Journal of Behavioral and Brain Science
Vol.2 No.2(2012), Article ID:19581,4 pages DOI:10.4236/jbbs.2012.22028
Brain Activity in Sleep Compared to Wakefulness: A Meta-Analysis
1School of Psychology & Psychiatry, Monash University, Melbourne, Australia
2Research Imaging Institute, The University of Texas Health Science Center, San Antonio, USA
3School of Psychology & Psychiatry, The Alfred and Monash University, Monash Alfred Psychiatry Research Centre, Melbourne, Australia
Email: *russell.conduit@monash.edu
Received August 23, 2011; revised September 8, 2011; accepted February 22, 2012
Keywords: Positron Emission Tomography (PET); Sleep; Meta-Analysis; Thalamus; Prefrontal Cortex
ABSTRACT
Objective: Neuroimaging studies using a variety of techniques have been conducted in sleep to explore the changes in brain activity during the different sleep stages. The current study employed a quantitative meta-analytic technique in an attempt to integrate the findings from such studies. Methods: Using an updated version of the Activation Likelihood Estimation (ALE) method, individual meta-analyses were carried out on: 1) studies contrasting REM sleep and wakefulness, and 2) studies contrasting NREM sleep and wakefulness. Results: Based on the results of the current meta-analyses, a number of cortical and subcortical brain regions appear to be involved in sleep and sleep processes, with both decreases and increases noted across NREM and REM sleep. Specifically, areas of decreased activity comprised thalamic structures (pulvinar, dorsomedial thalamus) and frontal regions (inferior, superior, and middle frontal gyrus). Furthermore, increased and decreased activity was noted in the anterior cingulate during sleep. Conclusions: Despite limited overlap across these sleep stages among regions identified, consistent decreases were revealed in NREM sleep (thalamus) and REM sleep (frontal cortex) when compared to wakefulness. Such findings suggest that these regions may ultimately play a key role in the loss of consciousness characteristic of sleep. Further research is needed to determine if and how such activity may be related to dreaming.
1. Introduction
Researchers have long been fascinated with sleep and since the discovery of rapid eye movement (REM) sleep in 1953 [1], the measurement of brain activity has moved beyond the use of the electroencephalogram (EEG) to include more advanced techniques such as neuroimaging. Currently a number of neuroimaging studies exist in the published literature that have attempted to reveal the patterns of brain activity occurring during sleep using methods such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). Such studies have investigated spontaneous changes in brain activity across different sleep stages, changes in brain activity during sleep following training on a cognitive task, as well as changes in brain activity during sleep following sleep deprivation or the administration of a pharmacological agent. There are also considerable differences in the participants sampled across such studies, the definitions employed for various sleep processes (i.e. dreaming), and sleep scoring procedures. Thus, achieving an accurate picture of the sleeping brain from such diverse methods and findings is difficult. Nevertheless, some researchers (e.g., [2,3]) have reviewed a collection of such studies and drawn conclusions regarding the likely cortical and subcortical regions involved in sleep.
In order to elucidate the nature of consistent activity within a certain class of imaging studies [4], functionlocation meta-analytic techniques such as the Activation Likelihood Estimation (ALE) technique [5] were developed. The ALE method essentially measures the overlap between a set of studies, which is achieved by modeling each significant location of activation (or deactivation) as the centre of a Gaussian probability distribution [5]. A visual map of the brain is then produced with estimates of the probability of activation (or deactivation) for voxels, based on the given set of studies. Thus, such an approach provides a considerable amount of information when compared to the traditional method of simply tabulating significant results. Recently, an updated version of the ALE technique has been developed to address limitations associated with the previous version, such as the need to specify the size of the probability distributions, the inclusion of significant activations/deactivations in deep white matter, and the emphasis on testing for above-chance clustering of individual foci (fixed-effects) [6]. To date, the ALE technique has not been applied to the area of sleep research. Thus, the purpose of this study was to use this updated ALE method to analyze sleep studies that have employed neuroimaging techniques. It was hypothesized that a quantitative meta-analysis of such studies would confirm a pattern of prefrontal hypoactivity during REM and NREM sleep, and hyperactivity in posterior brain regions (e.g. Parietal-Temporal-Occipital (PTO) junction) during REM sleep.
2. Methods
2.1. Literature Search
A PubMed search was conducted to identify neuroimaging studies during sleep available as of December 2010 using the terms “sleep”, “neuroimaging”, “imaging”, “fMRI”, and “PET”. The reference list of all identified studies were also examined. Studies that were carried out in clinical populations, administered cognitive tasks or pharmacological agents, or presented stimulation of any kind were excluded. Additionally, only studies that reported three-dimensional stereotactic coordinates extracted from single data sets (i.e. original studies) were included. The separate contrasts reported in these studies were then divided into the following three categories: 1) REM sleep versus NREM sleep; 2) REM sleep versus wakefulness; and 3) NREM sleep versus wakefulness. The contrasts in each of the above categories were subsequently screened to determine if there were groups of studies with similar methods (i.e. PET, fMRI, SPECT). Although there is no absolute minimum number of studies required for ALE analysis, a suitable number of studies were identified only for the latter two groups (five studies with similar imaging techniques). Thus, as there were an insufficient number of studies contrasting REM and NREM sleep using similar imaging methods, this comparison was not conducted.
2.2. Meta-Analysis Procedures
Coordinates originally published in MNI space (e.g., [7,8]) were converted to Talairach space using the Lancaster (icbm2tal) transformation [9]. The techniques applied in this analysis employed the updated version of ALE that has previously been described in detail [6]. The identified literature coordinates of maximum activation and maximum deactivation (significant at p < 0.05) were separately modeled with a three-dimensional Gaussian distribution, and convergence across experiments was quantitatively assessed. Rather than using a prespecified fullwidth half-maximum (FWHM) as in the original ALE approach, an algorithm was used to model the spatial uncertainty of each focus using an estimation of the inter-subject and inter-laboratory variability typically observed in neuroimaging experiments. This algorithm limits the meta-analysis to an anatomically constrained space specified by a gray matter mask, and includes a method that calculates the above-chance clustering between experiments (i.e. random-effects analysis), rather than between foci (i.e. fixed-effects analysis) [6]. The 3D modeled activation images are pooled to produce a statistical map that estimated the likelihood of activation for each voxel as determined by all the studies in the analysis. ALE was performed in Talairach space using GingerALE 2.1, distributed by the BrainMap Project [10,11]. The resultant ALE map was thresholded at a false discovery rate (FDR) corrected threshold of p < 0.05 [12]. A minimum cluster size of 200 mm3 was applied. Locations of the voxels with peak probabilities within clusters and the cluster sizes were identified. Peak locations for each ALE cluster were assigned an anatomical label using the Talairach Daemon ([13] www.talairach.org). ALE results were overlaid onto an anatomical template generated by spatially normalizing the International Consortium for Brain Mapping (ICBM) template to Talairach space [14]. Following the meta-analyses, an attempt was made to identify overlapping regions (i.e. brain areas activated during both REM and NREM sleep, brain areas deactivated during both REM and NREM sleep).
3. Results
The above literature search and review process yielded a number of PET studies which fell into two groups: 1) REM sleep versus wakefulness, and 2) NREM sleep versus wakefulness (Table 1). For the first group, forty-three foci from five studies were identified which reported areas of increased activation during REM sleep compared to wakefulness, and 37 foci from five studies reporting decreased activation. Consistent increases in brain activity were found in the anterior cingulate as well as other subcortical areas. Decreased brain activity was consistently observed in a number of frontal regions, such as the middle, inferior and superior frontal gyrus (Table 2). For the second group, 30 foci were identified from four studies that reported increased activation in NREM sleep, as well as 62 foci identified in five studies which reported decreased activation. In NREM sleep, consistent increases in brain activity were found in temporal and occipital regions (superior temporal gyrus and cuneus, respectively), as well as the anterior cingulate. Consistent decreases in activity were found in various subcortical structures such as the thalamus (i.e. pulvinar, dorsal nucleus, ventral posterior medial nucleus) and anterior cingulate, along with circumscribed frontal (inferior frontal gyrus) decreases (Table 3). Figure 1 presents the results of all four comparisons superimposed together over an image of a standard brain.
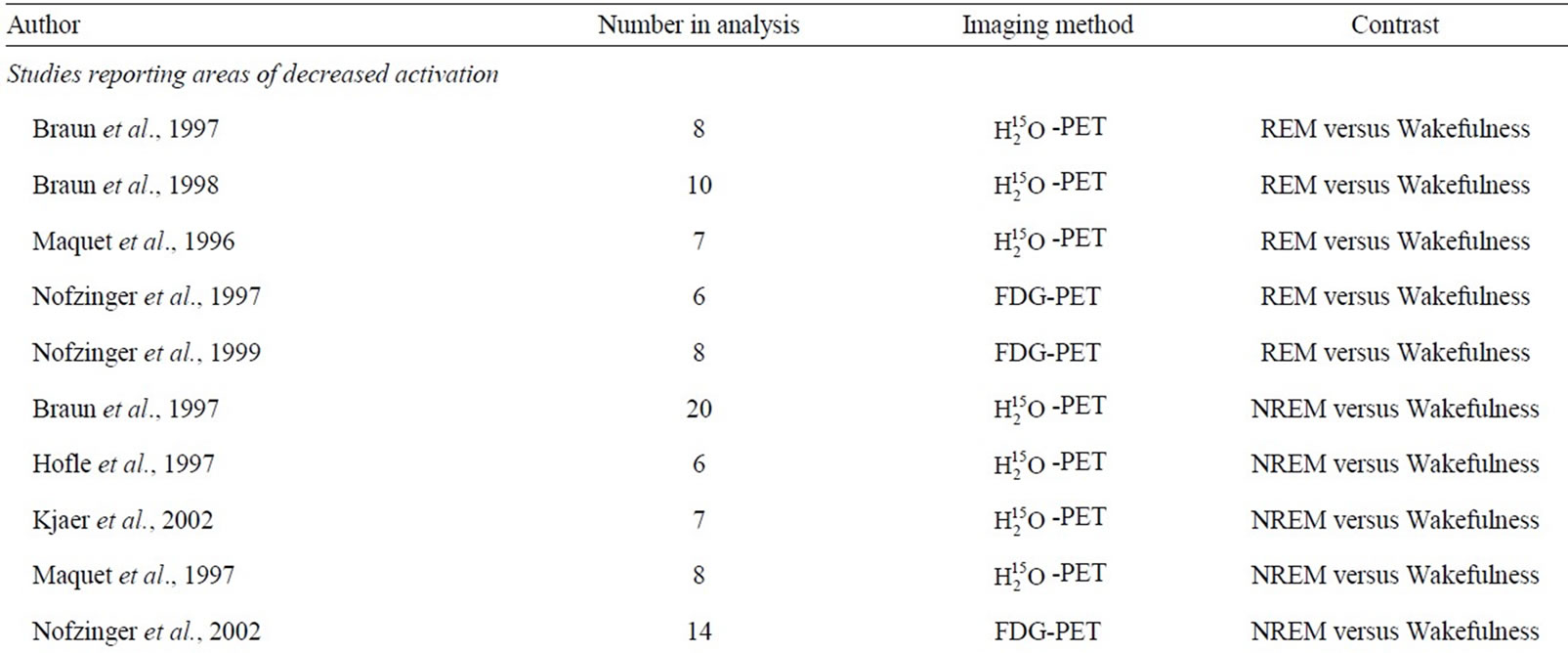
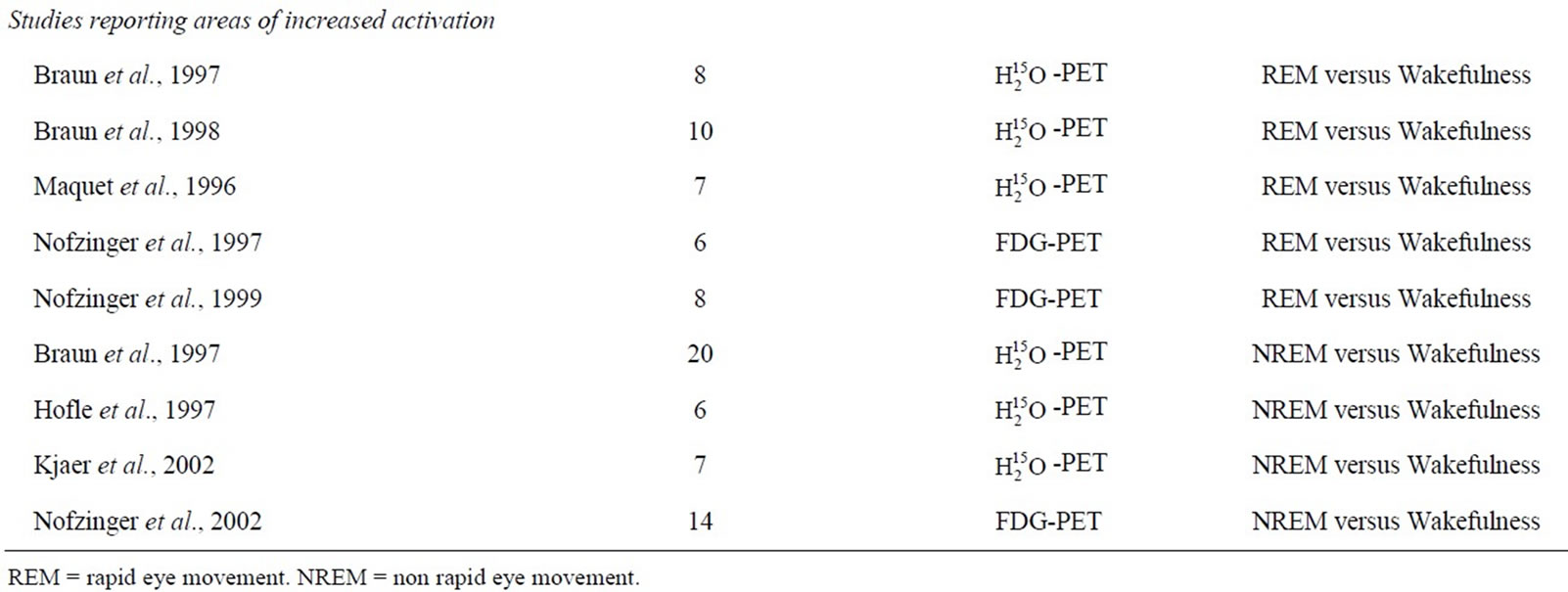
Table 1. Included studies.
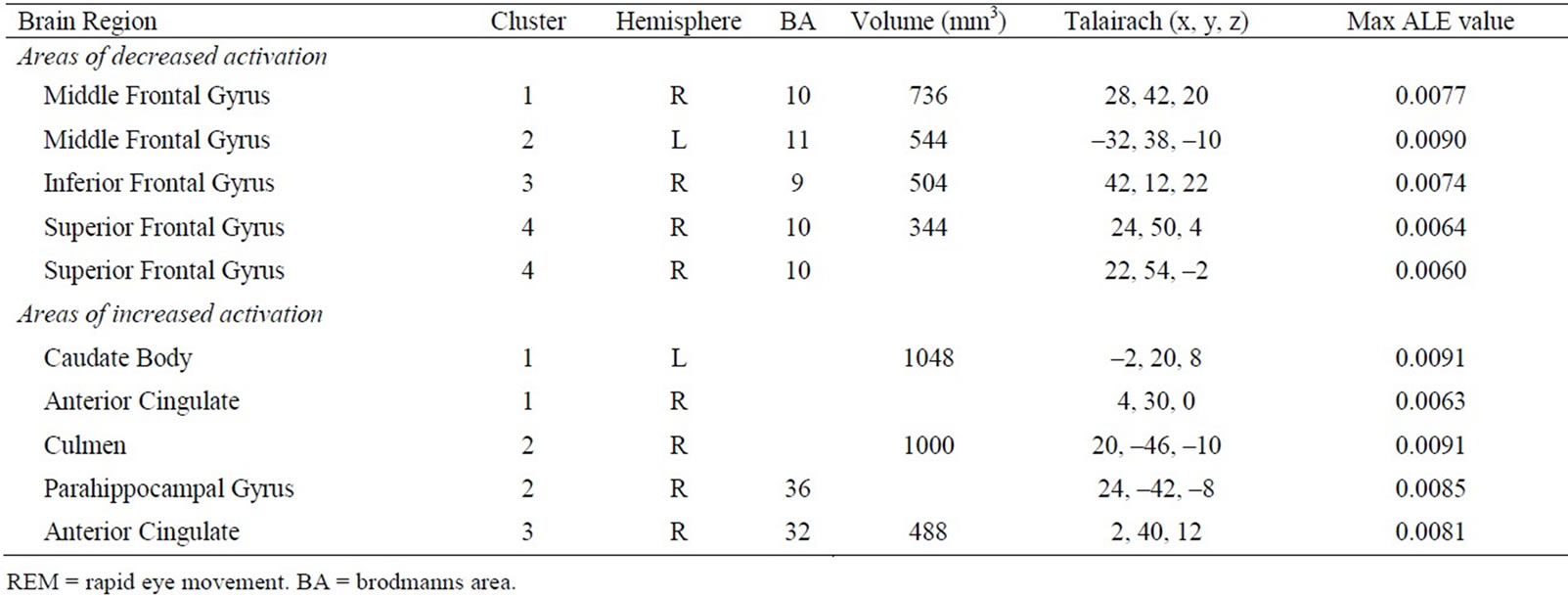
Table 2. Results of included PET studies investigating regional brain activity during REM sleep compared to wakefulness.
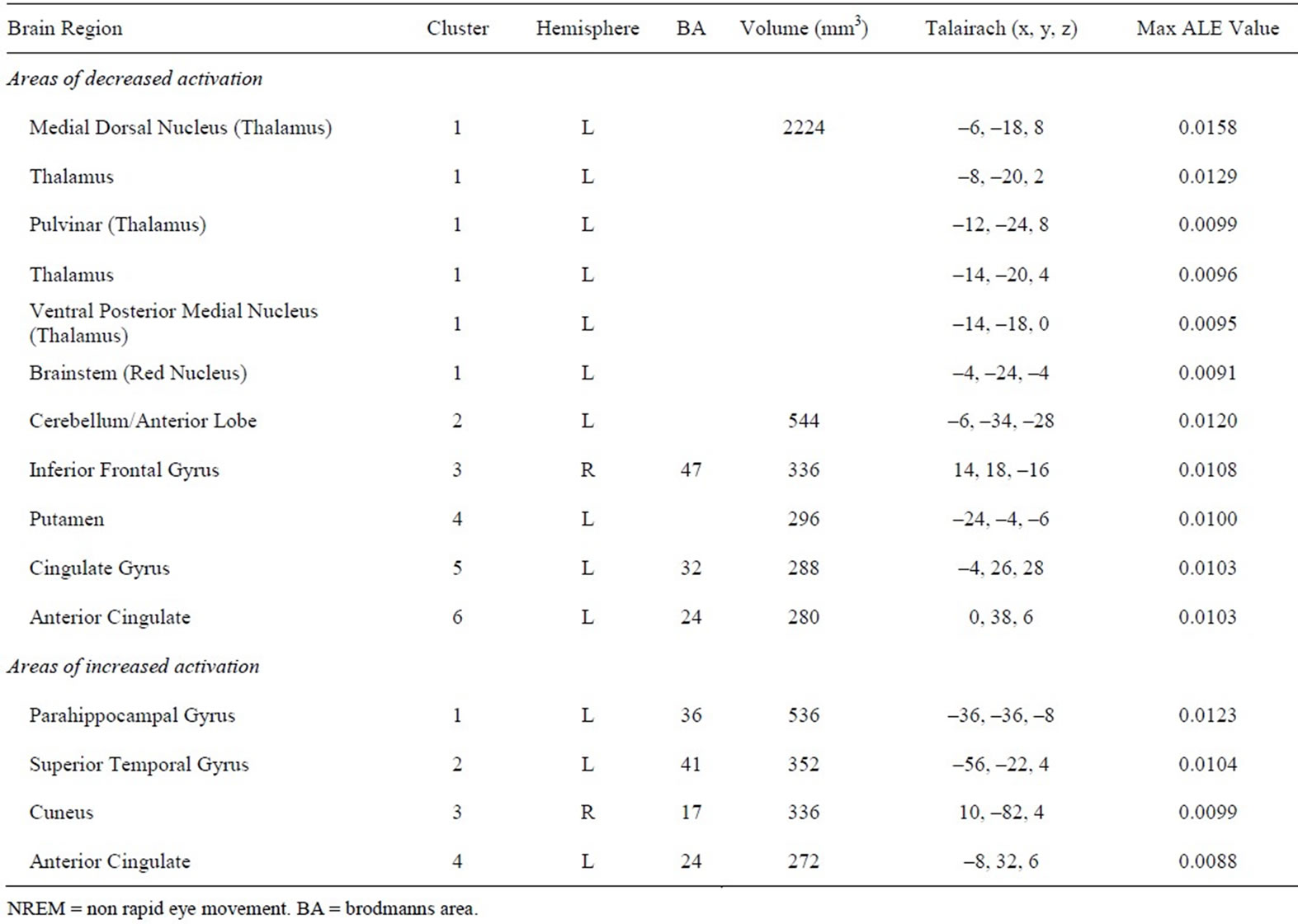
Table 3. Results of included PET studies investigating regional brain activity during NREM sleep compared to wakefulness.
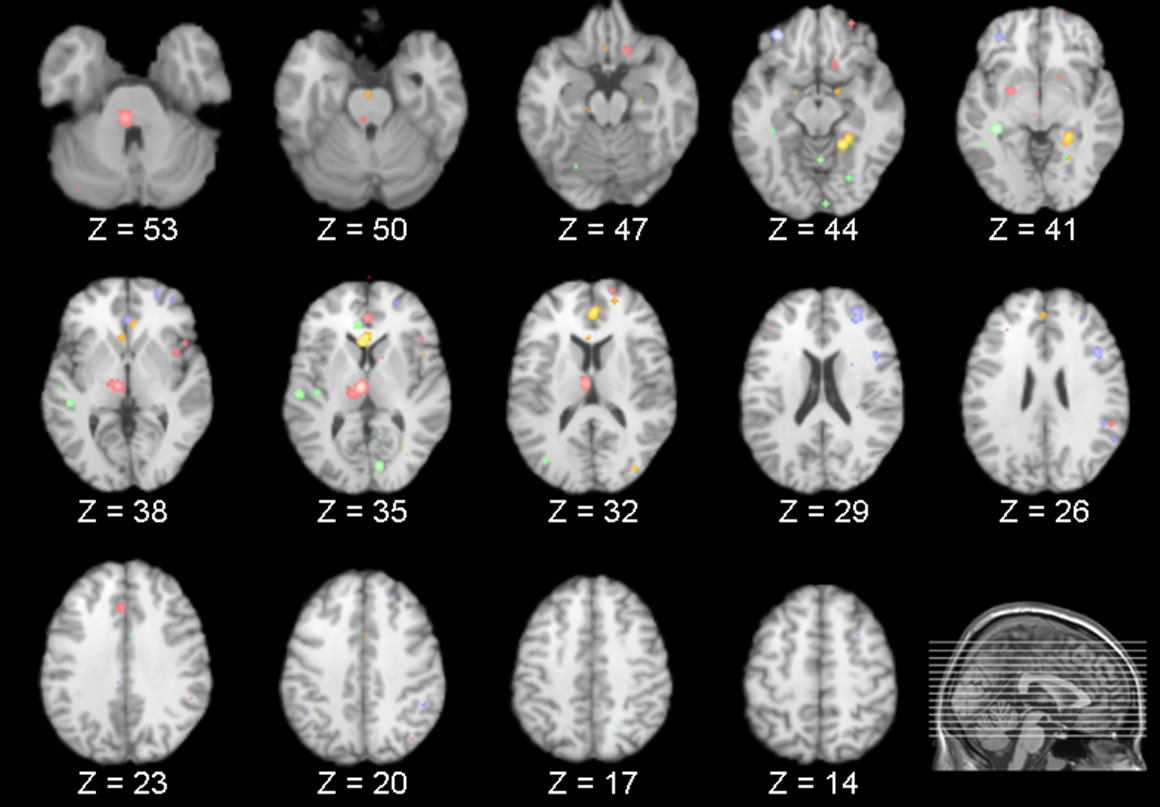
Figure 1. Each slice shows areas of increased (green) and decreased activity (red) in NREM and increased (gold) and decreased activity (blue) in REM sleep compared to wakefulness. Consistent decreases in the thalamus and frontal areas can be seen in both REM and NREM sleep.
Overlapping Regions
By superimposing the aforementioned comparisons, a limited set of brain areas were found to overlap. Specifically, from the results of the meta-analyses it was revealed that the inferior frontal gyrus exhibited significantly decreased activity during both REM and NREM sleep when compared to wakefulness, with a degree of overlap noted from the subsequent overlays. In addition, compared to wakefulness, the anterior cingulate exhibited significant increases during REM sleep as well as significant increases and decreases in NREM sleep. These areas were also found to overlap.
Although the meta-analyses found that the thalamus was significantly decreased during NREM and the superior and medial frontal gyrus significantly decreased in REM sleep, no overlap was noted for these areas as no significant increases/decreases were identified in the corresponding brain regions during REM and NREM sleep, respectively.
4. Discussion
This study aimed to analyze the results of neuroimaging studies investigating the neurophysiology of sleep. The findings from this study suggest that although there were a limited number of studies included in the analyses, there appears to be a specific set of brain regions involved in sleep, broadly related to the thalamus, frontal cortex and the anterior cingulate gyrus. In addition, although there was limited overlap across REM and NREM sleep among regions identified, it is important to note that the neurophysiology of sleep is complex with a number of brain areas likely involved, each with inherent differences in the level of activity during different sleep stages. Consequently, such activity may not be effectively captured using PET techniques or easily identified using the applied meta-analytic methods.
4.1. Thalamic Activation Patterns during Sleep
The results of the above meta-analysis of neuroimaging studies demonstrate that the thalamus is involved in sleep processes, with activity across a large portion of the thalamus found to be decreased during NREM sleep (i.e. dorsomedial thalamus, pulvinar, ventral posterior medial thalamus). Such findings are consistent with its known role in sleep regulation. Specifically, the thalamus is involved in the generation and propagation of sleep spindles [15] and k-complexes in NREM sleep [16]. In disorders that involve disruptions of the sleep-wake cycle (i.e. fatal familial insomnia [FFI]), such activity is absent. For instance, even before individuals with FFI become symptomatic (e.g. insomnia, stupors) the first significant abnormality observed is an absence of sleep spindles and k-complexes, as well as a concomitant decrease in thalamic metabolism [17]. These alterations in thalamic activity (reduced thalamic metabolism and the loss of these sleep rhythms) have been attributed to the marked reduction in a number of thalamic neuronal populations, particularly within anterior ventral and dorsomedial regions [18]. The involvement of these thalamic areas in sleep rhythms and the pathophysiology of FFI is further supported by studies which have revealed that bilateral lesions of the dorsomedial thalamus result in the loss of slow waves and sleep spindles [19]. Thus, the pattern of thalamic deactivation observed during NREM sleep in the current study is strikingly similar to the pattern observed in FFI, suggesting a common underlying mechanism.
The thalamus is a key structure involved in the relay of sensory and motor information received from the external environment to primary visual, auditory, and somatosensory cortex. It is the site where such information is initially processed before being relayed to the cerebral cortex for further processing (and in what form) [20]. Furthermore, as the thalamus shares reciprocal connections with the basal ganglia, cerebellum, neocortex, and limbic structures, it is also involved in many other functions, such as the relay of information concerning movement, learning, memory, and emotion [20,21]. Finally, specific regions of the thalamus subserve certain functions. For instance, the dorsomedial nucleus of the thalamus is involved in executive functions such as memory [22], and the observed decrease in this region is consistent with the view put forth by Hobson and colleagues [2] that relative to waking, working memory and other executive processes are deficient whilst dreaming. In addition, the pulvinar is an important thalamic structure, implicated in attentional processing associated with its reciprocal connections with cortical regions such as the posterior parietal, temporal, and areas of the visual cortex [20,21]. As such, the thalamus is involved in regulating arousal and attention, and consequently, the level of consciousness [23,24].
Thus, in light of the aforementioned functions of the thalamus, its deactivation during NREM sleep would result in deficits in these processes. For example, since thalamic nuclei are responsible for the transfer of information (whether from the external environment or internally generated) to the cortex, such deactivation may be related to slow thalamocortical oscillations generated by the thalamus [23]. Slow oscillations are involved in regulating the sleep cycle by increasing arousal thresholds [23,25]. This increased arousal threshold serves to block subjective awareness of the external environment and promote the deepening of sleep [26]. In sum, the lack of information reaching the cortex via thalamic projections combined with increased arousal thresholds may explain the loss of consciousness characteristic of sleep, particularly NREM sleep [23]. With respect to dreaming, all this is likely to suppress “the experience of perception and mentation during NREM sleep” ([2], p. 824).
However, the results of the current meta-analysis did not reveal any significant increase or decrease in thalamic activity in REM sleep when compared to wakefulness. Such a result is not altogether surprising since the imaging techniques employed in these studies may not effectively capture brief, sporadic ponto-geniculo-occipital activity in structures such as the lateral geniculate nucleus (LGN) which, to date, has only been detected using depth electrodes in animals. Such activity (generated by the pontine brainstem and relayed to the cortex via the LGN) conveys pseudosensory information that is subsequently elaborated by the cortex and experienced as dreams [2].
4.2. Frontal Cortex Activation Patterns during Sleep
In addition, compared to wakefulness there was a decrease in activity in frontal regions during NREM (inferior frontal gyrus) and REM sleep (middle, inferior, and superior frontal gyrus). These regions correspond to the dorsolateral [27] and orbitofrontal cortex [28]. This has lead theorists to postulate that such decreased activity may provide an essential role in dream recall [2,23]. Specifically, as these frontal cortical areas are predominantly involved in executive functions during wakefulness (such as directed thought and decision making, memory and attention) [28-31], such decreased activity during NREM and REM sleep has been linked to the observed disruptions in such processes during sleep [23, 32,33]. For instance, working memory is deficient from REM sleep dream reports, with scene shifts often experienced without conscious reflection by the dreamer [34]. Hence, such disruptions in memory and other executive processes may explain the unique qualities of dreams from REM sleep like their characteristic bizarreness, disorientation, and loss of self-reflective awareness [2,23,35].
4.3. Anterior Cingulate Activation Patterns during Sleep
Finally, the meta-analyses also revealed significant patterns of regional activity in the anterior cingulate. The anterior cingulate gyrus is involved in the processing of emotional information, particularly the emotions and memories evoked in response to fearful stimuli as well as the fear potentiated startle response [36]. This information processing in turn facilitates the behavioral and autonomic responses to such stimuli [36]. In addition to its role in processing emotion, the anterior cingulate gyrus is also involved in executive functions such as attention [37], including response monitoring [38] and error detection [39]. These various functions may be due to the location of the anterior cingulate gyrus, situated between the frontal cortex (executive functions) and limbic regions (emotion) [20].
In the current study, two areas of the anterior cingulate were found to be involved in sleep, exhibiting selective activation/deactivation patterns across REM and NREM. Specifically, decreases were observed in the left anterior cingulate gyrus (BA 24 and 32) during NREM. These regions of the anterior cingulate roughly correspond to the perigenual anterior cingulate cortex and midcingulate cortex, which are implicated in the aforementioned processes involving affect and response selection, respectively [40]. Thus, the decreases observed are consistent with the known deficits in such processes and overall brain deactivation inherent during this stage of sleep. However, there was also a circumscribed area of increased activity identified in the left anterior cingulate (BA 24). It is possible that such activity may reflect the transient appearance of certain sleep rhythms. For example, increased activity in the anterior cingulate cortex was found to coincide with periods of spindle activity [15] and slow waves [41]. These findings fit well with the results of the current meta-analysis, and suggest that the pattern of activity observed may reflect the activity of such NREM rhythms. Alternatively, the activation observed in our meta-analysis may reflect increased activity, which coincides with periods of dreaming in NREM. However, not all of the included studies systematically investigated dreaming, so such an explanation is purely speculative.
Interestingly, during REM sleep increases comprised areas of the right anterior cingulate cortex (i.e. BA 32). Such activity is not surprising since the anterior cingulate cortex has substantial connections to other structures in the forebrain limbic system such as the amygdala, a major centre involved in emotion [20,21,42,43]. Thus, such activation during REM sleep (combined with the aforementioned frontal deactivation during this stage of sleep) is consistent with the exaggerated affect and bizarre content, disorientation, as well as a lack of reflection and awareness characteristic of dreams from REM [44].
4.4. Methodological Considerations
The results of the aforementioned meta-analyses as well as the subsequent discussion and conclusions drawn must be interpreted in light of various limitations. Specifically, the major limitation concerns the type of studies included in the current review. Attempts were made to obtain a relatively homogenous sample of studies for inclusion in the analyses. As a result, a number of studies that may otherwise have provided valuable information regarding brain activity during sleep were excluded. For instance, studies that administered an external stimulus or pharmacological agent prior to or during sleep were excluded as such manipulation may produce different profiles of brain activity when compared to spontaneous sleep. However, despite such attempts the included studies were not strictly homogenous due to variability in the methods employed across studies. For example, although only PET studies were analyzed, the two PET techniques (as well as methods used in their analyses) differ. Furthermore, some studies included in the meta-analyses employed sleep deprivation procedures, a procedure which is likely to alter global as well as regional brain activity. Thus, the inclusion of such studies may have skewed the observed results. Finally, some of the included studies involved awakening participants in order to collect dream reports. These awakenings may have altered sleep integrity and architecture, and potentially the brain activity observed in subsequent scans. These issues highlight how the inclusion of such studies may influence the final result. However, had these studies been excluded, this would have further reduced the already limited sample size. These issues aside, significant clusters were revealed using the updated ALE technique.
4.5. Summary
The current study revealed a network of brain regions implicated in the neurophysiology of REM and NREM sleep. Specifically, in REM sleep this network included the anterior cingulate as well as the dorsolateral and orbitofrontal cortex. In NREM sleep, the network comprised these same areas as well as the thalamus. Further research is needed to elucidate the role of these regions in sleep and whether such activity coincides with periods of dreaming.
REFERENCES
- E. Aserinsky and N. Kleitman, “Regularly Occurring Periods of Eye Motility and Concomitant Phenomena during Sleep,” Science, Vol. 118, No. 3062, 1953, pp. 273-274. doi:10.1126/science.118.3062.273
- J. A. Hobson, E. F. Pace-Schott and R. Stickgold, “Dreaming and the Brain: Toward a Cognitive Neuroscience of Conscious States,” Behavioral and Brain Sciences, Vol. 23, No. 6, 2000, pp. 793-842. doi:10.1017/S0140525X00003976
- M. Solms, “Dreaming and REM Sleep Are Controlled by Different Brain Mechanisms,” Behavioral and Brain Sciences, Vol. 23, No. 6, 2000, pp. 843-850. doi:10.1017/S0140525X00003988
- P. T. Fox, L. M. Parsons and J. L. Lancaster, “Beyond the Single Study: Function/Location Metanalysis in Cognitive Neuroimaging,” Current Opinion in Neurobiology, Vol. 8, No. 2, 1998, pp. 178-187. doi:10.1016/S0959-4388(98)80138-4
- P. E. Turkeltaub, G. F. Eden, K. M. Jones and T. A. Zeffiro, “Meta-Analysis of the Functional Neuroanatomy of Single-Word Reading: Method and Validation,” Neuroimage, Vol. 16, No. 3, 2002, pp. 765-780. doi:10.1006/nimg.2002.1131
- S. B. Eickhoff, A. R. Laird, C. Grefkes, L. E. Wang, K. Zilles and P. T. Fox, “Coordinate-Based ALE MetaAnalysis of Neuroimaging Data: A Random-Effects Approach Based on Empirical Estimates of Spatial Uncertainty,” Human Brain Mapping, Vol. 30, No. 9, 2009, pp. 2907-2926. doi:10.1002/hbm.20718
- N. Hofle, T. Paus, D. Reutens, P. Fiset, J. Gotman, A. C. Evans and B. E. Jones, “Regional Cerebral Blood Flow Changes as a Function of Delta and Spindle Activity during Slow Wave Sleep in Humans,” Journal of Neuroscience, Vol. 17, No. 12, 1997, pp. 4800-4808.
- T. W. Kjaer, I. Law, G. Wiltschiøtz, O. B. Paulson and P. L. Madsen, “Regional Cerebral Blood Flow during Light Sleep—A H(2)(15)O-PET Study,” Journal of Sleep Research, Vol. 11, No. 3, 2002, pp. 201-207. doi:10.1046/j.1365-2869.2002.00303.x
- J. L. Lancaster, D. Tordesillas-Gutiérrez, M. Martinez, F. Salinas, A. Evans, K. Zilles, J. C. Mazziotta and P. T. Fox, “Bias between MNI and Talairach Coordinates Analyzed Using ICBM-152 Brain Template,” Human Brain Mapping, Vol. 28, No. 11, 2007, pp. 1194-1205. doi:10.1002/hbm.20345
- P. T. Fox and J. L. Lancaster, “Mapping Context and Content: The BrainMap Model,” Nature Reviews Neuroscience, Vol. 3, No. 4, 2002, pp. 319-321. doi:10.1038/nrn789
- A. R. Laird, J. L. Lancaster and P. T. Fox, “BrainMap: The Social Evolution of a Functional Neuroimaging Database,” Neuroinformatics, Vol. 3, No. 1, 2005, pp. 65-78. doi:10.1385/NI:3:1:065
- A. R. Laird, P. M. Fox, C. J. Price, D. C. Glahn, A. M. Uecker, J. L. Lancaster, P. E. Turkeltaub, P. Kochunov and P. T. Fox, “ALE Meta-Analysis: Controlling the False Discovery Rate and Performing Statistical Contrasts,” Human Brain Mapping, Vol. 25, No. 1, 2005, pp. 155-164. doi:10.1002/hbm.20136
- J. L. Lancaster, M. G. Woldorff, L. M. Parsons, M. Liotti, C. S. Freitas, L. Rainey, P. V. Kochunov, D. Nickerson, S. A. Mikiten and P. T. Fox, “Automated Talairach Atlas Labels for Functional Brain Mapping,” Human Brain Mapping, Vol. 10, No. 3, 2000, pp. 120-131. doi:10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8
- P. Kochunov, J. Lancaster, P. Thompson, A. W. Toga, P. Brewer, J. Hardies and P. Fox, “An Optimized Individual Target Brain in the Talairach Coordinate System,” Neuroimage, Vol. 17, No. 2, 2002, pp. 922-927. doi:10.1006/nimg.2002.1084
- M. Schabus, T. T. Dang-Vu, G. Albouy, E. Balteau, M. Boly, J. Carrier, A. Darsaud, C. Degueldre, M. Desseilles, S. Gais, C. Phillips, G. Rauchs, C. Schnakers, V. Sterpenich, G. Vandewalle, A. Luxen and P. Maquet, “Hemodynamic Cerebral Correlates of Sleep Spindles during Human Non-Rapid Eye Movement Sleep,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 104, No. 32, 2007, pp. 13164-13169. doi:10.1073/pnas.0703084104
- M. Steriade, “The Corticothalamic System in Sleep,” Frontiers in Bioscience, Vol. 8, 2003, pp. 878-899. doi:10.2741/1043
- P. Cortelli, D. Perani, P. Montagna, R. Gallassi, P. Tinuper, F. Provini, P. Avoni, F. Ferrillo, D. Anchisi, R. M. Moresco, F. Fazio, P. Parchi, A. Baruzzi, E. Lugaresi and P. Gambetti, “Pre-Symptomatic Diagnosis in Fatal Familial Insomnia: Serial Neurophysiological and [18F] FDG PET Studies,” Brain, Vol. 129, No. 3, 2006, pp. 668-675. doi:10.1093/brain/awl003
- V. Manetto, R. Medori, P. Cortelli, P. Montagna, P. Tinuper, A. Baruzzi, G. Rancurel, J. J. Hauw, J. J. Vanderhaeghen, P. Mailleux, O. Bugiani, F. Tagliavini, C. Bouras, N. Rizzuto, E. Lugaresi and P. Gambetti, “Fatal Familial Insomnia: Clinical and Pathologic Study of Five New Cases,” Neurology, Vol. 42, No. 2, 1992, pp. 312- 319.
- P. Montagna, F. Provini, G. Plazzi, R. Vetrugno, R. Gallassi, G. Pierangeli, M. Ragno, P. Cortelli and D. Perani, “Bilateral Paramedian Thalamic Syndrome: Abnormal Circadian Wake-Sleep and Autonomic Functions,” Journal of Neurology, Neurosurgery, and Psychiatry, Vol. 73, No. 6, 2002, pp. 772-774. doi:10.1136/jnnp.73.6.772
- J. Nolte, “The Human Brain: An Introduction to Its Functional Anatomy,” 6th Edition, Mosby, Edinburgh, 2008.
- J. H. Martin, “Neuroanatomy: Text and Atlas,” 3rd Edition, McGraw-Hill, New York, 2003.
- Y. D. Van der Werf, J. Jolles, M. P. Witter and H. B. Uylings, “Contributions of Thalamic Nuclei to Declarative Memory Functioning,” Cortex, Vol. 39, No. 4-5, 2003, pp. 1047-1062. doi:10.1016/S0010-9452(08)70877-3
- A. R. Braun, T. J. Balkin, N. J. Wesensten, R. E. Carson, M. Varga, P. Baldwin, S. Selbie, G. Belenky and P. Herscovitch, “Regional Cerebral Blood Flow throughout the Sleep-Wake Cycle: An H215O PET Study,” Brain, Vol. 120, No. 7, 1997, pp. 1173-1197. doi:10.1093/brain/120.7.1173
- C. M. Portas, G. Rees, A. M. Howseman, O. Josephs, R. Turner and C. D. Frith, “A Specific Role for the Thalamus in Mediating the Interaction of Attention and Arousal in Humans,” Journal of Neuroscience, Vol. 18, No. 21, 1999, pp. 8879-8989.
- M. Mancia and G. Marini, “Thalamic Mechanisms in Sleep Control,” In: O. Hayaishi and S. Inoue, Eds., Sleep and Sleep Disorders: From Molecule to Behavior, Academic Press, London, 1997, pp. 377-393.
- P. Maquet, D. Dive, E. Salmon, B. Sadzot, G. Franco, R. Poirrier and G. Franck, “Cerebral Glucose Utilization during Stage 2 Sleep in Man,” Brain Research, Vol. 571, No. 1, 1992, pp. 149-153. doi:10.1016/0006-8993(92)90522-B
- P. D. Zelazo and U. Muller, “Executive Function in Typical and Atypical Development,” In: U. Goswam, Ed., Blackwell Handbook of Child Cognitive Development, Blackwell Publishing, Oxford, 2002, pp. 445-469. doi:10.1002/9780470996652.ch20
- M. L. Kringelbach, “The Human Orbitofrontal Cortex: Linking Reward to Hedonic Experience,” Nature Reviews Neuroscience, Vol. 6, No. 9, 2005, pp. 691-702. doi:10.1038/nrn1747
- A. Baddely, “Recent Developments in Working Memory,” Current Opinion in Neurobiology, Vol. 8, No. 2, 1998, pp. 234-238. doi:10.1016/S0959-4388(98)80145-1
- A. Bechara, A. R. Damasio, H. Damasio and S. W. Anderson, “Insensitivity to Future Consequences Following Damage to Human Prefrontal Cortex,” Cognition, Vol. 50, No. 1-3, 1994, pp. 7-15. doi:10.1016/0010-0277(94)90018-3
- J. B. Brewer, Z. Zhao, J. E. Desmond, G. H. Glover and J. D. Gabrieli, “Making Memories: Brain Activity that Predicts How Well Visual Experience Will Be Remembered,” Science, Vol. 281, No. 5380, 1998, pp. 1185- 1187. doi:10.1126/science.281.5380.1185
- P. Maquet and G. Franck, “REM Sleep and Amygdale,” Molecular Psychiatry, Vol. 2, No. 3, 1997, pp. 195-196. doi:10.1038/sj.mp.4000239
- R. Stickgold, E. F. Pace-Schott and J. A. Hobson, “Subjective Estimates of Dream Duration and Dream Recall Process,” Sleep Research, Vol. 26, 1997, p. 279.
- J. A. Hobson, R. Stickgold and E. F. Pace-Schott, “The Neuropsychology of REM Sleep Dreaming,” Neuroreport, Vol. 9, No. 3, 1998, pp. 1-14. doi:10.1097/00001756-199802160-00033
- J. A. Hobson, S. A. Hoffman, R. Helfand and D. Kostner, “Dream Bizarreness and the Activation-Synthesis Hypothesis,” Human Neurobiology, Vol. 6, No. 3, 1987, pp. 157- 164.
- A. Pissiota, O. Frans, A. Michelgard, L. Appel, B. Langstrom, M. A. Flaten and M. Fredrikson, “Amygdala and Anterior Cingulate Cortex Activation during Affective Startle Modulation: A Pet Study of Fear,” European Journal of Neuroscience, Vol. 18, No. 5, 2003, pp. 1325- 1331. doi:10.1046/j.1460-9568.2003.02855.x
- M. Corbetta, F. M. Miezin, S. Dobmeyer, G. L. Shulman and S. E. Petersen, “Selective and Divided Attention during Visual Discriminations of Shape, Color and Speed: Functional Anatomy by Positron Emission Tomography,” Journal of Neuroscience, Vol. 11, No. 8, 1991, pp. 2383- 2402.
- J. D. Cohen, M. Botvinick and C. S. Carter, “Anterior Cingulate and Prefrontal Cortex: Who’s in Control?” Nature Neuroscience, Vol. 3, No. 5, 2000, pp. 421-423.
- S. Dehaene, M. I. Posner and D. M. Tucker, “Localization of a Neural System for Error Detection and Compensation,” Psychological Science, Vol. 5, 1994, pp. 303-305. doi:10.1111/j.1467-9280.1994.tb00630.x
- B. A. Vogt, P. R. Hof and L. J. Vogt, “Cingulate Gyrus,” In: G. Paxinos and J. K. Mai, Eds., The Human Nervous System, 2nd Edition, Academic Press, San Diego, 2004, pp. 915-949. doi:10.1016/B978-012547626-3/50025-9
- M. Murphy, B. A. Riedner, R. Huber, M. Massimini, F. Ferrarelli and G. Tononi, “Source Modeling Sleep Slow Waves,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 106, No. 5, 2009, pp. 1608-1613. doi:10.1073/pnas.0807933106
- P. Maquet, J. M. Peters, J. Aerts, G. Delfiore, C. Degueldre, A. Luxen and G. Franck, “Functional Neuroanatomy of Human Rapid-Eye-Movement Sleep and Dreaming,” Nature, Vol. 383, No. 6596, 1996, pp. 163-166. doi:10.1038/383163a0
- P. Maquet, C. Degueldre, G. Delfiore, J. Aerts, J. M. Peters, A. Luxen and G. Franck, “Functional Neuroanatomy of Human Slow Wave Sleep,” Journal of Neuroscience, Vol. 17, No. 8, 1997, pp. 2807-2812.
- A. R. Braun, T. J. Balkin, N. J. Wesensten, F. Gwadry, R. E. Carson and M. Varga, “Dissociated Pattern of Activity in Visual Cortices and Their Projections during Human Rapid Eye Movement Sleep,” Science, Vol. 279, No. 5347, 1998, pp. 91-95. doi:10.1126/science.279.5347.91
NOTES
*Corresponding author.

