Advances in Bioscience and Biotechnology
Vol.3 No.7A(2012), Article ID:25172,9 pages DOI:10.4236/abb.2012.327128
Oxidative stress in brickmakers of Juárez City, Chihuahua, México: Case-control study
![]()
1Laboratorio de Toxicología Ambiental, Facultad de Química, Universidad Autónoma del Estado de México, Toluca, México
2Laboratorio de Ciencias Ambientales, Instituto de Ciencias Biomédicas, Universidad Autónoma de Ciudad Juárez, Juárez, México
3Facultad de Enfermería y Nutriología, Universidad Autónoma de Chihuahua, Chihuahua, México
4Laboratorio de Toxicología Acuática, Departamento de Farmacia, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, México City, México
Email: *lmgomezo@uaemex.mx
Received 20 August 2012; revised 27 September 2012; accepted 13 October 2012
Keywords: Oxidative Stress; Brickmakers; Chronic Exposure; Biomarkers; Juárez City
ABSTRACT
A case-control study was conducted in a brickmaker’s community in Juarez City, Chihuahua in Mexico. This population has been chronically exposed to a wide spectrum of potentially health-damaging pollutants that include coarse, fine and ultrafine particles, carbon monoxide (CO), oxides of nitrogen and sulphur, transitional metals, polycyclic aromatic hydrocarbons (PAHs), volatile organic compounds and bioaerosols. Lipid peroxidation level (LPX) and protein carbonyl content (PCC) help to evaluate oxidized protein content and activity of the antioxidant enzymes: superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) were evaluated in blood samples of study participants. The group of occupationally exposed (OE) workers consisted of 30 individuals ranging in age from 25 to 55 years, with a mean of 15 years in a brick production-related job. The control group included 30 volunteers who were neither environmentally nor occupationally exposed to brick production activities and whose sociodemographic characteristics were similar to the OE group. Results indicate that none of workers used any type of protective equipment (respirator or face mask, gloves, coveralls) during brick manufacturing. LPX and PCC significantly increased (p < 0.5) in the OE group compared to the control group. SOD, CAT and GPx activity was significantly increased (p < 0.5) in the OE group compared to the control group. Therefore, evaluation of stress oxidative biomarkers is advisable in order to assay chronic exposure to workers in brick manufacturing.
1. INTRODUCTION
Air pollution is a significant cause of morbidity and mortality. The greatest health impacts from air pollution worldwide occur among the poorest and most vulnerable populations. The amount of exposure in terms of the number of people, exposure intensity and time spent exposed is far greater in the developing world [1]. Continuous exposure to airborne pollutants leads to cellular damage that may lead to development of airway diseases. Since most of the airway diseases, including cancer, take a long latent period to develop, the cellular changes are immensely helpful to identify persons at risk so that medical intervention can be initiated at an early stage for better therapeutic response. There is now evidence linking an increased risk of respiratory tract infections, exacerbations of inflammatory lung conditions, cardiac events, stroke, eye disease, tuberculosis, and cancer with hospital admissions and with air pollution levels [2-4].
Respiratory health issues are of great concern in the Paso del Norte region and many respiratory diseases are attributed to air pollution in the area. Specifically, main contributors to air pollution in this area are the brick kilns [5]. This is a very serious problem due to the 313 brick kilns distributed in Juarez City and the hazardous fuels that are combusted for brick manufacturing. It is suspected that the pollutants generated by the kilns may even be precursors to several types of cancer. The brickmakers and their families are directly exposed to air pollution, which may affect their respiratory system just as smoking does or even worst. The malignant effects do not stop at the border, but affect the population in the US albeit a more dilute effect. However, there are few studies regarding the effect of the kiln emissions directly onto the brickmakers’ health. In terms of smoke inhalation, the brickmakers can be viewed as compulsive smokers who eventually are a high cancer risk group.
The production process of bricks can roughly be divided into six main steps, which are: digging the clay, preparing the clay, forming, drying, firing and distributing the bricks; although many technological combinations are possible during the brick production. Depending on the type of fuel that is used, the products released to the atmosphere are various. It contains known human carcinogens like benzene, 1,3-butadine and benzo(a)pyrene [6-9], a wide spectrum of potentially healthdamaging pollutants that include coarse, fine and ultrafine particles, carbon monoxide (CO), oxides of nitrogen and sulphur, transitional metals, polycyclic aromatic hydrocarbons, volatile organic compounds and bioaerosols. Most of these compounds are considered to be toxic for the human health.
There are few studies on the quantification of gaseous and particulate emissions [10,11] However, a thorough characterization and quantification of the brick kiln emissions have not been reported to date. Although the presence of all these combustion by-products is a health concern for the brickmakers and nearby residents, they are not aware of the shortand long-term-toxicity of these pollutants. One way to evaluate the damage induced by contaminants present in both air and water, is the use of biomarkers, which are defined as any measurable biochemical, physiological or morphologic changes associated with exposure to a toxic agent. Some biomarkers include assays at the biochemical level to evaluate oxidative stress in terms of the damage induced by reactive oxygen species (ROS) on macromolecules. Oxidative stress is produced by an imbalance of ROS and antioxidant systems in the body [12]. The toxic effects of many xenobiotics are mediated by ROS formation as a result of redox cycling [13]. The antioxidant enzymes that organisms use to offset oxidative damage include superoxide dismutase (SOD), which converts  to H2O2; catalase (CAT), which reduces H2O2 to water; and glutathione peroxidase (GPx) which detoxifies H2O2 or organic hydroperoxides to water [12]. Organisms are able to adapt to increased ROS production by increasing regulation by antioxidant defenses [14]. The failure of antioxidant defenses to detoxify excess ROS production may lead to significant oxidative damage including enzyme inactivation, protein degradation, DNA damage and lipid peroxidation (LPX) [15]. The latter in particular is considered the primary mechanism through which oxyradicals can potentially damage tissues, hampering cell function and inducing alterations in the physicochemical properties of the cell membrane leading to disruption of vital functions [16].
to H2O2; catalase (CAT), which reduces H2O2 to water; and glutathione peroxidase (GPx) which detoxifies H2O2 or organic hydroperoxides to water [12]. Organisms are able to adapt to increased ROS production by increasing regulation by antioxidant defenses [14]. The failure of antioxidant defenses to detoxify excess ROS production may lead to significant oxidative damage including enzyme inactivation, protein degradation, DNA damage and lipid peroxidation (LPX) [15]. The latter in particular is considered the primary mechanism through which oxyradicals can potentially damage tissues, hampering cell function and inducing alterations in the physicochemical properties of the cell membrane leading to disruption of vital functions [16].
The purpose of the present study was to conduct a case-control study in the brick makers in the region of Juarez City, Chihuahua, Mexico, where there is chronic exposure to different xenobiotics release in the brick manufacturing using and oxidative stress as biomarker.
2. MATERIALS AND METHODS
2.1. Study Population
The research protocol complied with the ethical princeples in the Declaration of Helsinki and was approved by the Ethics in Research Committee of the Centro de Investigación en Ciencias Médicas (CICMED) of the Universidad Autónoma del Estado de México (UAEM) and the Instituto de Ciencias Biomédicas (ICB) of the Universidad Autónoma de Ciudad Juárez (UACJ) .
The study population lives in Estrella del Poniente, Juarez City, Chihuahua, México. It is located at North East side of Juarez with geographic coordinate’s 31˚44′05.92″N latitude and 106˚32′04.20″W longitude, at an altitude of 4007 ft (Figure 1). The main economic activity in this region is brick manufacturing activities. There is also an inadequate disposal of the refuse generated by this activity (container and packaging materials) that are usually burned on the kilns.
All brickmakers were invited to participate in the study. They were informed about the research characteristics and the need to take a blood sample from each. Persons agreeing to take part in the study signed an informed consent letter and answered a questionnaire on their demographic characteristics (age, place of residence, origin) and employment history (years in a brick production-related job and protective equipment used).
2.2. Study Groups
Based on questionnaire responses, study participants were classified into two groups: occupationally exposed (OE) and unexposed or control. Participants in the OE group were selected according to the following criteria: more than ten years on a brick production-related job and 20 to 50 years of age. Individuals receiving medical treatment or who had chronic degenerative diseases (cardiovascular disease, hypertension, and diabetes), smokers, alcoholics, and persons receiving radiation treatment or chemotherapy were excluded from the study. The control group was formed by individuals who did not come in contact with brickmaking activities and were similar in socioeconomic characteristics and age to OE participants, but whose work activity was not brick production-related. These volunteers were initially contacted at the ICB of the UACJ, where they

Figure 1. Location of the brickmaker’s community in Juarez City.
had gone to obtain health certificates.
2.3. Sampling
Blood samples were taken from participants following a 12-h fast in two collection tubes: one containing heaprin as an anticoagulant, the other with EDTA for use in hemoglobin level determination. Samples were placed in an ice bath for transport to the laboratory and immediate analysis.
2.4. Analyses
The following biomarkers were evaluated: lipid peroxidation (LPX), protein carbonyl content (PCC) to evaluate oxidized protein content and activity of the antioxidant enzymes: superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx). Hemoglobin levels and total protein content were used to express results.
2.4.1. Determination of LPX
LPX was determined by the Büege and Aust (1978) method [17], which is based on quantification of malondialdehyde (MDA), the end product of lipid peroxidetion. To 500 µL blood was added Tris-HCl buffer pH 7.4 until a 1-mL volume was attained, plus 2 mL TCATBA reagent [15% trichloroacetic acid and 0.375% thiobarbituric acid (w/v)]. The sample was heated to boiling for 45 min, allowed to cool and the precipitate removed by centrifugation at 3000 rpm for 5 min. Absorbance was read at 535 nm against a reagent blank. Results were expressed as mmols MDA/g protein, and determination was made using a MEC of 1.56 × 105 mol/cm.
2.4.2. Determination of PCC
PCC was determined by the method of Levine et al. (1994) [18]. Soluble proteins were obtained by centrifugation of samples at 10,500 rpm for 30 min. To 100 µL of this supernatant was added 150 µL of 10 mM dinitrophenylhydrazine in 2 M HCl (Sigma) prior to incubation at room temperature for 1 h in the dark. Subsequently, 500 µL of 20% trichloroacetic acid was added and the sample was kept at 4˚C for 15 min. Next, this was centrifuged at 16,000 rpm for 5 min. The bud was rinsed three times in 1:1 ethanol:ethyl acetate (Baker), dissolved in 150 µL of 6 M guanidine (Sigma) pH 2.3, and incubated at 37˚C for 30 min. Absorbance was read at 366 nm and results were expressed as nmols of reactive carbonyls (C=O) formed/g protein based on their molar extinction coefficient of 21,000 M–1·cm–1.
2.4.3. Determination of SOD
SOD activity was determined by the modified Misra and Fridovich (1972) method [19], which is based on inhibition of autoxidation of adrenaline at pH 10.2 in hemoglobin-free lysates from erythrocytes and other proteins. In a quartz cuvette were placed 150-μL aliquots of homogenate (obtained from 500 μL total blood in 2 mL distilled water, sonicated for 15 min, then supplemented with 2.5 mL of 1:1 ethanol:chloroform). Addition was then made of 750 μL carbonate buffer solution pH 10.2 (50 mM sodium bicarbonate, 0.1 mM EDTA, adjusted to pH 10.2 with Na2CO3 in powdered form) and 600 μL adrenaline (30 mM) in 0.05% acetic acid. Absorbance was read at 0 s, 30 s and 5 min, at 480 nm. Results were expressed as µmols SOD/g protein. Estimates were derived by the formula [SOD] = (A30s − A5min) * (A0/MEC), where the molar extinction coefficient (MEC) of adrenaline is 21/M/cm.
2.4.4. Determination of CAT
CAT activity was quantified according to the Radi et al. (1991) method [20], which is based on disappearance of H2O2 as a result of CAT action through change in absorbance per minute. To 20 μL erythrocyte homogenate plus 1 mL isolation buffer solution (0.3 M sucrose; 1 mM HEPES; 5 mM KH2PO4 adjusted to pH 7.4) was added 200 µL H2O2 (20 mM), reading absorbance at 0 and 60 s, at 240 nm in quartz cuvettes. Results were expressed as µmols H2O2/mg protein. Estimates were obtained using the formula [H2O2] = (A0s − A60s)/MEC, where the MEC of H2O2 is 0.043/mM/cm.
2.4.5. Determination of GPx Activity
GPx activity was determined by the Gunzler and FloheClairborne (1985) method [21] as modified by Stephensen et al. (2000). To 100 μL of supernatant was added 10 μL glutathione reductase (2 U glutathione reductase, Sigma-Aldrich), plus 290 μL reaction buffer [50 mM K2HPO4 (Vetec), 50 mM KH2PO4 (Vetec) pH 7.0, 3.5 mM reduced glutathione (Sigma-Aldrich), 1 mM sodium azide (Sigma-Aldrich) and 0.12 mM NADPH (Sigma-Aldrich)] and 100 µL H2O2 (0.8 mM, Vetec). Absorbance was read at 340 nm at 0 and 60 s. Enzyme activity was estimated using the equation: GPx concentration = (A0s − A60s)/MEC), where the MEC of NADPH = 6.2 mM/cm. Results were expressed as mM NADPH/g protein.
2.4.6. Determination of Total Protein Content
Total protein content was quantified by the Bradford (1976) method [22]. To 25 µL of sample (blood or erythrocyte homogenate) was added distilled water until a volume of 100 µL was attained. Next, 2.5 mL was added of Bradford’s reagent (100 mg Coomassie Blue dye, 50 mL of 95% ethanol, 100 mL H2PO4) plus distilled water to attain a final volume of 1 L. The sample was shaken, and then it was allowed to rest for 5 min. Absorbance was read at 595 nm and results were interpolated on a standard bovine serum albumin (BSA) curve at a concentration range of 10 to 200 µg/mL.
2.4.7. Determination of Hemoglobin
Hemoglobin was determined using a Beckman Coulter AcT Diff hematology analyzer.
2.5. Statistical Analysis
Descriptive analysis was performed and measures of central tendency were determined, subsequently testing the normality and homoscedasticity of the values obtained. To find significant differences among study group variables, Kruskal-Wallis one-way analysis was applied followed by Dunn’s multiple comparisons test. The level of significance was set at p ≤ 0.05.
3. RESULTS AND DISCUSION
3.1. General Characteristics of the Study Population
The main characteristics of participants in the study are summarized in Table 1. Of the total number of OE individuals, 100% were men, with a mean age of 40.9 years (range 25 - 55 years). Control group individuals numbered 30; 100% were men, with a mean age of 38.5 years (range 22 - 53 years) (Table 1).
The mean residence time of study participants in the brick production region of Juarez city, in the State of Chihuahua was 20 years (range 1 - 55 years) and the mean time in a brickmaker-related job was 15 years (range 5 years to 40 years), indicating chronic exposure to a wide spectrum of potentially health-damaging pollutants that include coarse, fine and ultrafine particles, carbon monoxide (CO), oxides of nitrogen and sulphur, transitional metals, polycyclic aromatic hydrocarbons, volatile organic compounds and bioaerosols. As regards the use of protective equipment during work, 100% of OE participants said that they did not use face masks, gloves, respirators, coveralls, and protective eyewear.
Activities carried out by brick makers workers were grouped into one category: field work. The majority of individuals in the OE group do not use protective equipment. As a result, they come in greater contact with different xenobiotics via any one of the potential absorption routes (dermal, inhalatory, digestive, or through the mucosa) which, combined with different temperature gradients and poor ventilation, poses increased risks to their health.
Brick making involves environmental release of dif-
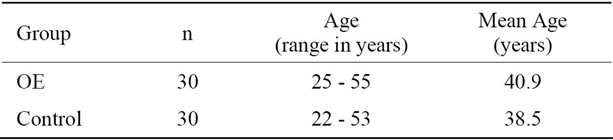
Table 1. Gender and age of the study population.
ferent substances, some of them have already been characterized. Gaseous effluents in the Estrella del Poniente MK2 kilns were sampled only for selected burns of the total series. For these cases, the effluents from the active and filter (passive) kiln were sampled every hour. Three of the collected filters were analyzed to determine the presence of semivolatile compounds. The Polycyclic Aromatic Hydrocarbons (PAHs), with low molecular weight were detected with GC/mass spectrometry, and the PAHs with high molecular weight were detected by HPLC. The PAHs most frequently found were naphthalene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(b)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, dibenzo(a,h)anthrancene, and indeno (1,2, 3-cd)pyrene [10]. The concentration of metals (micrograms per cubic meter) produced as effluents during the oil burn in the Estrella del Poniente kiln were lead (Pb), chromium (Cr), cadmium (Cd) and barium (Ba) [10].
Metals play important roles in a wide variety of biological processes of living systems. Homeostasis of metal ions, maintained through tightly regulated mechanisms of uptake, storage and secretion is therefore critical for life and is maintained within strict limits [23]. Metal ion transporters participate in maintaining the required levels of the various metal ions in the cellular compartments.
Detailed studies in the past two decades have shown that redox active metals like iron (Fe), copper (Cu), cobalt (Co), Cr, Pb, Cd and other metals undergo redox cycling reactions and possess the ability to produce reactive radicals such as superoxide anion radical and nitric oxide in biological systems [24-28]. Disruption of metal ion homeostasis may lead to oxidative stress, a state where increased formation of reactive oxygen species (ROS) overwhelms body antioxidant protection and subsequently induces DNA damage, lipid peroxidetion, protein modification and other effects, all symptomatic for innumerable diseases, involving cancer, cardiovascular disease, diabetes, atherosclerosis, neurological disorders (Alzheimer’s disease, Parkinson’s disease), chronic inflammation and others [29-32].
3.2. Stress Oxidative Biomarkers
3.2.1. LPX and PCC
Biomarker results show a significant variation in the OE group compared to the control group. LPX results of our study obtained in blood samples of the study population (Figure 2) show significant increases (p ≤ 0.05) in the OE group (14.2%) compared to the control group. As well as PCC results (Figure 3) obtained in OE group show significant increases of 5% compared to the control group (p ≤ 0.05).
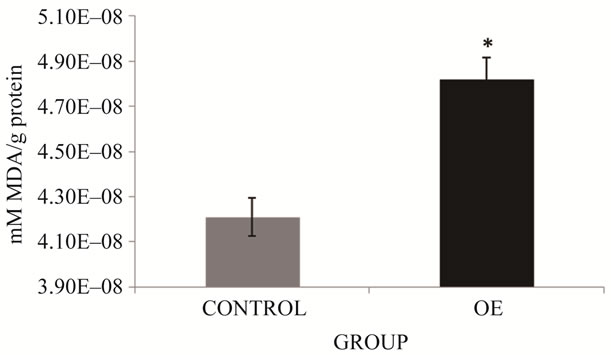
Figure 2. LPX in brick maker workers from Juarez City, Chihuahua, Mexico. The mean for the total number of individuals in each study group is shown. Error bars indicate standard deviation. *Significantly different from the control group at p ≤ 0.05.
These results could be explained by the presence of metals emitted in the kiln (Pb, Cd, Cr y Ba). The underlying mechanism of action for all these metals involves formation of the superoxide radical, hydroxyl radical (mainly via Fenton reaction) and other ROS, finally producing mutagenic and carcinogenic malondialdehyde (MDA), 4-hydroxynonenal (HNE) and other exocyclic DNA adducts. On the other hand, the redox inactive metals, such as Cd, Pb and arsenic (As) show their toxic effects via bonding to sulphydryl groups of proteins and depletion of glutathione. More specifically, intermediate oxidation states of Cr (V an IV) are also proposed to play a role in chromium genotoxicity and carcinogenicity, either directly or through reaction (e.g. via the Fenton reaction) with other cellular components, resulting in the generation of reactive oxygen species [33,34].
Cd itself is unable to generate free radicals directly, however, indirect formation of ROS and RNS involving the superoxide radical, hydroxyl radical and nitric oxide has been reported [35]. Some experiments also confirmed the generation of non-radical hydrogen peroxides which itself in turn may be a significant source of radicals via Fenton chemistry. An interesting mechanism explaining the indirect role of Cd in free radical generation was presented, in which it was proposed that Cd can replace iron (Fe) and Cu in various cytoplasmic and membrane proteins (e.g. ferritin, apoferritin), thus increasing the amount of unbound free or poorly chelated Cu and Fe ions participating in oxidative stress via Fenton reactions [36]. These results are supported by recent findings by Watjen and Beyersmann [37].
Also, free radical-induced damage by Pb is accomplished by two independent, although related mechanisms [28]. The first involves the direct formation of ROS including singlet oxygen, hydrogen peroxides and hydroperoxides and the second mechanism is achieved

Figure 3. PCC in brick maker workers from Juarez City, Chihuahua, Mexico. The mean for the total number of individuals in each study group is shown. Error bars indicate standard deviation. *Significantly different from the control group at p ≤ 0.05.
via depletion of the cellular antioxidant pool. Interrelations between these two mechanisms exist so that the increase in ROS on one side simultaneously leads to depletion of antioxidant pools on the other [38].
On the other hand, it is now well known that the metabolic activation by enzyme-catalyzed reactions of the PAHs (Cycloheptene, benzo(b+k)Xuoranthene, benzene, benzo(a)pyrene, dimethylbenzene, phenanthrene, ethenylethylbenzene, pyrene, ethylmethylbenzene) could result in the excessive production of ROS capable of interfereing with cell homeostasis and produce oxidative stress [39,40]. Some of these compounds were also found in the study site [10] and could contribute to the increased level of lipid peroxidation and protein oxidation found in our study.
3.2.2. Antioxidant Enzymes
During lifetime, an antioxidant-sophisticated network counteracts the deleterious action of ROS on macromolecules [41] Cells synthesize some of their antioxidants, as the enzymes SOD, CAT and GPx, as well as the peptides with thiols groups, as glutathione (GSH) and thioredoxin (TRX) family. These systems play an important role in the ability of the body to respond to the oxidant challenge of using molecular oxygen to drive reactions that yield the necessary energy. In eukaryotic organism, several ubiquitous primary antioxidant enzymes, such as SOD, CAT, and different forms of peroxidases work in a complex series of integrated reactions to convert ROS to more stable molecules, such as water and molecular oxygen. Secondary enzymes act in concert with small molecular-weight antioxidants to form redox cycles that provides necessary cofactors for primary antioxidant enzymes functions. The small molecular weight antioxidants (e.g. GSH, NADPH, TRX, vitamins E and C, trace metals, such as selenium) can also function as direct scavengers of ROS. This complex system has the ability to both maintain an intracellular redox balance and prevent or reduce molecular damage by ROS [41, 42].
Various antioxidants (both enzymatic and non-enzymatic) provide protection against deleterious metal-mediated free radical attacks. Generally, antioxidants can protect against redox-metal (Fe, Cu) toxicity by i) chelating ferrous ion and preventing the reaction with molecular oxygen or peroxides, ii) chelating iron and maintaining it in a redox state that makes iron unable to reduce molecular oxygen and iii) trapping any radicals formed. One of the most effective classes of antioxidants are thiol compounds, especially glutathione, which provide significant protection by trapping radicals, reduce peroxides and maintain the redox state of the cell [43].
It is known that increased ROS production is related to the increase of antioxidant enzymes. A marked increase of SOD activity was found in OE group (8.9%) compared to controls (*p < 0.05) (Figure 4). Comparison of CAT activity results among study groups found a 16.4% increase of this activity in the OE group, which differed significantly from activity in the control group (p < 0.05) (Figure 5).
Finally, GPx results (Figure 6) obtained in OE group show significant increases of 209.7% compared to the control group (p ≤ 0.05). The increase of antioxidant enzymes in our study could be due to the presence of ROS and NOS. In the case of SOD, the presence of superoxide anion radical might stimulate increased activity. It is well known that superoxide dismutase (SOD) converts O2* to H2O2; as well as, catalase (CAT), which reduces H2O2 to water; and glutathione peroxidase (GPx) which detoxifies H2O2 or organic hydroperoxides to water [12]. The products of the metabolism of metals and PAH (superoxide anion radical and hydrogen peroxide) could be responsible for the increased activity of the enzymes tested.
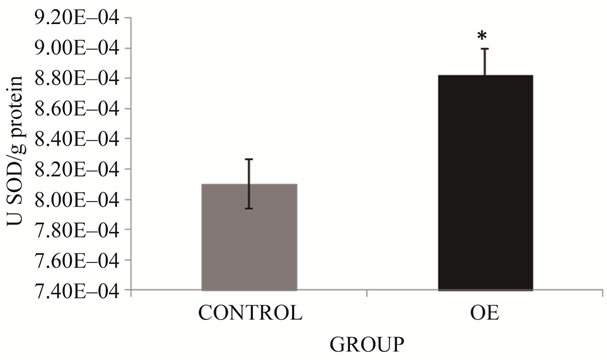
Figure 4. SOD activity in brick maker workers from Juarez City, Chihuahua, Mexico. The mean for the total number of individuals in each study group is shown. Error bars indicate standard deviation. *Significantly different from the control group at p ≤ 0.05.
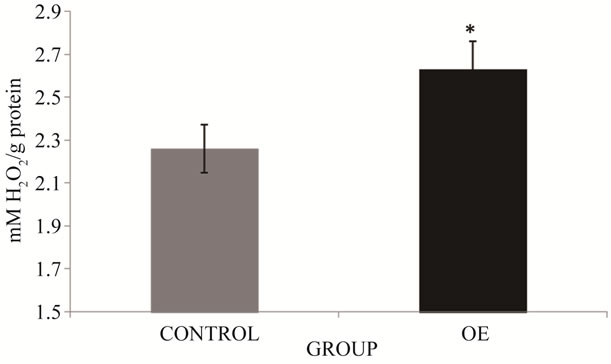
Figure 5. CAT activity in brick maker workers from Juarez City, Chihuahua, Mexico. The mean for the total number of individuals in each study group is shown. Error bars indicate standard deviation. *Significantly different from the control group at p ≤ 0.05.
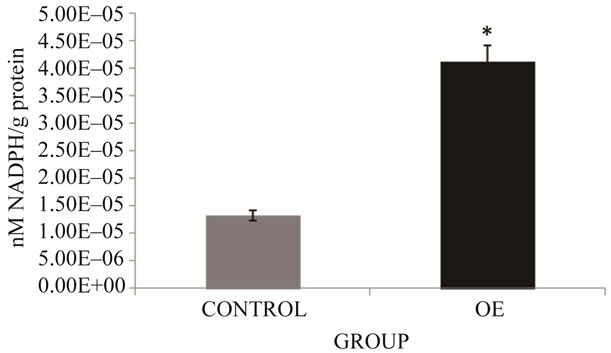
Figure 6. GPx activity in brick maker workers from Juarez City, Chihuahua, Mexico. The mean for the total number of individuals in each study group is shown. Error bars indicate standard deviation. *Significantly different from the control group at p ≤ 0.05.
4. CONCLUSION
The population of Estrella del Poniente in Juárez City, Chihuahua, Mexico is at potential risk, since brickmakers show high exposure to extremely toxic substances (PAH and metals) as a result of unsafely performed work. They do not use protective equipment such as face masks, gloves, respirators, coveralls, nor protective eyewear. Determination of a set of biomarkers for oxidative stress is important for early detection of the effects of the pollutants in order to prevent health damage on the exposed population.
5. ACKNOWLEDGEMENTS
The authors acknowledge the participation and attitude of the brickmakers in Juarez City, Chihuahua to collaborate in this study.
REFERENCES
- Fullerton, D.G., Bruce, N. and Gordon, S.B. (2008) Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Transactions of the Royal Society of Tropical Medicine and Hygiene, 102, 843-851. doi:10.1016/j.trstmh.2008.05.028
- Lin, H.H., Ezzati, M. and Murray, M. (2007) Tobacco smoke, indoor air pollution and tuberculosis: A systematic review and metaanalysis. PLoS Med, 4, e20. doi:10.1371/journal.pmed.0040020
- Laden, F., Schwartz, J., Speizer, F.E. and Dockery, D.W. (2006) Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. American Journal of Respiratory and Critical Care Medicine, 173, 667-672. doi:10.1164/rccm.200503-443OC
- Pope III, C.A., Burnett, R.T., Thun, M.J., Calle, E.E., Krewski, D., Ito, K. and Thurston, G.D. (2002). Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Journal of the American Medical Association, 287, 1132-1141. doi:10.1001/jama.287.9.1132
- Corral-Avitia, A.Y. and De la Mora-Covarrubias, A. (2012) Environmental assessment of brick kilns in Chihuahua State, Mexico, using digital cartography. In: Mahamane, A., Ed., The Functioning of Ecosystem, Rijeka, Croatia, 271-282.
- Sinha, S.N., Kulkarni, P.K., Shah, S.H., Desai, N.M., Patel, G.M., Mansuri, M.M. and Saiyed, H.N. (2006) Environmental monitoring of benzene and toluene produced in indoor air due to combustion of solid biomass fuels. Science Total Environmental, 357, 280-287. doi:10.1016/j.scitotenv.2005.08.011
- Naeher, L.P. Brauer, M., Lipsett, M., Zelikoff, J.T., Simpson, C.D., Koenig, J.Q. and Smith, K.R. (2007) Woodsmoke health effects: A review. Inhalation Toxicology, 19, 67-106. doi:10.1080/08958370600985875
- Straif, K., Baan, R., Grosse, Y., Secretan B., Ghissassi, F. and Cogliano, V. (2006) WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of household solid fuel combustion and of high-temperature frying. Lancet Oncology, 7, 977-978. doi:10.1016/S1470-2045(06)70969-X
- Danielsen, S., Loft, A., Kocbach, P., Schwarze, E. and Møller, P. (2009) Oxidative damage to DNA and repair induced by Norwegian wood smoke particles in human A549 and THP-1 cell lines. Mutation Research, 674, 116- 122. doi:10.1016/j.mrgentox.2008.10.014
- Bruce, C.W., Corral, A.Y. and Lara, A.S. (2007) Development of cleaner burning brick kilns in Ciudad Juarez, Chihuahua, México. Journal of the Air & Waste Management Association, 57, 444-456. doi:10.3155/1047-3289.57.4.444
- Blackman, A., Newbold, S., Shih, J.-S. and Cook, J. (2000) The benefits and costs of informal sector pollution control: Mexican brick kilns. Resources for the Future. Washington DC.
- Barata, C., Varo, I., Navarro, J.C., Arun, S. and Porte, C. (2005) Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds. Comparative Biochemistry and Physiology, 140, 175-186.
- Abdollahi, M., Ranjbar, A., Shadnia. S., Nikfar, S. and Rezaie, A. (2004) Pesticides and oxidative stress: A review. Medical Science Monitor, 10, 141-147.
- Livingstone, D.R. (2003) Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Revue de Medicine Veterinaire, 154, 427-430.
- Halliwell, B. and Gutteridge, J.M.C. (1999) Free radicals in biology and medicine. 3rd Edition, Oxford University Press, Oxford.
- Rikans, L.E. and Hornbrook, K.R. (1997) Lipid peroxidetion, antioxidant protection and aging. Biochimical et Biophysical Acta, 1362, 116-127. doi:10.1016/S0925-4439(97)00067-7
- Büege, J.A. and Aust, S.D. (1978) Microsomal lipid peroxidation. Methods in Enzymology, 52, 302-310. doi:10.1016/S0076-6879(78)52032-6
- Levine, R.L., Williams, J.A., Stadtman, E.R. and Shacter, E. (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods in Enzymology, 233, 346-357. doi:10.1016/S0076-6879(94)33040-9
- Misra, H.P. and Fridovich, I. (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological Chemistry, 247, 3170-3175.
- Radi, R., Turrens, J.F., Chang, L.Y., Bush, K.M., Carpo, J.D. and Freeman, B.A. (1991) Detection of catalase in rat heart mitochondria. Journal of Biological Chemistry, 266, 22028-22034.
- Gunzler, W. and Flohe-Clairborne, A. (1985) Glutathione peroxidase. In: Green-Wald, R.A., Ed., Handbook of Methods for Oxygen Radical Research, CRC Press, Boca Ratón, 285-290.
- Bradford, M. (1976) A rapid and sensitive method for the quantitation of microorganism quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry, 72, 248-254. doi:10.1016/0003-2697(76)90527-3
- Bertini, I. and Cavallaro, G. (2008) Metals in the “omics” world: Copper homeostasis and cytochrome c oxidase assembly in a new light. Journal of Biological Inorganic Chemistry, 13, 3-14. doi:10.1007/s00775-007-0316-9
- Cairo, G., Recalcati, S., Pietrangelo, A. and Minotti, G. (2002) The iron regulatory protein: Targets and modulators of free radical reactions and oxidative damage. Free Radical Biology and Medicine, 32, 1237-1243. doi:10.1016/S0891-5849(02)00825-0
- Cornejo, P., Tapia, G., Puntarulo, S., Galleano, M., Videla, L.A. and Fernandez, V. (2001) Iron-induced changes in nitric oxide and superoxide radical generation in rat liver after lindane or thyroid hormone treatment. Toxicology Letters, 119, 87-93. doi:10.1016/S0378-4274(00)00295-2
- Bagchi, D., Stohs, S.J., Downs, B.W., Bagchi, M. and Preuss, H.G. (2002) Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology, 180, 5-22. doi:10.1016/S0300-483X(02)00378-5
- Cuypers, A., Plusquin, M., Remans, T., Jozefczak, M., Keunen, E., Gielen, H., Opdenakker, K., Nair, A.R., Munters, E., Artois, T.J., Nawrot, T., Vangronsveld, J. and Smeets, K. (2010) Cadmium stress: An oxidative challenge. Biometals, 23, 927-940. doi:10.1007/s10534-010-9329-x
- Ercal, N., Gurer-Orhan, H. and Aykin-Burns, N. (2001) Toxic metals and oxidative stress. Part 1. Mechanisms involved in metal-induced oxidative damage. Current Topics in Medicinal Chemistry, 1, 529-539. doi:10.2174/1568026013394831
- Gackowski, D., Kruszewski, M., Jawien, A., Ciecierski, M. and Olinski, R. (2001) Further evidence that oxidative stress may be a risk factor responsible for the development of atherosclerosis. Free Radical Biology and Medicine, 31, 542-547. doi:10.1016/S0891-5849(01)00614-1
- Fraga, C.G. and Oteiza, P.I. (2002) Iron toxicity and antioxidant nutrients. Toxicology, 180, 23-32. doi:10.1016/S0300-483X(02)00379-7
- Bush, A.I. (2008) Drug development based on the metals hypothesis of Alzheimer’s disease. Journal of Alzheimer’s Disease, 15, 223-240.
- Durackova, Z. (2010) Some current insights into oxidative stress. Physiological Research, 59, 459-469.
- O’Brien, T., Mandel, H.G., Pritchard, D.E. and Patierno, S.R. (2002) Critical role of chromium (Cr)-DNA interacttions in the formation of Cr-induced polymerase arresting lesions. Biochemistry, 41, 12529-12537. doi:10.1021/bi020452j
- Quievryn, G., Peterson, E., Messer, J. and Zhitkovich, A. (2003) Genotoxicity and mutagenicity of chromium (VI)/ ascorbate-generated DNA adducts in human and bacterial cells. Biochemistry, 42, 1062-1070. doi:10.1021/bi0271547
- Waisberg, M., Joseph, P., Hale, B. and Beyersmann, D. (2003) Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology, 192, 95-117. doi:10.1016/S0300-483X(03)00305-6
- Price, D.J. and Joshi, J.G. (1983) Ferritin. Binding of beryllium and other divalent metal ions. Journal of Biological Chemistry, 258, 10873-10880.
- Watjen, W. and Beyersmann, D. (2004) Cadmium-induced apoptosis in C6 glioma cells: Influence of oxidative stress. Biometals, 17, 65-78. doi:10.1023/A:1024405119018
- Gurer, H. and Ercal, N. (2000) Can antioxidants be beneficial in the treatment of lead poisoning? Free Radical Biology and Medicine, 29, 927-945. doi:10.1016/S0891-5849(00)00413-5
- Garçon, G., Garry, S., Gosset, P., Zerimech, F., Martin, A., Hannothiaux, M.H. and Shirali, P. (2001) Benzo(a)- pyrene-coated onto Fe2O3 particles induced lung tissue injury: role of free radicals. Cancer Letters, 167, 7-15. doi:10.1016/S0304-3835(01)00474-8
- Garçon, G., Ledoux, F., Hannothiaux, M.H., Zerimech, F., Puskaric, E. and Shirali, P. (2002) Urban particulate air pollution and evaluation of its toxicity on human pulmonary cells in culture. 6th International Aerosol Conference Taiwan.
- Tilman, G. (2005) Oxidants and antioxidants defense systems. In: The handbook of environmental chemistry 2.0. Springer Berlin Heidelberg, New York.
- Bonnefont-Rousselot, D., Therond, P., Beaudeux, J.L., Peynet, J., Legrand, A. and Delatrade, J. (2001) Aging and oxidative stress. Which potential markers? Annales de Biologie Clinique, 59, 453-459.
- Jomova, K. and Valko, M. (2011) Advances in metalinduced oxidative stress and human disease. Toxicology, 283, 65-87. doi:10.1016/j.tox.2011.03.001
NOTES
*Corresponding author.

