Journal of Biomaterials and Nanobiotechnology
Vol.4 No.3(2013), Article ID:35331,8 pages DOI:10.4236/jbnb.2013.43038
Enzymatic Removal of Phenol from Industrial Wastewaters
![]()
1Department of Chemical Engineering, Faculty of Engineering, Ege University, Izmir, Turkey; 2Department of Bioengineering, Faculty of Engineering, Ege University, Izmir, Turkey.
Email: *sayit.sargin@ege.edu.tr
Copyright © 2013 Artun Sukan, Sayit Sargin. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 27th, 2013; revised April 26th, 2013; accepted May 15th, 2013
Keywords: Industrial Wastewater; Phenol; Laccase; Immobilization; RSM
ABSTRACT
Phenol, as a pure substance, is used in many fields due to its disinfectant, germicidal, local anaesthetic and peptizing properties. Aqueous solutions of phenol are produced as a waste of these industries and are discharged into the environment. Therefore, elevated concentrations of phenol may be found in air or water due to industrial discharge or use of phenolic products. The aim of this study was to evaluate the phenol removal capability of enzymes from low-phenolcontent (up to 5%) industrial wastewaters and to optimize the reaction conditions. For this purpose, two different enzymes namely, Laccase and Peroxidase were investigated with respect to their phenol removal capacities. The enzymatic reaction conditions were optimized using Response Surface Methodology (RSM). As a result 78% phenol removal was achieved with laccase using a model wastewater. In the studies where the enzyme was immobilized, a 50% removal was achieved indicating that further optimization was needed in this area.
1. Introduction
Phenolic contaminants are found in wastewaters of various industries such as petroleum refining, coal conversion, plastics, textiles, iron and steel manufacturing as well as pulp and paper manufacturing [1,2]. They represent a potential danger to human health because almost all are toxic and many of them are known or suspected to be carcinogenous [3,4]. It is very important to remove phenolic compounds from contaminated waters before discharging them into the environment because of their toxicity [5]. Conventional processes for removal of phenols from industrial wastewaters include extraction, adsorption on activated carbon, bacterial and chemical oxidation, electrochemical techniques, irradiation, etc. All of these methods have serious shortcomings such as high costs, incomplete removal, formation of hazardous byproducts, low efficiencies and applicability to limited concentration ranges [6], therefore enzyme-based treatment methods have been developed. Enzymatic methods have several advantages due to their specificity, efficiency in removing targeted compounds and ease of handling [7].
Peroxidases, horse radish peroxidase, lipase, laccase and bilirubin oxidase, oxidise the aromatic compounds to form aromatic radicals, which in turn combine to form polymeric structures that precipitate spontaneously from the solution due to their low solubility. Peroxidase is a more widely studied enzyme while the potential of laccase has been investigated by a few workers [8-11]. Horseradish peroxidase has been widely used in the presence of H2O2, for the polymerisation of phenol and its derivatives [12]. However, since H2O2 causes inhibition and deactivation of horseradish peroxidase, the use of laccase, in the presence of dissolved molecular oxygen for its catalytic activity, may be more advantageous than the peroxidase [8, 13-15]. Currently, there are a number of studies describing phenol removal from aqueous wastes using laccases and it is known that laccase activity is inhibited at high concentrations of phenol. Many researchers [2,4,5,11,16, 17] have reported very promising results at low concentrations. All reported results on enzymatic removal of phenol in literature, are with low concentrations of phenol, ranging between 1 mM to 10 mM (0.01% - 0.10%) [2,4,5,11,16,17]. The present work describes the removal of phenol by enzymatic methods in batch and continuous processes using free and immobilized laccase. The main novelty of the present work is the relatively high concentrations of aqueous phenol solutions ranging between 50 mM to ~500 mM (0.5% - ~5.0%), representing the typical phenol concentration range of industrial wastewaters in Turkey since diluting the wastewater to reach the cited values of phenol concentration is not preferred due to environmental concerns and reluctance to pollute clean water with a toxic compound.
2. Materials and Methods
2.1. Materials
Two different enzymes, namely peroxidase and laccase were used in this study. Peroxidase (Novozyme® 51004) was produced by submerged fermentation of a high-producing genetically modified strain of Aspergillus oryzae. The calculated activity is 1.93 U/ml the physical form of the enzyme is liquid, with an approximate density of 1.28 g/ml. Laccase (Novozymes® 51003) was also produced by submerged fermentation of a genetically modified strain of Aspergillus oryzae. The calculated activity the enzyme used in this study is 2.1 U/ml. All other chemicals such as; phenol used in the preparation of model wastewater and Potassium Ferrocyanide (K3(Fe(Cn)6)), hydrogen peroxide H2O2 (35%), 4-Aminosntipyrine (4- AAP), PEG 6000, and ammonia, used in the analyses; were analytical grade and were purchased from Merck. The phenolic wastewater was taken from the phenolic resins production plant of Cukurova Kimya Sanayi A.S. The phenol content of this water varied between 2 to-5% (w/v). The immobilized enzyme column was specifically designed using a 60-ml plastic column and was covered with a metal grid.
2.2. Methods
The Model industrial waste waters containing different concentrations of phenol ranging from 0.5% - 8.0%, were prepared by dissolving measured amounts of phenol in distilled water.
Phenol content was measured according to the modified method of Martin (1949) [18]. Solution containing phenol was mixed with 2N NH4OH and 2%w/v 4-AAP, mixed, 8% K3Fe((CN)6) added mixed and measured at 500 nm.
Peroxidase activity was determined as U/ml. 400 µl enzyme, 1.5 ml phenol (50 mM), 750 µl 4-AAP (24 mM), 1.5 ml H2O2 (1 mM) and 750 µl phosphate buffer (pH 7.4 - 10 mM) were mixed to produce a 6 ml reaction volume and the change in absorbance was measured at 510 nm at kinetic mode in order to find ΔAbs value.
Laccase activity was determined as U/ml. 100 µl enzyme, 100 µl ABTS, 800 µl citrate buffer (pH 4.5 - 10 mM) were mixed to produce a 1 ml reaction volume and the change in absorbance was measured at 420 nm at kinetic mode in order to find ΔAbs value.
For enzyme immobilization, enzyme solution was mixed with 3% (w/v) alginate solution at a ratio of 1.5 - 2.5 U/ml. The enzyme solution containing alginate was mixed with glass beads of 4.5 mm in diameter providing a rigid support for the alginate beads. The surface of the glass beads were covered with alginate-enzyme mixture and dropped into CaCl2 (5% w/v) solution to solidify.
Different enzyme solutions were tested for their efficiency in different phenol concentrations. The model solution or the original industrial wastewater was used as the source of phenol solution depending on the requirements of the experiment. Batch experiments for enzymetic treatments were conducted at room temperature (approximately 25˚C) in 250 ml Erlenmeyer flasks containing 45 mL of synthetic waste water (phenol—distilled water).
Peroxidase reaction mixtures were prepared by adding predetermined doses of horseradish Peroxidase e (HRP), hydrogen peroxide (H2O2) and poly ethylene glycol (PEG) to phenol containing (5%w/v) wastewater solution.
Laccase reaction mixtures were prepared by simply adding predetermined doses of laccase into phenol containing (5%w/v) wastewater solution. The final concentration of the reaction mixture was adjusted according to the requirements of the experiments by adding distilled water. After treatment the resulting solution was centrifuged at 11,000 rpm for 6 minutes. The supernatant was analysed for phenol. The phenol concentrations were measured twice.
A central composite design (CCD) was applied using Design-Expert 6.0 (trial version), with five variables at three levels was performed in order to explore the effect of variables on the response in the region of investigation. Enzyme concentration, phenol concentration, rates of agitation, temperature and reaction times were considered as the most effective independent variables according to preliminary experiments and to literature [11]. The total number of experiments with five variables was 50. Forty two experiments were augmented with eight replications at the centre points to evaluate the pure error. The experiments were conducted in shake flasks due to the oxygen dependency of the reaction. Percentage phenol removal was taken as the response. Experiments were performed according to experimental design matrix given on Table 1, within the ranges indicated.
The matrix for five variables was varied at five levels (−α, −1, 0, +1, +α). The higher level of variable was designed as “+1”, the lower level was designed as “−1”, centre point was designed as “0” and star points were designed as “−α and +α”. In the optimization process the response can be related to chosen variables by linear or quadratic models. A quadratic model is given as;
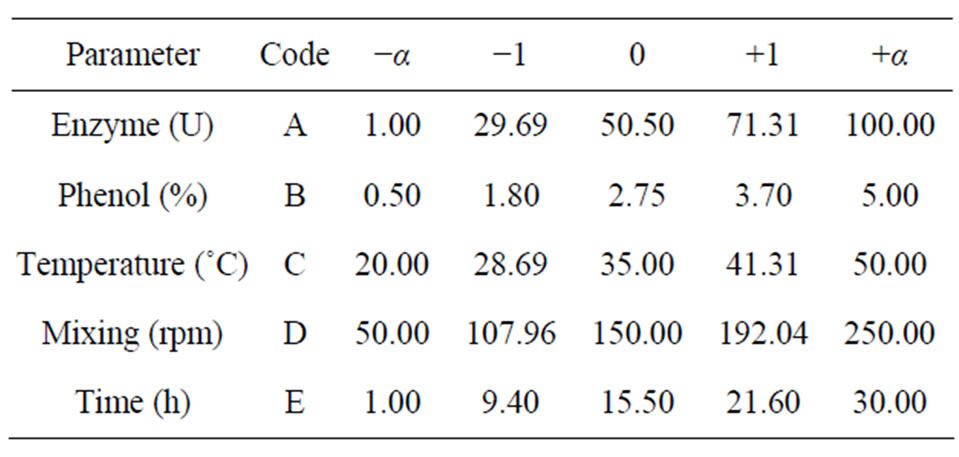
Table 1. Points selected for RSM design.
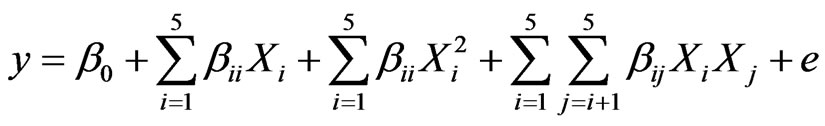 (1)
(1)
where y is the response, β0 the constant coefficient, Xi (i = 1 – 5) are non-coded variables, βis are the linear, and βiis are the quadratic, and βijs are the second-order interaction coefficients. The parameters in our study are coded only with letters rather than Xi for simplicity. Data were processed for Equation (1) using Design-Expert 6.0 program including ANOVA to obtain the interaction between the process variables and the response. The quality of the fit of polynomial model was expressed by the coefficient of determination R2.
3. Results and Discussion
3.1. Preliminary Experiments
The preliminary experiments were started in tubes and continued in shake flasks due to the oxygen dependency of the reaction. The effect of pH on phenol removal was determined in reaction mediums containing pure water (no pH control) or Citrate buffer (pH controlled at pH 7.5). The effect of mixing on the efficiency of the enzymatic reaction, was determined by monitoring the time course of two different reactions; one in a test tube without any mixing and the other in a stirred vial, and comparing the results. The combined effect of pH and mixing were investigated in stirred vials using a magnetic stirrer. One vial contained pure water (initial pH 6.0), the second contained citrate buffer (pH 7.5) and in the third one, the pH value was set at pH 7.5 at the onset of the reaction. For the effect of oxygen, the experiments were conducted in closed flasks with a magnetic stirrer. The phenol content in one flask was measured against time by opening the lid of the tube thereby allowing it to aerate intermittently, and the other one was kept closed during the entire reaction. In all experiments phenol contents were reported as “% phenol” and determined in duplicate.
Although many researchers have reported good results for phenol removal using peroxidase [11], in our experiments the phenol reduction from high content phenol solutions using different dosages of Peroxidase (ranging between 0.2 U to 4 U) was not statistically significant and enzyme denaturation was observed at the end of two hours. A similar initial experiment was conducted using laccase with concentrations ranging between 0 U - 4 U. Approximately 30% higher removal of phenol was achieved with a dose of 4 U enzyme. Thus, laccase was found to be a better catalyst for the removal of phenol at the concentrations tested, although the removal of phenol appeared to be a reversible reaction due to the sudden increases in phenol concentration during the reaction (data not shown). This reversibility comes from the polymerization of the phenol, which has a mechanism that has not been completely explained [9,19]. Fluctuations observed in all graphs due to this reversibility. The phenol concentration tends to increase in some points. This reversibility was also considered in the selection of enzyme and further experiments were conducted using laccase. However, the results obtained in this study were not satisfactory in terms of absolute removal therefore some preliminary experiments were also conducted regarding the environmental conditions that might relate the phenol polymerization with laccase concentrations
3.2. Effect of Environmental Conditions
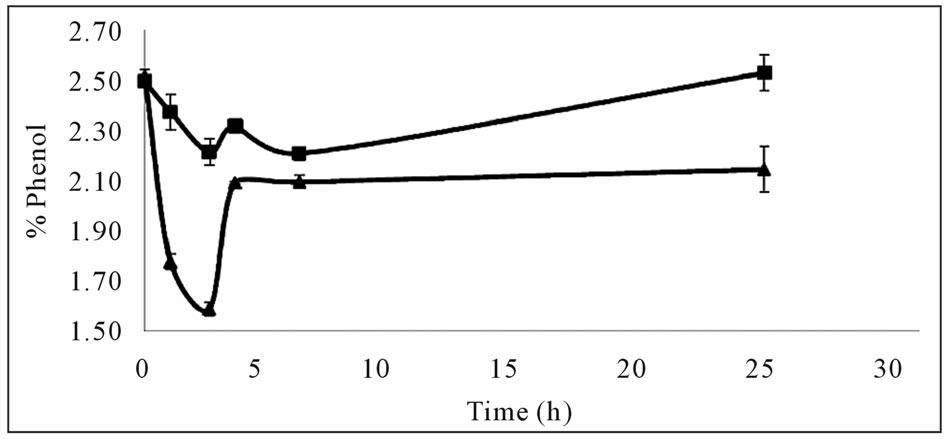
Initially, the effects of pH, mixing and their combined effects on phenol removal, were investigated. The use of a buffer solution had a negative effect on phenol removal and a lower concentration was obtained when the pH of the reaction medium was not controlled (Figure 1(a)). In
 (a)
(a)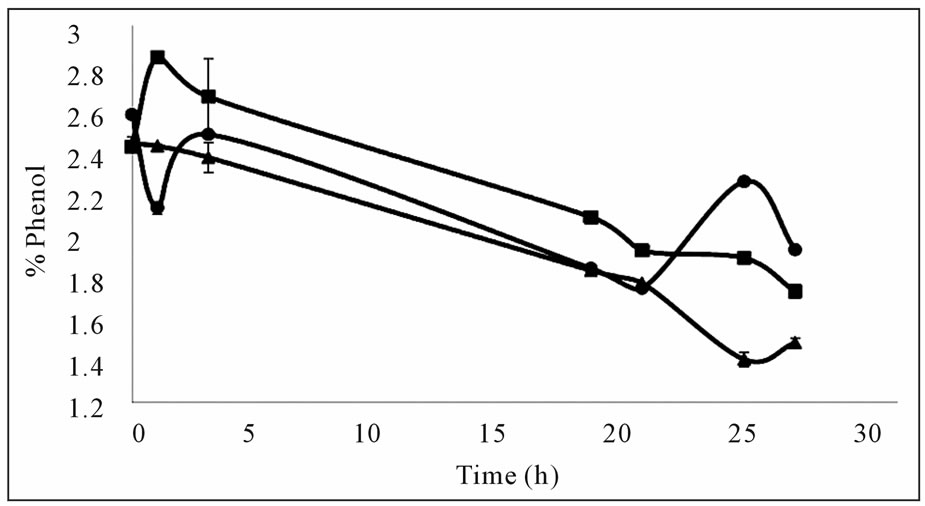 (b)
(b)
Figure 1. Effects of pH and mixing on phenol removal (a) Effect of pH, with buffer (■) and water (▲) (b) Effect of pH and mixing together, buffer (■), water (▲), initial (●).
the work of Aktas and Tanyolac (2003) [8], the effect of pH was found to be significant and an optimum around pH 5.0 was reported for the removal of catechol. But in this study the effect of pH on phenol removal with laccase was found to be negative (Figure 1(b)). This could be due to the different substrates used (phenol vs catechol).
This negative effect of pH was also verified with the combined effect experiment (Figure 1(b)). Phenol removal was better in the medium without any pH control indicating that excess H+ ions in the reaction medium effect the reaction mechanism negatively through the recombination of phenoxy radicals H+ ions. This may be interfering with the reaction by filling the empty radical and stopping it. However, further experimentation is needed to elucidate the exact mechanism of phenol removal from aqueous solutions and this was outside the scope of the present study.
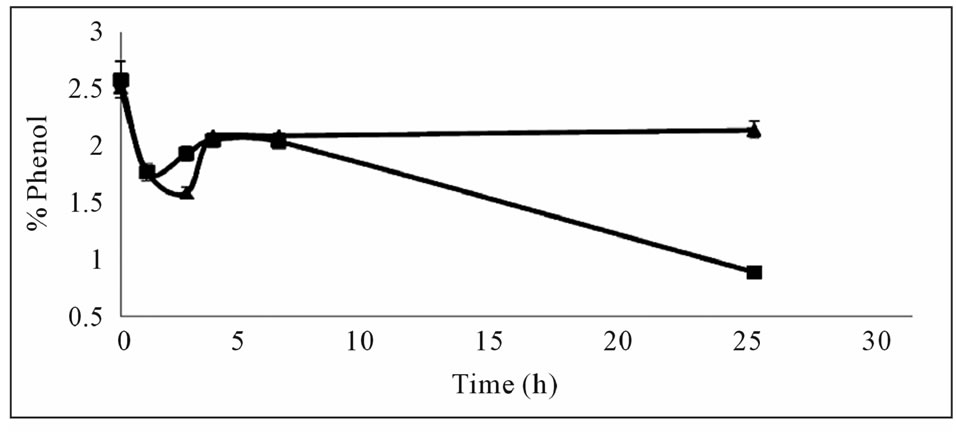
Mixing was observed to have a positive effect on phenol removal (Figure 2(a)) leading to a considerable decrease at the end of 24 hours. This indicated that aeration and a certain amount of time was required for the reaction. The results of this experiment were also verified in further experiments where the phenol content in the aerated flask decreased more than that of the closed flask at the end of 30 hours (Figure 2(b)). The amount of precipitate formed was larger in the aerated flask indicating that the polymerization reaction is dependent on the
 (a)
(a)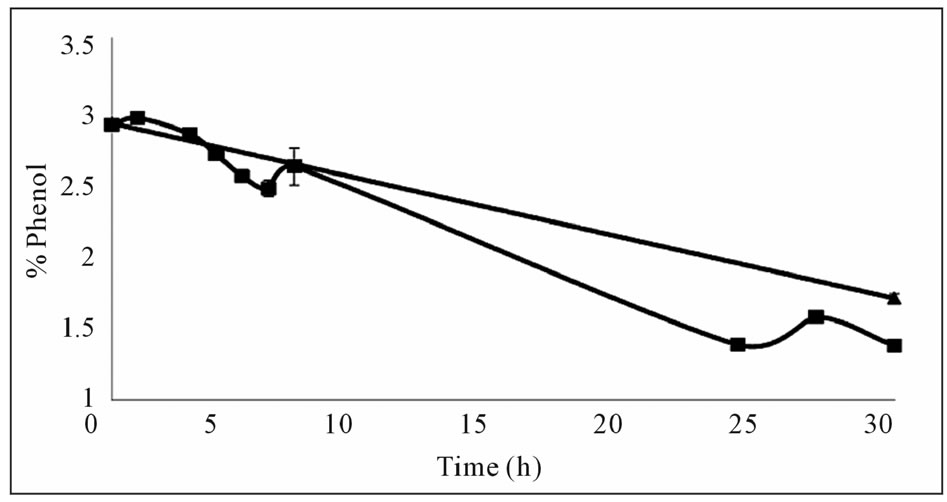 (b)
(b)
Figure 2. Effects of mixing and oxygen on phenol removal (a) Effect of mixing, closed square; mixed, closed triangle; static (b) Effect of oxygen, with oxygen (■), without oxygen (▲).
oxygen content. The findings of this study on oxygen dependency of the reaction agree with the results of Aktas and Tanyolac (2003) [8] where polymerisation showed a high dependency on oxygen. Therefore subsequent experiments were conducted with cotton wool stoppers allowing for oxygen to transfer into the reaction medium.
3.3. Optimization of Reaction Conditions with RSM
As was mention in the Methods section, the parameters subject to optimization were selected through the initial experiments for the statistical optimization step. Enzyme concentrations and phenol concentrations were the crucial parameters together with temperature, reaction time and agitation (due to the oxygen dependency of the reaction).
As a result of the analysis (Table 2), the following model was obtained (Equation (2)).
 (2)
(2)
In the statistical optimisation results were quite promising. Approximately 75% removal was achieved with the optimum conditions determined through the statistical analysis.
According to experimental design method, enzyme and phenol concentrations were found to be statistically significant due to their P-values are lower than 0.0001. The positive coefficient value of enzyme concentration indicates that it has a positive effect on the removal of phenol in contrast to phenol concentration which has a negative coefficient.
The effect of phenol and enzyme concentrations showed that, percent phenol removal increases with increasing enzyme concentrations and decreases with increasing phenol concentrations. An increase in removal at increased enzyme concentration is a known fact of enzymatic reactions [2,4,5,11,16,17], according to our model 100 U - 60 U enzyme dose appears to yield a 100% removal from the water between 1.6% - 0.5% phenol concentrations.
Time was found to be statistically non-significant because of high P-value. But when the graph showing the
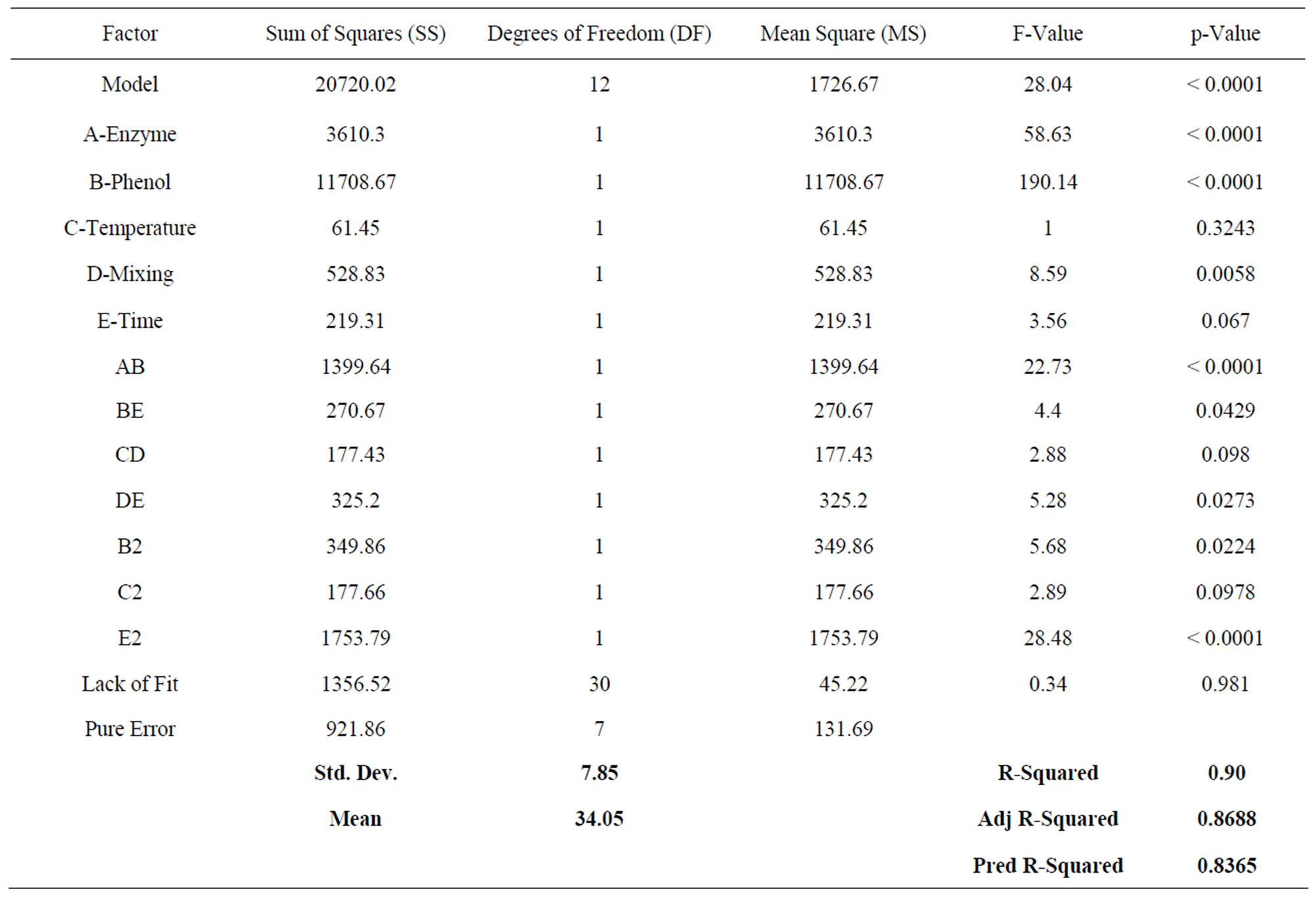
Table 2. Results of ANOVA for response surface reduced quadratic model.
interaction between the phenol concentration and contact time is analysed, it can be seen that the phenol removal shows a slight improvement between 1 hour and 20 h as if it has an optimum value in between (Figure 3(a)).
Furthermore, although the effects of mixing and time were found to be non-significant on removal of phenol, (Figures 3(a) and (d)) their combined effect was significant (Figure 3(b)).
Removal of phenol increased until optimum point (around 22.75 hours) with increasing contact time interval in all mixing values (Figure 3(b)). This is an indication of clear interaction in between parameters.
In the study of Ghasempur et al. (2007) [20] with HRP enzyme, the effect of pH on phenol removal was found to be non-significant although it clearly affected phenol removal. They reported an interaction between pH and H2O2, arguing that H2O2 influenced the effect of pH because it has a crucial role on phenol removal as an electron donor. Similarly in our study oxygen is the electron donor for laccase therefore an interaction was observed between time and mixing where mixing facilitated oxygen transfer in the reaction medium and affected the speed of reaction.
Although the enzyme activity is normally highly dependent on temperature, the results of this study indicated that temperature was not a significant parameter in phenol removal within the range of values tested. Figures 3(d) and (c) show no distinct optimum point for temperature, although a slight curve is observed with the changing time. However, although temperature was found to be not-significant in the statistical analysis, it is clear that temperature is a parameter in phenol removal, and in this case the entire experimental range for temperature was suitable for the reaction. This is further supported by the fact that no low percentage removal areas (indicated in blue) are observed in the graph. Similarly, percentage phenol removal increases slightly with increasing mixing intensity showing no significant effect. In the study of Aktas (2005) [21] catechol removal with laccase showed a strong temperature dependency with a distinct optimum point. Ghasempur et al. (2007) [20], observed that phenol removal with HRP clearly improved when the temperature increased although it was not statistically important. This shows that the reaction between laccase and phenol has a unique behaviour compared to the other enzymatic removal reactions. This might be due to the different molecular structures of the two molecules.
The conditions of the optimum point predicted by the program can be seen in Table 3. In order to verify this
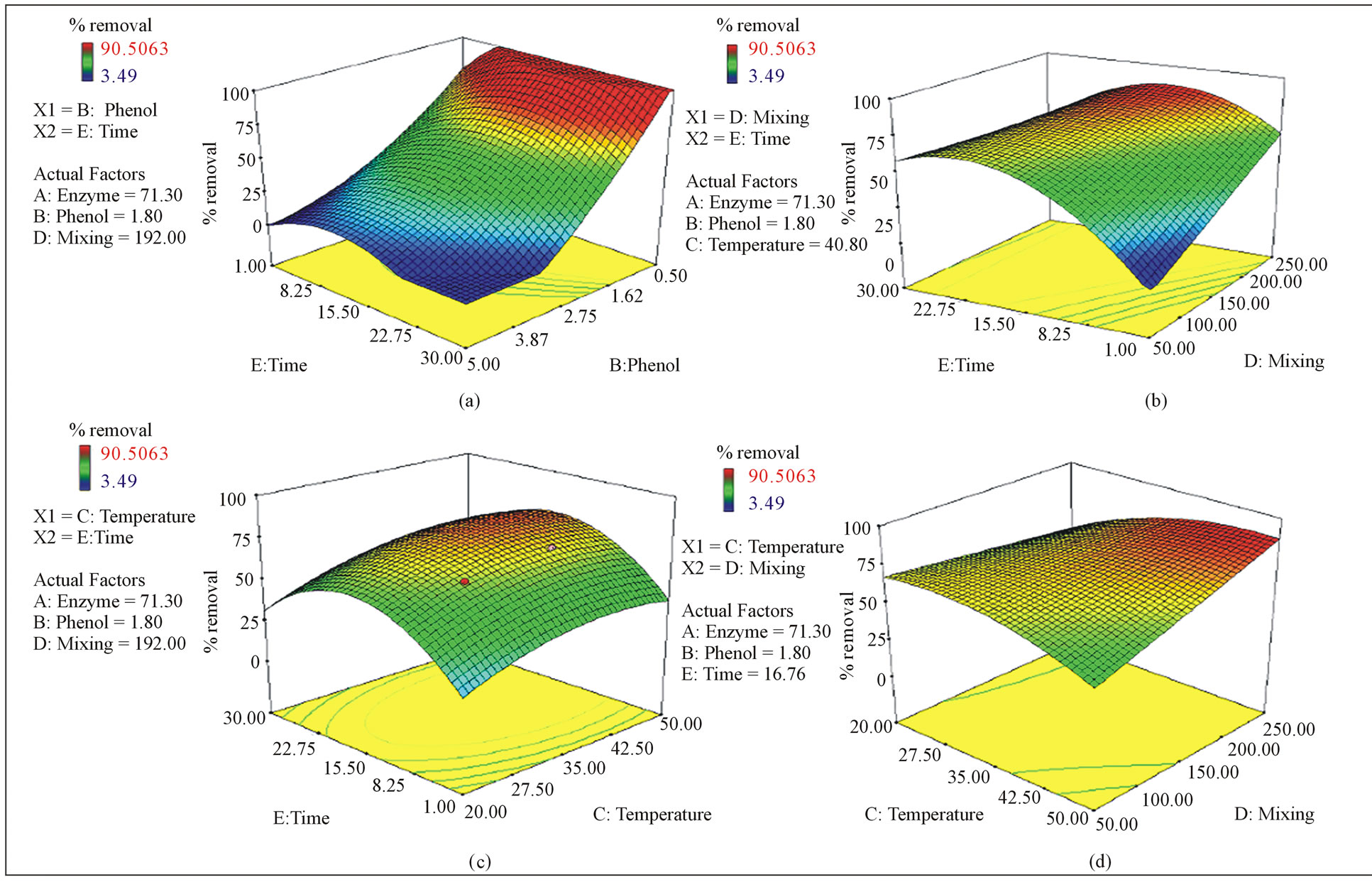
Figure 3. 3D graphs of parameters effecting phenol removal. (a) Effect of time and phenol concentration, (b) Effect of time and mixing, (c) Effect of time and temperature, (d) Effect of temperature and mixing.
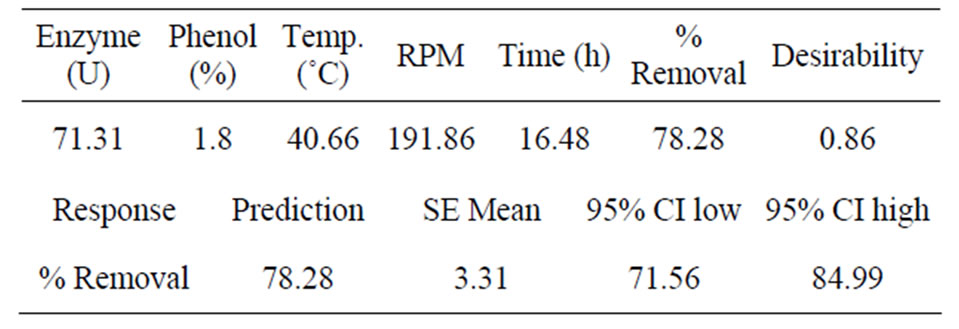
Table 3. Optimisation result of statistical analysis and confidence levels.
prediction, confirmation experiments were performed in the same way as the optimization experiments (Table 4).
The results obtained were between the confidence levels (Table 3) of the predicted % phenol removal. This indicated that the model is useful within the given range of conditions, verifying the reliability of the model.
The results clearly show that parameters influence each other, and the ideal value for one depends on the values of the others. This is a unique feature of the experimental design methodology compared to the traditional methods where simultaneous observation of two parameters is very difficult.
3.4. Treatment of Industrial Wastewater with Laccase
Subsequent to optimization using model wastewater, the

Table 4. Confirmation experiment conditions and results.
optimum conditions determined were tested using the industrial wastewater obtained from Cukurova Kimya Ind. The industrial wastewater contained ~2.5% phenol similar to the model wastewater and ~2.0% formaldehyde. laccase was added in the same ratio and phenol removal was measured at t = 0 and t = 16 h. All experiments were done in duplicates.
In the original sample containing formaldehyde almost no reduction was obtained at the end of 16 h. Therefore in the next experiment formaldehyde was evaporated in a rotary vacuum evaporator and the mixture was then treated with laccase. This yielded 17% phenol removal. Subsequently, the effect of temperature was tested to determine the potential of enzymatic removal at ambient temperature in order to reduce operational costs. It was found that 22% reduction could be achieved at 25˚C in comparison to 17% obtained at 40˚C.
3.5. Treatment with Immobilized Enzyme
Based on the results obtained from the statistical optimization, it was decided to immobilise the laccase used, to enable multiple-utilisation of the enzyme. The phenol solution was passed through the reaction column containing immobilised enzyme-alginate beads, at a flow rate of 75 ml/h and the remaining solution was analysed for phenol content. Two different approaches were investigated. First one was the cycle method, where the phenol solution passed through the column completely and analysed for phenol content (“cycle 1”) and then was passed again for the next cycle. In the other method, phenol solution was continuously passed through the column in a closed circuit and samples were taken against time throughout the process. When the synthetic wastewater was passed through the immobilized enzyme column in cycles, polymerization of phenol occurred on the surface of the packing material. The most drastic change in phenol content was observed in first cycle (20%) while the percent removal was not so promising.
In the circuit approach, phenol concentration showed a profile similar to the non-immobilized enzyme removal and more than 50% removal was achieved. This indicated that phenol removal with the immobilized enzyme system could be possible in a continuously operated column.
Further optimization studies are needed to improve the percentage removal and to determine feasible working conditions. Within our knowledge phenol removal with immobilized enzymes has not been reported by other workers making this study the first, showing that phenol removal can be achieved successfully with an immobilized enzyme column.
4. Conclusion
In this study removal of phenol by enzymatic reaction using laccase has been demonstrated. Optimized conditions for the laccase and synthetic phenol-water system were determined and were applied to a real industrial wastewater. Successful phenol removal was demonstrated with an immobilized enzyme column for the first time, presenting preliminary results indicating that immobilized enzyme in a packed column can be a possible alternative for phenol removal from industrial waste waters, improving the economics of the whole process.
In the future studies, addition of a suitable solvent may be investigated for removal with laccase. There are studies with Peroxidase where significant improvement has been reported with the addition of a solvent in the reaction medium. Also the polyphenolic product has not yet been characterized. Therefore, since the value of the produced material is not known it is hard to decide whether it is feasible to use this method to remove phenol.
Furthermore, the characterization of the polyphenolic product of the enzymatic reaction (with laccase) and the determination of its commercial value may improve the attraction and economics of the proposed enzymatic removal system.
REFERENCES
- H. M. Freeman, “Standard Handbook of Hazardous Waste Treatment and Disposal,” McGrawhill, New York, 1995.
- K. F. Mossallam, F. M. Sultanova and N. A. Salemova, “Peroxidase Catalyzed Removal of Phenol from Sythetic Wastewater,” 13th International Water Technology Conference, IWTC 13, Egypt, 2009.
- A. Sakurai, M. Masuda and M. Sakakibara, “Effect of Surfactants on Phenol Removal by the Method of Polymerization and Precipitation Catalysed by Coprinus Cinereus Peroxidase,” Journal of Chemical Technology and Biotechnology, Vol. 78, 2003, pp. 952-958. doi:10.1002/jctb.891
- Bódalo, L. Gómez, E. Gómez, J. Bastida and M. F. Máximo, “Comparison of Commercial Peroxidases for Removing Phenol from Water Solutions,” Chemosphere, Vol. 63, No. 4, 2006, pp. 626-632. doi:10.1016/j.chemosphere.2005.08.007
- M. Stanisavljevic and L. Nedic, “Removal of Phenol from Industrial Wastewaters by Horseradish Peroxidase,” Working and Living Environmental Protection, Vol. 2, No. 1, 2004, pp. 345-349.
- D. A. Villalobos and I. D. Buchanan, “Removal of Aqueous Phenol by Arthromyces Ramosus Peroxidase,” Journal of Environmental Engineering and Science, Vol. 1, 2002, pp. 65-73. doi:10.1139/s01-003
- K. Wilberg, C. Assenhaimer and J. Rubio, “Removal of Aqueous Phenol Catalysed by a Low Purity Soybean Peroxidase,” Journal of Chemical Technology and Biotechnology, Vol. 77, No. 7, 2002, pp. 851-857. doi:10.1002/jctb.646
- N. Aktas and A. Tanyolac, “Reaction Conditions for Laccase Catalyzed Polymerization of Catechol,” Biosource Technology, Vol. 87, No. 3, 2003, pp. 209-214. doi:10.1016/S0960-8524(02)00254-7
- A. Kunamneni, F. J. Plou, A. Ballesteros and M. Alcalde, “Laccases and Their Applications: A Patent Review,” Recent Patents on Biotechnology, Vol. 2, 2006, pp. 10-24. doi:10.2174/187220808783330965
- H. Ceylan, S. Kubilay, N. Aktas, and N. Sahiner, “An Approach for Prediction of Optimum Reaction Conditions for Laccase-Catalyzed Bio-Transformation of 1-Naphthol by Response Surface Methodology (RSM),” Bioresource Technology, Vol. 99, No. 6, 2008, pp. 2025-2031. doi:10.1016/j.biortech.2007.03.018
- J. Driver, “Degradation of Phenolic Compounds Using Laccase or Peroxidase Enzymatic Treatment,” MSc. Thesis, Auburn University, Faculty of Civil Engineering, Auburn, 2010.
- Y. Wu, K. E. Taylor, N. Biswas and J. K. Bewtra, “Kinetic Model Aided Reactor Design for Peroxidase Catalyzed Removal of Phenol in the Presence of Polyethylene Glycol,” Journal of Chemical Technology and Biotechnology, Vol. 74, 1999, pp. 19-26.
- L. Gianfreda, F. Xu and M. Bollog, “Laccase a Useful Group Oxidoreducdtive Enzymes,” Bioremediation Journal, Vol. 3, 1999, pp. 1-26. doi:10.1080/10889869991219163
- A. I. Yaropolov, O. V. Skorobogatko, S. S. Vartanov, S. D. and Varfolomeyev, “Laccase Properties, Catalytic Mechanism and Applicability,” Applied Biochemistry and Biotechnology, Vol. 49, 1994, pp. 257-279. doi:10.1007/BF02783061
- N. Aktas, G. Kibarer and A. Tanyolac, “Effects of Reaction Conditions on Laccase-Catalysed 1-Naphthol Polymerisation,” Journal of Chemical Technology and Biotechnology, Vol. 75, No. 9, 2000, pp. 840-846. doi:10.1002/1097-4660(200009)75:9<840::AID-JCTB292>3.0.CO;2-9
- J. Yu, K. E. Taylor, H. Zou, N. Biswas and J. K. Bewtra, “Phenol Conversion and Dimeric Intermediates in Horseradish Peroxidase-Catalyzed Phenol Removal from Water,” Environmental Science and Technology, Vol. 28, No. 12, 1994, pp. 2154-2160. doi:10.1021/es00061a025
- I. D. Buchanan and J. A. Nicell, “Model Development of Horseradish Peroxidase Catalyzed Removal of Aqueous Phenol,” Biotechnology and Bioengineering, Vol. 54, No. 3, 1997, pp. 251-261. doi:10.1002/(SICI)1097-0290(19970505)54:3<251::AID-BIT6>3.0.CO;2-E
- R. W. Martin, “Rapid Colorimetric Estimation of Phenol,” J. Sos. Chem, Vol. 21, 1949, pp. 1-3.
- M. Reihmann and H. Ritter, “Synthesis of Phenol Polymers Using Peroxidase,” Advances in Polymer Science, Vol. 194, 2006, pp. 1-49. doi:10.1007/12_034
- S. Ghasempur, S. F. Torabi, S. O. R. Siadat, M. J. Heravi, N. Ghaemi and K. Khajeh, “Optimization of PeroxidaseCatalyzed Oxidative Coupling Process for Phenol Removal from Wastewater Using Response Surface Methodology,” Environmental Science and Technology, Vol. 41, No. 20, 2007, pp. 7073-7079. doi:10.1021/es070626q
- N. Aktas, “Optimization of Biopolymerization Rate by Response Surface Methodology (RSM),” Enzyme and Microbial Technology, Vol. 37, No. 4, 2007, pp. 441-447. doi:10.1016/j.enzmictec.2005.03.010
NOTES
*Corresponding author.

