoparticulated system able to deliver a Barbatimão standardized fraction. Nanoparticles were prepared by ionotropic gelation method involving the mixing of two aqueous phases at room temperature. The product was characterized by infrared absorption spectrometry, scanning electron microscopy, quantification of fraction in the formulation, in vitro release profile, size of particles and polydispersity index. The level of Barbatimão fraction obtained in the formulation was 38.23%, with 30% of encapsulated fraction released in 7 days. No chemical reaction between the fraction and the matrix could be noticed. The particles containing fraction presented an average size of 241 nm and polydispersity index of 0.484. It seems that the phytotherapic formulation could be used as drug delivery system for sustained release of Barbatimão fraction.
1. INTRODUCTION
The oxidation reactions are essential to metabolism since oxygen is the final electron acceptor in ATP production. ATP is the energy donor molecule of various physiological reactions [1]. Thus, a small amount of oxygen (2%) is continuously reduced to reactive oxygen species (ROS) or free radicals [2].
In some human diseases, there is an increased activity of ROS, either by a primary event (excessive exposure to radiation) or a secondary one (tissue damage by trauma) [3]. Among these diseases are those which affect the skin such as psoriasis, dermatitis and burns.
Recent researchs have investigated the potential of plant products as antioxidants for the prevention of diseases induced by ROS. Some plants are sources of natural antioxidants as phenolics, tannins, flavonoids, among others. These compounds can act as abductors of radical and sometimes as metal chelators, interfering both at the stage of initiation and propagation of the oxidative process [4].
In this context, the use of Barbatimão (Stryphnodendron obovatum Benth.) extracts or fractions in pharmaceutical formulations seems to be promising as an alternative in antioxidant therapy. Pharmacological and biological studies of Barbatimão extracts have shown antibacterial, anti-inflammatory, healing and antioxidant action. These properties are attributed to bioactive tannins, considered his chemical markers. Several tannins were identified in the standardized Barbatimão fraction, including gallic acid (GA), catechin (C), gallocatechin (GC), epigallocatechin (EGC) and epigallocatechin gallate (EGCG). The selected markers GC and EGCG were used for simultaneous chromatographic quantification. The Barbatimão fraction was standardized in 12.2 µg/mL (1.22% w/w) and 14.2 µg/mL (1.42% w/w) for GC and EGCG, respectively [5]. These results encourage the development of phytotherapic formulation for dermatological diseases treatment.
The pharmaceutical forms commonly used for this treatment are semi-solid preparations. However, because there is no effective penetration in the stratum corneum to allow an easier passage of the drug, the use of such preparations is difficult. On the other hand, the use of nanoparticulated systems facilitates the delivery of active compound due to its ability to protect the encapsulated substance while facilitating their transport to the body compartments. In addition, they allow a controlled release of the substance [6].
Chitosan, in the pharmacotechnical context, is an interesting polymer matrix, due to its biocompatibility, biodegradability and mucoadhesive properties. It is a polysaccharide formed by monomers of D-glucosamine and N-acetyl-D-glucosamine derived from chitin, which is found in the exoskeleton of crustaceans and insects [7]. Its cationic nature allows gel formation by contact with multivalent polyanions such as sodium tripolyphosphate (TPP) [6]. This method, known as ionotropic gelation, is based on electrostatic interaction of the amino residues of the positively charged polymer with negative charges of the polyanion [8,9].
Since various skin disorders, such as psoriasis and dermatitis, are pathologies associated with production of ROS and the traditional treatment is relatively ineffective (topical agents), inconvenient (phototherapy) or toxic (methotrexate) [3], the present work aimed to prepare, investigate the characteristics and antioxidant activity of chitosan-based nanoparticles loaded with standardized fraction of S. obovatum (Barbatimão fraction) for future antioxidant therapy.
2. MATERIALS AND METHODS
The Barbatimão fraction was obtained and standardized according to the method described by Nascimento et al. [5]. The method used for the production of nanoparticles was based on work of Calvo et al. [10]. The autors produced nanoparticles of 200 to 1000 nm successfully. The nanoparticles were prepared by an ionotropic gelation method involving the mixing of two aqueous solutions, the first containing chitosan and Barbatimão fraction at a ratio of 4.5:1, respectively, and the second containing TPP 1 mg/mL. The system was submitted to magnetic stirring at room temperature protected from light for 24 h. The dispersion obtained was subjected to sonication for 5 min to reduce the particle size. Blank samples were also prepared without Barbatimão fraction. The nanoparticles samples were naturally dried at room temperature allowing the formation of a thin film which was submitted to the caracterization analysis.
Barbatimão fraction, blank nanoparticles and Barbatimão-loaded nanoparticles were analyzed by infrared absorption spectrometry with attenuated total reflectance (ATR-FTIR) on spectrometer PerkinElmer FTIR, model Spectrum One (USA). The scanning electron micrographs were obtained for morphological characterization of the systems on electron microscope JEOL, model JSM- 6360 LV, operated at 15 kV in low vacuum.
The diameter of the nanoparticles and the polydispersity index (PI) was determined by dynamic light scattering on Zetasizer 3000 MHS (Malvern, England). The PI estimates the size variability of the particles in comparison with the average. The analysis was carried out in suspension containing nanoparticles, avoiding the possible formation of aggregates attributed to the drying stage. The zeta potential was not measured.
The quantification of the Barbatimão fraction content in the formulation was made by antioxidant DPPH assay in suspension containing the nanoparticles (quintuplicate) by spectrophotometry (l 516 nm) on spectrometer Shimadzu, model UVmini 1240 (Japan). This method use the stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical reagent. It is widely described in the literature [11-14]. However, the application in suspensions of nanoparticles was adapted and validated [15].
The in vitro release profile was determined by placing 0.5 mL of the formulation in a Spectrapore® dialysis membrane (12,000 Da) to ensure only the quantification of the Barbatimão fraction free. It was added 2.5 mL of water in the bottles containing the membranes. The liquid was changed every predetermined time and the antioxidant activity of aliquots was evaluated.
3. RESULTS AND DISCUSSION
The nanoparticulate drug delivery systems offer numerous advantages over the conventional dosage forms. These include improved efficacy, reduced toxicity and improved patient compliance [6]. In this work, it has been prepared and characterized nanoparticles carrier of standardized Barbatimão fraction that combines the properties of chitosan with plant extract which have therapeutical antioxidant potential. The method of preparation was very simple, with mild conditions.
In this context, the ATR-FTIR spectras of the nanoparticles produced are represented in Figure 1. As can be seen, the characteristic absorption bands of chitosan have been preserved in the Barbatimão-loaded formulation without considerable shifts, suggesting no obvious reaction between polymer and Barbatimão fraction. However, there was intensification of the band at 1406 cm−1 (stretching of C=C bonds of the aromatic rings of the Barbatimão fraction) which suggest that the extract was eficiently loaded in nanoparticles. Moreover, a broadening and overlap of bands from 3000 to 3400 cm−1 suggest that there was hydrogen bonding interaction between components of the Barbatimão fraction and the polymer.
From the electron micrographs obtained (Figure 2), it was observed a cluster of particles in the micro and nanometer scale. It might be inferred that this agglomeration occurs during drying of the material for production the film analyzed. This fact difficults the determination of particle size.
Through the technique of dynamic light scattering, particles carrying Barbatimão fraction had an average size of 241 nm ± 25 nm and 0.484 PI (acceptable values to topical use).
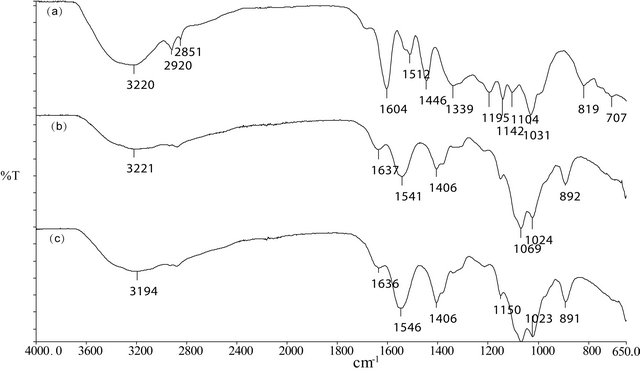
Figure 1. ATR-FTIR spectras of the Barbatimão fraction (a), blank nanoparticles (b) and Barbatimão-loaded nanoparticles (c).
 (a)
(a)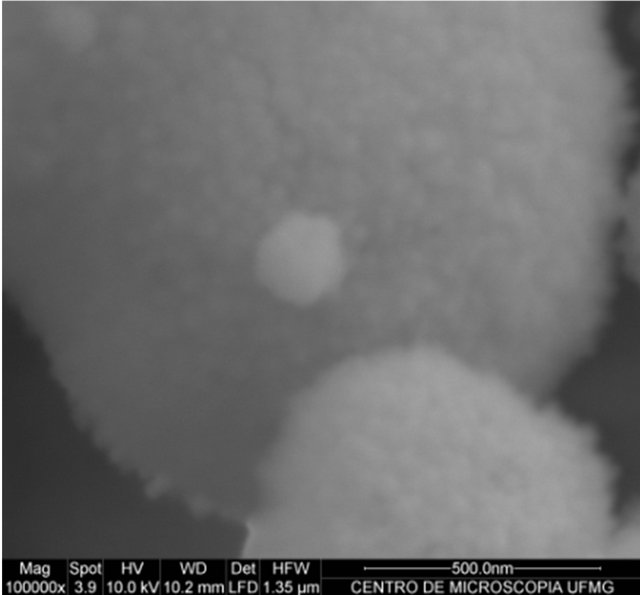 (b)
(b)
Figure 2. Electron micrographs of Barbatimão-loaded nanoparticles increase in 10,000 (a) and 100,000 (b) times.
The concentration of Barbatimão fraction in the final formulation was 54.62 ± 0.25 mg/mL. It means that 38.23% of the fraction initially used in the formulation were incorporated in the form of encapsulated and soluble fraction.
The result obtained for the first test release is shown in Figure 3. As can be observed after 960 min, only about 20% of the fraction in the formulation was released.
The plateau observed suggested that it would be necessary increase the interval between each change of medium (water) in the bottles. Thus, there would be a greater accumulation of extract, improving its detection by the method chosen. The test was repeated in a larger interval of time (360 h). The result is shown in Figure 4.
It can be seen that about 30% of the fraction were released after 7 days of incubation and there was no further release of material after.
It is suggested that the amount of extract not released is linked to the polymeric matrix by hydrogen bonds, as seen in Figure 1.
4. CONCLUSION
Polymeric nanoparticles based on chitosan have been developed and characterized. They showed potential to be used as drug carriers with controlled release, including Barbatimão fraction for skin diseases treatment. However, more studies are being made to increase the yield of the fraction encapsulated as well as the amount
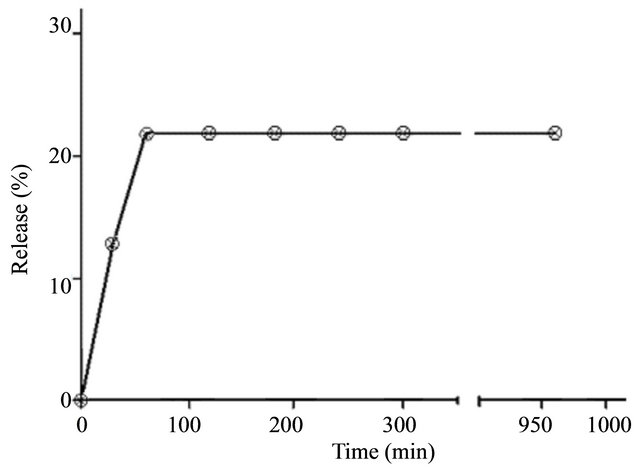
Figure 3. In vitro release profile of the Barbatimão fraction from Barbatimão-loaded nanoparticles for 960 min.
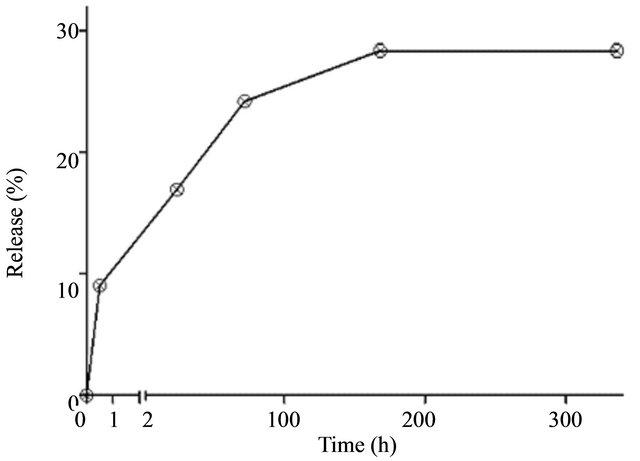
Figure 4. In vitro release profile of the Barbatimão fraction from Barbatimão-loaded nanoparticles for 360 hours.
released by the system.
5. ACKNOWLEDGEMENTS
The authors thank to financial support of CNPq (577102/2008-7) and FAPEMIG.
REFERENCES
- Davies, K.J. (1995) Oxidative stress: The paradox of aerobic life. Biochemical Society Symposia, 61, 1-31.
- Halliwell, B. (1999) Antioxidant defense mechanisms: From the beginning to the end (of the beginning). Free Radical Research, 31, 261-272. doi:10.1080/10715769900300841
- Favier, A.E., Cadet, J., Kalyanaraman, B., Fontecave, M. and Pierre, J.L. (1995) Analysis of free radicals in biological systems. 1st Edition, Birkhauser Verlag, Basel. doi:10.1007/978-3-0348-9074-8
- Ramalho, V.C. and Jorge, N. (2006) Antioxidantes utilizados em óleos, gorduras e alimentos gordurosos. Química Nova, 29, 755-760. doi:10.1590/S0100-40422006000400023
- Nascimento, A.M. (2008) Avaliação da qualidade de extratos de Stryphnodendron adstringens (Martius) Coville. Master Dissertation, Federal University of Minas Gerais, Belo Horizonte.
- Fuente, M., et al. (2010) Chitosan-based nanostructures: A delivery platform for ocular therapeutics. Advanced Drug Delivery Reviews, 62, 100-117. doi:10.1016/j.addr.2009.11.026
- Rodrigues, L.B., et al. (2009) In vitro release and characterization of chitosan films as dexamethasone carrier. International Journal of Pharmaceutics, 368, 1-6. doi:10.1016/j.ijpharm.2008.09.047
- Keawchaoon, L. and Yoksan, R. (2011) Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids and Surfaces B, 84, 163-171. doi:10.1016/j.colsurfb.2010.12.031
- Nagpal, K., Singh, S.K. and Mishra, D.N. (2010) Chitosan nanoparticles: A promising system in novel drug delivery. Chemical Pharmaceutical Bulletin, 58, 1423-1430. doi:10.1248/cpb.58.1423
- Calvo, P., Remuñán-López, C., Vila-Jato, J.L. and Alonso M.J. (1997) Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. Journal of Applied Polymer Science, 63, 125-132. doi:10.1002/(SICI)1097-4628(19970103)63:1<125::AID-APP13>3.0.CO;2-4
- Nuntanakorn, P., et al. (2007) Analysis of polyphenolic compounds and radical scavenging activity of four American Actaea species. Phytochemical Analysis, 18, 219-228. doi:10.1002/pca.975
- Brand-Williams, W., Cuvelier, M.E. and Berset, C. (1995) Use of a free radical method to evaluate antioxidant activity. Lebensmittel Wissenschaft & Technologie, 28, 25- 30. doi:10.1016/S0023-6438(95)80008-5
- Zhang, Y., Yang, Y., Tang, K., Hu, X. and Zou, G. (2008) Physicochemical characterization and antioxidant activity of quercetin-loaded chitosan nanoparticles. Journal of Applied Polymer Science, 107, 891-897. doi:10.1002/app.26402
- Jin, Z.Q. and Chen, X. (1998) A simple reproducible model of free radical-injured isolated heart induced by 1,1-diphenyl-2-picryl-hydrazyl (DPPH). Journal of Pharmacological and Toxicological Methods, 39, 63-70. doi:10.1016/S1056-8719(97)00093-2
- Costa, D.F.G., Franca, J.R., Faraco, A.A.G and Castilho, R.O. (2010) Método de atividade antioxidante (DPPH) para a quantificação do extrato padronizado de Barbatimão em formulação farmacêutica polimérica nanoparticulada. Proceedings of the 21th Simpósio de Plantas Medicinais do Brasil, João Pessoa, 14-17 October 2010.
NOTES
*Corresponding author.

