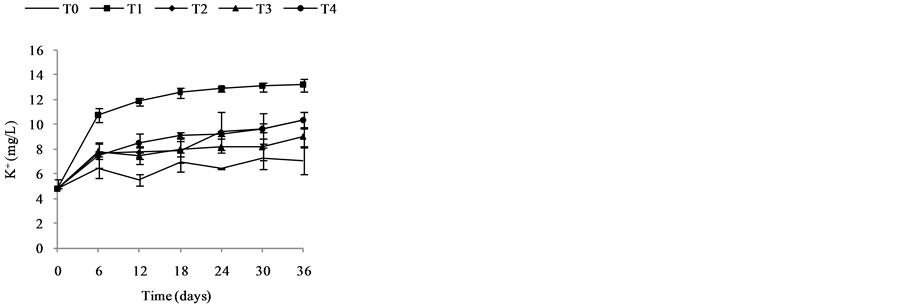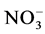Agricultural Sciences
Vol.05 No.12(2014), Article ID:51019,12 pages
10.4236/as.2014.512129
Decomposition and Mineralization Effect of Various Sources of Pig Manure on Water Quality and Nutrients Availability for Agro-Fish System in Benin
H. K. J. Bokossa1*, A. Saïdou2, E. Sossoukpe1, D. E. Fiogbé1, D. Kossou3
1Laboratory of Research on Wetlands (LRW), Department of Zoology, Faculty of Science and Technique, University of Abomey-Calavi, Cotonou, Benin
2Integrated Soil and Crop Management Unit (ISCM), Laboratory of Soil Sciences, Department of Crop Science, Faculty of Agronomic Science, University of Abomey-Calavi, Cotonou, Benin
3Laboratory of Plant Biology, Department of Crop Science, Faculty of Agronomic Science, University of Abomey-Calavi, Cotonou, Benin
Email: *bokossakouessiv@yahoo.fr
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 19 August 2014; revised 21 September 2014; accepted 23 October 2014
ABSTRACT
In spite of the relevance of current studies on the importance of organic fertilizers such as animal manure in improving the health of ecosystems, little is known about the biochemical mechanisms affecting the availability of nutrients released from the organic fertilizer in water. A litter bag study during 6 weeks was carried out in pots containing 25 liters of water with 15 g of pig dejec- tions as organic fertilizers. The experimental design was a completely randomized block design with three replications. The treatments consisted of dejections of pigs nourished with: recom- mended diet composition T1, partially improved diet with Azolla filiculoides T2, improved diet with Azolla filiculoides T3, improved diet with cereal bran T4. A control treatment without dejection (T0) was considered in the study for comparison purpose. Four pigs per type of diet were considered leading to 16 white landrace pigs of six months age followed for dejection collections. Strong release of nutrients for better yield for agro-fish system was obtained in the manure T1 with 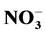 (10.85 ± 0.00) mg/L;
(10.85 ± 0.00) mg/L; 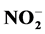 ( 0.011 ± 0.00) mg/L;
( 0.011 ± 0.00) mg/L; 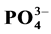 (2.13 ± 0.07) mg/L and K+ (10.76 ± 0.57) mg/L; Ca2+ (2.92 ± 0.11) mg/L and Mg2+ (2.53 ± 0.00) mg/L followed by manure T3 and T4 with high N content. The relatively low ratio C/N (14.25) for T1 and (15.84) for T3 induced more nutrients releasing. This study showed an important N loss probably due to microorganism activities which fluctuate nutrient availability. Also significant correlations were noted between the nutrient dynamics in water and physicochemical parameters showing the effect of abiotic factors on organic matter decomposition and mineralization which depend on microbial activities in water and pig manure composition.
(2.13 ± 0.07) mg/L and K+ (10.76 ± 0.57) mg/L; Ca2+ (2.92 ± 0.11) mg/L and Mg2+ (2.53 ± 0.00) mg/L followed by manure T3 and T4 with high N content. The relatively low ratio C/N (14.25) for T1 and (15.84) for T3 induced more nutrients releasing. This study showed an important N loss probably due to microorganism activities which fluctuate nutrient availability. Also significant correlations were noted between the nutrient dynamics in water and physicochemical parameters showing the effect of abiotic factors on organic matter decomposition and mineralization which depend on microbial activities in water and pig manure composition.
Keywords:
Pig’s Diet, Manure, Fertilization, Physico-Chemical Microbial Water Properties, Nutrients Availability

1. Introduction
The current population growth demands from the international community to increase and diversify agricultural and fisheries productivity. This can only be achieved through the improvement of crops and livestock by adopting more efficient farming techniques [1] . The recent decade has seen the growth of a particular attention to the issue of pollution from chemical fertilizers and pesticides [2] , which remains the increase in production costs and significantly reduce farmers’ incomes. In this context, manure with the organic matter they contain, are not only a guarantee for the physical, chemical and biological soil fertility but also the main pillars for achieving gains significant productivity [1] . Generally farmyard manure or composts are suggested as an alternative process for the management of these wastes [3] . Nitrogen release from organic wastes mineralization has been successfully tested in agriculture [3] - [7] . These authors concluded that, the supply of manure in agricultural land is an excellent way to recycle nutrients and organic matter to improve crop production. But according to [4] , knowledge of nutrients release from the mineralization of organic manure is critical in regulating net ecosystem productivity, especially in nutrient-limited systems. In fact, controlling the rate of mineralization including bio- chemical mechanisms affecting the availability of nutrients released is essential for rigorous prediction quanti- ties of organic fertilizers for rice-fish systems. Lu and Li [8] revealed that rice-fish farming systems constitute a unique agro-landscape across the world, especially in tropical and sub-tropical areas and the introduction of fish rearing to rice farming creates an integrated agro-ecological system practice. In that system, Haroon et al. [9] found that paddy yields were ranging 1.5 - 3.7 t∙ha−1∙crop−1 in rice-fish and 1.5 - 1.8 t∙ha−1∙crop−1 in rice-alone culture with insignificant differences between the years (P > 0.05). It was also proved that, when the pigs were nourished with diet of high nutritive value such as Azolla filiculoides, it also improves the quality of dejection produced which could be used to feed fish and to supply nutrient to the crop considering wetland production system [10] . In such way, developing strategies to recycle nutrient from these residues especially those from livestock and pig in the context of Benin agriculture is a challenge. However, in Benin more than 518,600 pigs are breed with an increase of 3% of the livestock the last four years [11] [12] leading to an important quantities of dejection produced which are source of environmental pollution. Zooplankton productions systems based on the use of pig manures have been previously reported [13] but there is lack of knowledge on the biochemical mechanisms affecting the availability of nutrients released during the mineralization of organic fertilizers such as manure from pig fed with Azolla filiculoides and rice bran in wetland condition for fish and crop production system.
This paper reports the results of a study of pig dejection decomposition and nutrient mineralization in wetland condition using pots experiment. It aims to (i) evaluate the influence of diet on the nutritional quality of applied manure, (ii) evaluate the effect of the nutritional quality of different sources of pig manure on nutrient dynamics in wetland, (iii) determine the effect of abiotic parameters on nutrient availability in the water.
2. Materials and Methods
2.1. Experimental Site
The experiment was carried out on the site of the Laboratory of Research on Wetlands (LRW) Department of Zoology, Faculty of Science and Technique of University of Abomey-Calavi, Benin (Longitude E: 2˚20'18.7"; Latitude N 6˚24'53.4"). This site is located at an altitude of 17 ± 3 m. The area is characterized by sub-equatorial
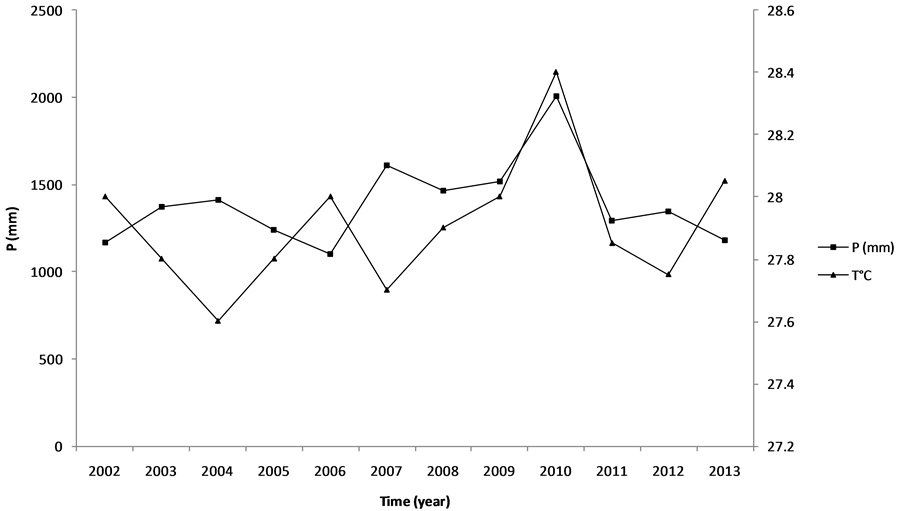
climate with two rainy seasons (March to end of July and mid-September to November) and two dry seasons (August to mid-September and December to March). The rainfall pattern and temperature during the experiment is presented in Figure 1.
Azolla filliculoides an aquatic fern used to enrich the pigs diet was produced during two months (June to July 2013) in ponds located at the experimental site.
2.2. Pigs Manure Collection and Process
Pigs feeding and manure collection according to the types of diet served to the animal were carried out in the LRW pigsty located on the experimental site. The manures were daily collected and mixed according to the diet served to the pigs. Experiment on pigs’ manure decomposition was carried out on the site of LRW. The dejections were collected from July 17th 2013 until sufficient quantity to carry out the experiment was obtained in 19th August 2013. The dejections collected were dried at ambient temperature. About 50 kg of pig dejection were collected per types of diet given to the pigs. The diets given to the pigs were made based on result of Azolla fili- culoides use from experiment carried out by Accodji et al. [11] and Djissou [14] .
The treatment were manure collected from pigs nourished with: T1 (recommended diet composition: 15% Azolla + 55% provender + 5% coconut copra + 5% oil palm + 5% soybean bran + 10% rice bran + 5% kitchen waste), T2 (partially improved diet with Azolla: 30% Azolla + 65% rice bran + 5% oil palm), T3 (improved dietwith Azolla: 47.5% Azolla + 47.5% rice bran + 5% oil palm), and T4 (improved diet with cereal bran: 15% Azolla + 40% rice bran + 40% wheat bran + 5% oil palm). A control treatment without manure (T0) was considered in the study as comparison purpose. The experiment was carried out during six weeks (from 19 September to 25 October 2013).
In total, four pigs per type of diet were considered leading to 16 white landrace pigs of six months age followed for dejection collection. Instead of plastic containers clay jars were used for the pot experiment as they create fresh conditions like in the wetland. Tap water was used to create wetland condition in the jars.
2.3. Experimental Design
The experimental design was a complete randomized block with three replications. Manure bags used in this experiment were constructed from nylon mosquito net with a mesh size of 1 mm. The nylon was tied 15 g of dejection and suspended in 25 liters of tap water representing 600 g/m3 [3] and also adapted from litter-bags techniques [15] - [17] .
Figure 1. Rainfall pattern and temperature during the experiment.
For each treatment (except the control), 12 bags with 15 g oven dried manure material were filled. Manure bags were randomly placed in the 12 clay jars of one lot to be sampled weekly. In total, 6 lots of 12 clay jars leading to 72 clay jars. A control (T0) consists of jars containing 25 liters of water without dejection in addition to jars containing water and dejection. Overall, 15 water samples were successively collected at 6, 12, 18, 24, 30 and 36 days and transported to the laboratory for the analysis. So for each sampling time, there were three ma- nure bags per treatment.
2.4. Manure and Water Analysis
Analyses concerned firstly dejection quality after feeding and secondly water containing these dejections during mineralization process. Methods used for the dejection analysis were that described by NF EN 14082 norm [18] . The analyses were performed on: organic carbon (After drying, powder reduction and incineration at 550˚C for 24 h, the organic matter is determined by the difference between the treated sample and the mass of ash ob- tained assigned an empirical coefficient of 2.) The ash obtained after dissolution in HCl, 6N, after evaporation at 125˚C gives a residue which, dissolved in HNO3 were used to measure Ca, Mg, K and P by spectrophotometric essay, total N using Kjeldahl digestion in a mixture of H2SO4 selenium method followed by distillation and titra- tion, total P using AFNOR NFT 90-023, 1982 spectrophotometric assay after persulphate digestion in Kjeldahl flask method. Water sample collected were stored at the laboratory in a fridge at 4˚C in order to assess enrich- ment with nutrient released during the mineralization process. Analyses included suspended solids, 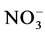 ,
, 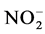 and
and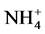 . The suspended solids were measured using Standard NF EN 14082 method after water evapo- ration in an oven at 105˚C for 24 hours.
. The suspended solids were measured using Standard NF EN 14082 method after water evapo- ration in an oven at 105˚C for 24 hours. 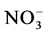 using sodium salicylate method [19] , nitrate ions in the presence of sodium salicylate form sodium paranitrosalicylate colored in yellow which is measured colorimetrically at a wavelength of 420 nm.
using sodium salicylate method [19] , nitrate ions in the presence of sodium salicylate form sodium paranitrosalicylate colored in yellow which is measured colorimetrically at a wavelength of 420 nm. 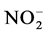 using reagent of Zambely method [19] , where ammonium ions in presence of sul- fanilic acid and phenol form a yellow colored complex whose intensity is proportional to the concentration of
using reagent of Zambely method [19] , where ammonium ions in presence of sul- fanilic acid and phenol form a yellow colored complex whose intensity is proportional to the concentration of 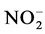 and the ions concentration is measured colorimetrically at a wavelength of 435 nm.
and the ions concentration is measured colorimetrically at a wavelength of 435 nm.  by NESSLER method [19] , ammonia in the presence of this reagent for reddish-brown color which is measured colorimetri- cally at a wavelength of 425 nm.
by NESSLER method [19] , ammonia in the presence of this reagent for reddish-brown color which is measured colorimetri- cally at a wavelength of 425 nm. 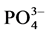 (In strongly acidic solution, the ammonium molybdate reacts with the orthophosphate to form phosphoric acid molybdo reduced by amino acid reactive to form a blue compound capable for spectrophotometric assay at 700 and 880 nm.)
(In strongly acidic solution, the ammonium molybdate reacts with the orthophosphate to form phosphoric acid molybdo reduced by amino acid reactive to form a blue compound capable for spectrophotometric assay at 700 and 880 nm.)
K+, Ca2+ and Mg2+ content in water were determined with Atomic Absorption Spectrophotometer. Tempera- ture and dissolved oxygen were measured in situ using Oxythermometer brand OXI 197i-N27455, the conduc- tivity using conductivimeter brand N27444-197i, and pH with pH meter 197i brand-N27499.
2.5. Method of Statistical Analysis
The variance analysis test followed by the homogeneity test of Student-Newman-Keuls at the 5% was performed with the SAS 9.2 software. These tests were performed after inspection of the normality and homoscedasticity conditions of variance, to compare the means of physico-chemical data. Also using Minitab, the Pearson corre- lation coefficient was used to determine possible correlations between the temperature, dissolved oxygen, pH, conductivity and nutrient dynamics.
3. Results
3.1. Diets and Pig Manure Chemical Composition
The chemicals characteristics of pigs’ manures used for the experiment are presented in Table 1. It appears from the results that carbon and nitrogen content in the manure collected from pigs nourished with improved diet with Azolla (T3) significantly (P < 0.05) increase by 1.2 and 1.3 times compared with that collected from pigs nou- rished with the recommended diet composition (T1). In general for carbon, one can rank the manures richness according: T3 > T2 > T1 > T4 and T3 > T1 > T4 > T2 for nitrogen content. These results lead to a lowest C/N ratio for treatment T3 while the highest C/N ratio was obtained with T1 and T4.
The manure collected from pigs nourished with recommended diet composition (T1) presents significantly higher P content compared with that collected from treatment T2, T3 and T4 (Table 1). The addition of Azolla in the diet seems to decrease significantly by 1.1 and 1.3 P content in the treatment T3 and T2 respectively compared with T1. Furthermore, K, Ca and Mg concentrations were high in the manure T1 compared with the
Table 1. Average value (±standard errors) of the chemical characteristics of pigs’ manures regarding the composition of the diets (T1 = Manure of pigs nourished with recommended diet composition; T2 = manure of pigs nourished with diet partially improved with Azolla; T3 = manure of pigs nourished with diet improved with Azolla; T4 = manure of pigs nourished with diet improved with cereal bran) used to feed the animal.
Note: means followed by the same letter are not significantly different (P > 0.05) according to the Student Newman-Keuls test.
other treatments. However, significant decrease by 2.3, 2.4 and 1.9 were noticed with K concentration in the manures respectively with treatments T2, T3 and T4 compared with T1. The decrease is less (1.4 for treatments T2 and T3 and 1.5 with T4 compared with treatment T1) with Ca content in the manures. Concerning Mg con- tent, manure collected from pigs’ nourished with improved diet with Azolla did not vary compare with that from pigs nourished with recommended diet composition. However, significant decrease by 1.4 and 1.3 in Mg content in the manure is noticed with treatments T2 and T4 compared with that of pigs nourished with recommended diet composition (treatment T1). The coefficient of variation calculated for all treatments is somewhat elevated for N and K (CV > 20) and somewhat lower for P, Ca, Mg and C (CV < 20) revealing the difference in composition manure and in conjunction with the chemical composition of the tested diets. We can conclude that nutrients content in the manure depends on the chemical composition of the diet used to feed the animals.
3.2. Water Quality and Nutrient Dynamics
3.2.1. Physical and Chemical Parameters Measured in Situ
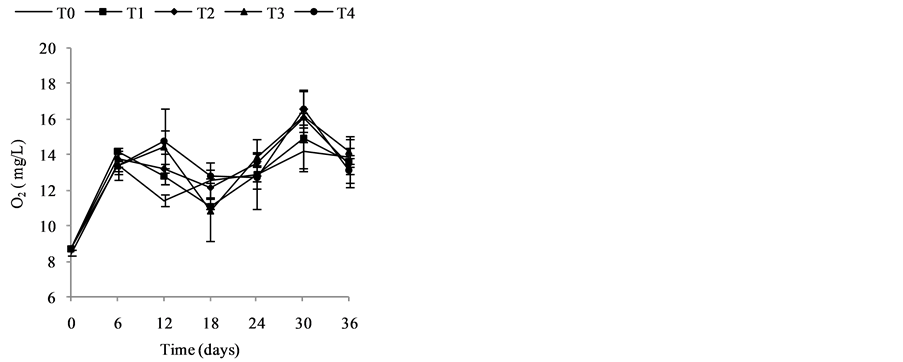
Change in temperature (Figure 2(c)), dissolved oxygen (Figure 2(a)), conductivity (Figure 2(d)), and Ph (Figure 2(b)) during the 36 days of experiment is depicted in Figure 2. In general, the values of the water temperature fluctuate between 25.90˚C and 33.77˚C approximately. The values observed are similar in all treatments and vary according to the weeks of experiment. However, there is a drop in global temperature after a week of fertilization in all treatments and increase during the 3rd and 6th week. These temperatures observed are those of mesophilic microorganisms therefore support the biodegradability of organic matter in pig manure. The temperature rise observed the 3rd and 6th week shows activation of metabolic reactions of nutrients released by microorganisms. Dissolved oxygen affects life in ecosystems. The average values of dissolved oxygen registered during the experiment are between 8.36 mg/L and 16.57 mg/L. However, in the 3rd and 6th weeks a decrease in the concentration of dissolved oxygen in all treatments was observed; this can be correlated with the increase in temperature observed in the third and sixth week of experimentation. In fact, aerobic microorganisms whose metabolic activity is activated by the rise in temperature consume then enough oxygen for faster degradation of organic matter. pH values at the start of experiment were low and indicate relatively acidic water (5.13). These values range 5.13 to 8.14 at the end of experiment, whereas in the control treatment T0, the pH remained rela- tively low. The contribution of manure resulted in a gradual increase in the pH which reached 8.14 especially in T4. This indicate a propensity to alkalinity in water containing pig manure mainly due to a release of NH3 from 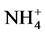 in the area during pig manure mineralization in the water. Water conductivity is a global but rough para- meter to estimate water mineralization. Except treatment T0 (90.98 µS/cm) where conductivity values were low and constant, those observed in T1 to T4 increased from the first to the sixth week. Generally, water conductivi- ty in jars range 40.66 µS/cm to 174, 93 µS/cm reflecting progressive mineralization of water due to the activity of pig manure decomposition by microorganisms
in the area during pig manure mineralization in the water. Water conductivity is a global but rough para- meter to estimate water mineralization. Except treatment T0 (90.98 µS/cm) where conductivity values were low and constant, those observed in T1 to T4 increased from the first to the sixth week. Generally, water conductivi- ty in jars range 40.66 µS/cm to 174, 93 µS/cm reflecting progressive mineralization of water due to the activity of pig manure decomposition by microorganisms
3.2.2. Nutrient Dynamics in Water (mg/L)
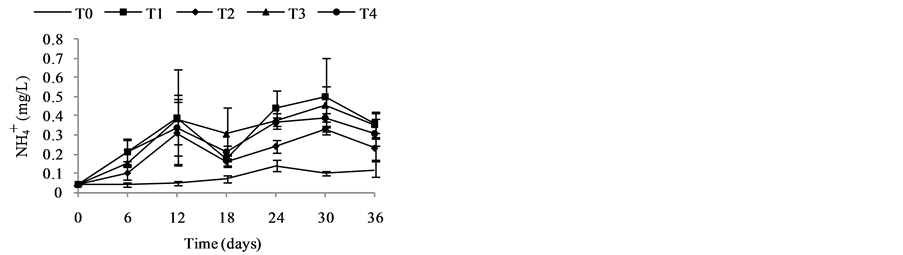
Nutrient dynamics during the experiment are presented in Figure 3. 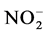 rates (Figure 3(a)) in the different treatments were increased over time with variations ranging between (0.003 ± 0.00) and (0.11 ± 0.00) mg/L ob-
rates (Figure 3(a)) in the different treatments were increased over time with variations ranging between (0.003 ± 0.00) and (0.11 ± 0.00) mg/L ob-
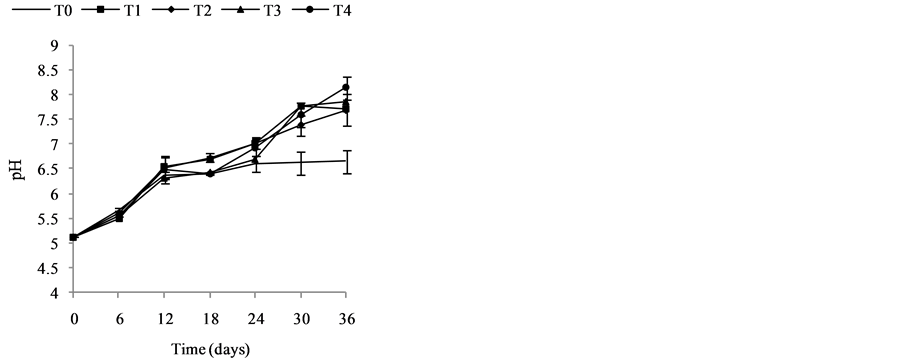

Figure 2. Change in temperature, dissolved oxygen, conductivity, and pH during the 36 days of experiment. (a) Change in Dissolved Oxygen (mg/L) during 36 days of experiment; (b) Change in pH during 36 days of experiment; (c) Change in Temperature (˚C) during 36 days of experiment; (d) Change in conductivity (µS/cm) during 36 days of experiment.
tained with T4 during the fourth week. Also there is a decrease of nitrite content in treatments T1 to T4 during the 3rd and 6th weeks. These variations observed are related to the activities of micro-organisms in the water that influences nitrogen availability with 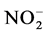
Nitrates (Figure 3(b)) are the most stable form of the nitrogen in the water from the nitrification and organic nitrogen. During the test, the values of the nitrates content are between (6.04 ± 0.00) mg/L and (22.95 ± 3.19) mg/L with a high value observed in T1 with (22.95 ± 3.19) mg/L after five weeks fertilization. Decreases levels were observed during the third and sixth weeks. Indeed, as the temperature increases, the biochemical reactions are activated and result in a loss of NH3 by transformation of nitrogen or denitrifying bacteria where the decrease observed in the third and sixth weeks. It was noted that the low value recorded at the control treatment T0 is due to the fact that this treatment has received no manure.
The values of 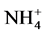
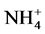
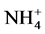
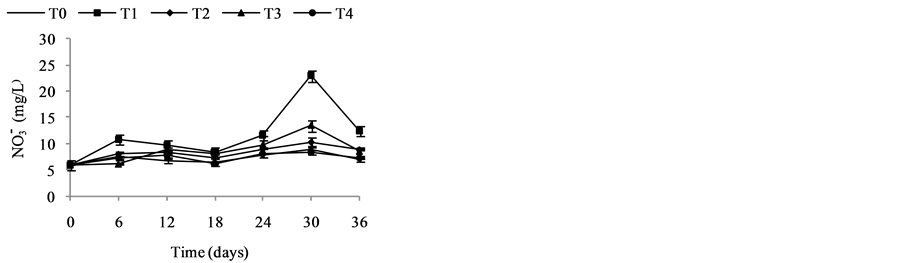



Figure 3. Variation of the nutrient (


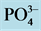



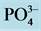
isms present in the water, the pH and properties of manure in water.
The values of the 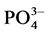
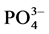
3.2.3. Correlations between in Situ Measured Parameters and Nutrient Dynamics in Water
Abiotic parameters including pH, conductivity, temperature and dissolved oxygen have strongly influenced the dynamics of nutrient released in the water during the mineralization process of manure from pig mainly fed with Azolla filiculoides and rice bran. Thus according to the Pearson correlation test, the pH is correlated with the concentration of 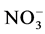
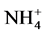
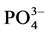
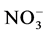
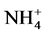
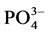
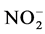
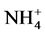
Indeed, the biochemical mechanisms of degradation of organic matter depend on physical and chemical con- ditions of the environment. The lower rate of dissolved oxygen in the 3rd and 6th week coincid with the increase of temperature reflecting a high decomposition activity of aerobic bacteria that induce a large release of nutrients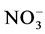
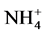
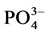
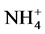
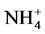
In total, the release of nutrients contained in the manure of pigs fed on Azolla filiculoides and rice bran was modulated by abiotic parameters namely dissolved oxygen, pH, conductivity and temperature in the aquatic environment.
4. Discussion
4.1. Influence of the Tested Diets on Manure Fertilizer Quality
Food is an essential parameter of the composition of the manure. Approximately 60% - 80% of the nitrogen and phosphorus ingested are not absorbed by the animal and about 90% for potassium [20] .
From the results obtained, the nutritional quality of manure varies significantly from one treatment to another. This is due to the composition of the diets and to the digestibility of applied rations for monogastric such as pigs. According to Corpen [21] , diet and discharge quality depends of physiological state as required for pig metabol- ism. The standard reference for nitrogen rejection sow is 17.50 kg/sow/year, post weaning 0.44 kg/pig fattening phase, 3.25 kg/pig. The high levels of nitrogen in manure (T3) are related to the high content of Azolla in this regime. Also, the differences between manure composition are related to the nature of the raw material which was used: Azolla filiculoides and rice bran at various doses. This confirm the results of Faure [22] which focused with regard to protein content, that the yield of digestion vary considerably from raw materials, and by the variety for the same raw material. Others factors responsible for the variability in the quality of manure were cited such as trypsin inhibitors, lectins and tannins responsible for the low digestibility of certain varieties of peas [23] .
Thus, the high rate of nitrogen released by the pig in the T3 can be explained by the low digestibility of the ingredients as Azolla filiculoides in the ration which is highly rich in fiber and lignin readily degradable. The C/N ratios recorded in T1 to T4 are well above the C/N ratio found in pig slurry whose value is 4 [24] . This difference could be explained by the composition of the diet applied to feed pigs.
4.2. Quality Effects of Manure on the Enrichment of Water in Nutrients (N, P, K, Ca and Mg)
During the decomposition of pig manure, the water temperature is an important factor not only for organic pro- duction but also for solubility gases such as oxygen [25] . Thus, the low value of the temperature observed after one week of fertilization is due to aeration of the area with oxygen. In fact, during this week of fertilization mi- crobial activity is much slower compared to the 3rd and 6th where it is faster. Basic pH values (8.14) obtained at the end of the experiment reflecting an enrichment of the area in NH3 are contrary to the values of pH acids ob- tained by Brault [26] due to the degradation of organic substances. This difference could be explained by the en- vironmental climatic conditions. However, the pH values recorded during these experiments are compatibles for fish farming which allows a value between 6.5 and 9 [27] .
High levels of suspended solids matters and stored water conductivity in T1 to T4 are due to the high release of nutrient in the area and confirm the results of Belghiti et al. [25] who proved the mineralization effect on wa- ter mineral contents. For good manure, organic matter decomposition process is performed by heterotrophic bacteria which consume dissolved oxygen to release the nutrients such as ammonium, nitrite, nitrate and phos- phate and carbon dioxide [28] The values recorded for nitrites during different sampling campaigns are in ac- cordance with the optimum value required 0.1 mg/L [29] for aquaculture water. However, high level of nitrites (1.11 mg/L) obtained with T4 after 4 weeks of fertilization might be due to physico-chemical and biological process taking place in this environment [30] . Indeed, during the degradation process of organic matter by mi- croorganisms there is an ammonia oxidation or reduction of the nitrate which induce a release of nitrous ions in the area. This could justify the findings observed in all the treatments except, To at the 3rd and 6th week of expe- riment in which there has been a substantial decline in the rate of nitrogenous nutrients in fertilized water based on pig waste level. As for nitrates, these values are much higher than the standard set for surface water is 0.1 mg/L for freshwater aquaculture [31] .
High nitrate levels in treatments T1 to T4 could be explained by the significant degradation of organic matter in the area under the influence of microorganisms [32] . As nitrates proceed from the nitrification of nitrogen in pig waste [28] , ammonium is a mineral which process usually results on an incomplete degradation of organic matter [28] . This could justify the fluctuating values nitrogen during the experiment where organic matter is gradually degraded. It is noted that the ammonium also appears in the area from the conversion of nitrates. It could also be noted that the different nitrogen forms and their evolution in ecosystems depend on biological activity, pedo-climatic conditions [33] .
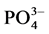
4.3. Correlations between Parameters Measured in Situ and Nutrient Dynamics in Water
According to our results, the Pearson correlation test revealed an interaction between abiotic parameters such as pH, conductivity, dissolved oxygen, temperature and nutrient dynamics in water containing pig manure during mineralization. In spite of the increase in the content of nitrogenous nutrients throughout the experiment, signifi- cant nitrogen losses were recorded for all treatments particularly during the third and sixth weeks of experi- mentation, periods corresponding to lower rate of dissolved oxygen and an increase in temperature. Many au- thors showed the influence of abiotic physico-chemical parameters on the dynamics of nutrients in the water. Thus, according to Hafiane et al. [36] , hot weather leads to increased net loss of nitrogen as NH3. In the same way, Dovonou et al. [37] prove that because NH4+proceed from the decomposition of excreta, it pass to gas form and lead to an elevation of pH. This loss of nitrogen observed at the 3rd and 6th weeks in NH3 corresponds to a transfer of ammonia gas present in the manure in the immediate atmosphere [38] . Indeed, according to the author, process of volatilization varies with the NH4+ concentration in the manure, the enzyme activity, the pH, the temperature, and the slurry properties. Complementary to this author, [39] describes in fact, an equilibrium which exists between the concentrations of 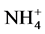
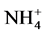
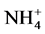
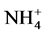
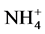
5. Conclusion
The studies on the decomposition of pig manure fed on with different foods based on Azolla filiculoides and rice bran prove that the treatments T1 containing Azolla filiculoides, provender and rice bran showed rapid minerali- zation because of their relative low C/N ratio. The composition of pig dejection varies from one treatment to another because of the composition of the diet applied. The treatments T2 and T3 which contained respectively 30% and 47.5% of Azolla (aquatic plant rich in nitrogen) in their food release significant rate of nitrogen in the manure. The C/N ratio in the treatment T2 slows the rate of mineralization of the organic material and thereby a very small quantity of nutrients is available in the water while those reports in the treatments T1, T3 and T4 were respectively 14, 25, 13.22 and 15.85.
The water quality varies according to the composition of the animal dejection, but it also depends on the dura- tion of fertilization, the abiotic parameters such as pH, temperature, conductivity and dissolved oxygen which modulate microorganism activities in the manure then nutrient availability for agro-fish system.
Acknowledgments
We express our gratitude to the Scientific Council of the University of Abomey-Calavi, which funded the pro- ject untitled “Optimization of Agricultural Production Integrated System without Inputs (OPASISI)” in which this research work has been undertaken.
References
- FAO (Food and Agriculture Organization) (2006) Utilisation des engrais par culture au Maroc. Première édition (Rome), 73 p.
- Pilar, F., Castellar, I. and Navarro, J. (2005) Nitrate Leaching in Pepper Cultivation with Organic Manure and Supplementary Additions of Mineral Fertilizer. Communications in Soil Science and Plant Analysis, 36, 2889-2899. http://dx.doi.org/10.1080/00103620500306072
- Amadji, L.G., Saidou, A. and Chitou, L. (2009) Recycling of Organic Residues in Compost to Improve Coastal Sandy Soil Properties and Cabbage shoot In Benin. International Journal of Biological and Chemical Sciences, 3, 192-202.
- Cordovil, C.M.S., Cabral, F. and Coutinho, J. (2007) Potential Mineralization of Nitrogen from Organic Wastes to Ryegrass and Wheat Crops. Bioresource Technology, 98, 3265-3268. http://dx.doi.org/10.1016/j.biortech.2006.07.014
- Evers, G.W. (2002) Ryegrass-Bermudagrass Production and Nutrient Uptake When Combining Nitrogen Fertilizer with Broiler Litter. Agronomy Journal, 94, 905-910. http://dx.doi.org/10.2134/agronj2002.9050
- Vagstad, N., Broch-Due, A. and Lyngstad, I. (2001) Direct and Residual Effects of Pulp and Paper Mill Sludge on Crop Yield and Soil Mineral N. Soil Use and Management, 17, 173-178. http://dx.doi.org/10.1079/SUM200172
- Vasconcelos, E., Cabral, F. and Cordovil, C.M.S. (1999) Wheat Yield and Leachability of Phosphorus and Nitrogen in Pig Slurry Amended Soils. Communications in Soil Science and Plant Analysis, 30, 2245-2257. http://dx.doi.org/10.1080/00103629909370369
- Lu, J.B. and Li, X. (2006) Review of Rice-Fish-Farming Systems in China—One of the Globally Important Ingenious Agricultural Heritage Systems (GIAHS). Aquaculture, 260, 106-113. http://dx.doi.org/10.1016/j.aquaculture.2006.05.059
- Haroon, A.K.Y. and Pittman, K.A. (1997) Rice-Fish Culture: Feeding, Growth and Yield of Two Size Classes of Puntius gonionotus Bleeker and Oreochromis spp. in Bangladesh. Aquaculture, 154, 26l-281.
- Youssouf, A., Saidou, A., Mama, D., Fiogbé, E.D. and Micha, J.-C. (2012) Evaluation of Nitrogen and Phosphorus Wastes Produced by Nile Tilapia (Oreochromis niloticus L.) Fed Azolla-Diets in Earthen Ponds. Journals of Environ- mental Protection, 3, 502-507.
- Accodji, J.M.M., Fiogbé, E.D. and Gangbazo, K.H. (2009) Essai de valorisation d’Azolla (Azolla microphylla Kaulf) dans la production porcine en zone humide. International Journal of Biological and Chemical Sciences, 3, 890-898.
- Fiogbé, E.D. and Gangbazo, K.H. (2005) Production porcine avec Azolla. Actes des 2èmes Journées Scientifiques Internationales des Universités Nationales du Bénin, 13-16 avril 2004, 142-154.
- Agadjihouèdé, H., Montchowi, E., Chikou, A. and Lalèyè, P.A. (2011) Libération comparée de sels dans l’eau par la minéralisation de l’azolla, la bouse de vache, la fiente de volaille et les sons de riz et de maïs utilisés en pisciculture. International Journal of Biological and Chemical Sciences, 5, 1883-1897.
- Djissou, A.S. (2012) Production d’aliments vivants (zooplancton) à partir des déjections de porcs pour nourrir les larves de poissons: Détermination des doses optimales. Mémoire de Master en Hydrobiologie Appliquée. Option: Aquaculture, Université d’Abomey-Calavi, Cotonou, 44 p.
- Qiu, S., Mc Comb, A.J. and Bell, R.W. (2013) Leaf Litter Decomposition and Nutrient Dynamics in Woodland and Wetland Conditions along a Forest to Wetland Hillslope. International Scholarly Research Network. ISRN Soil Science, 2012, Article ID: 346850.
- Hartemink, A.E. and O’Sullivan, J.N. (2001) Leaf Litter Decomposition of Piper aduncum, Gliricidia sepium, and Imperata cylindrica in the Humid Lowland of Papua New Guinea. Plant and Soil, 230, 115-124. http://dx.doi.org/10.1023/A:1004868502539
- Tian, G., Kang, B.T. and Brussaard, L. (1992) Biological Effects of Plant Residues with Contrasting Chemical Compositions under Humid Tropical Conditions-Decomposition and Nutrient Release. Soil Biology and Biochemistry, 24, 1051-1060. http://dx.doi.org/10.1016/0038-0717(92)90035-V
- Kanninkpo, C. (2013) Synthèse de quelques méthodes d’analyses utilisées par le Laboratoire des Sciences du Sol, Eaux et Environnement (LSSEE/CRA Agonkanmey/INRAB) pour les produits végétaux; Rédigé sur la base des documents de travail; 2 p.
- Rodier, J. (1996) L’Analyse de l’eau. eaux naturelles, eaux résiduelles, eau de mer. 8ème Edition, DUNOD, Paris, 1383 p.
- Dourmand, J.Y. and Henry, Y. (1994) Influence de l’alimentation et des performances sur les rejets azotés du porc. Institut National de la Recherche Agronomique (INRA), Production animale (ProdAnim), 7, 263-274.
- Corpen (1996) Estimation des rejets d’azote et de phosphore des élevages de porcs. 23 p.
- Faure, P. (2011) Digestion et Absorption des glucides. UE de biochimie métabolique. Université Joseph Fourrier Grenolde 1, Etude de Santé, 1ième année, 31 p.
- Crevieu, G. (1999) Digestion des protéines végétales chez les monogastriques. Exemple des protéines de pois. Institut National de la Recherche Agronomique (INRA), Production animale (ProdAnim), 12, 147-161.
- Bernard, C., Côté, C., Côté, D., Girou, M., Grégoire, R., Joncas, R. and Martin, D.Y. (2003) Commission sur le développement durable de la production porcine au Québec. Mémoire de l’Institut de recherche et de développement en agroenvironnement (Irda) Inc., Sainte-Marie, 41 p.
- Belghiti, M.L., Chahlaoui, A., Bengoumi, D. and EL Moustaine, R. (2013) Etude de la qualité physico ۔chimique et bactériologique des eaux souterraines de lanappe plio-quaternaire dans la région de meknès (maroc). Larhyss Journal, 14, 21-36.
- Brault, J.L. (1989) Mémento Technique de l’eau. Ed. Technique et Documentation, Paris, 3-119.
- Chikou, A. (2011) Pisciculture: Notes de cours à l’intention des étudiants du Master 1 en Hydrobiologie et Aquaculture. Université d’Abomey-Calavi, Cotonou, 86 p.
- Cornaz (2004) Evaluation du statut trophique d’un canal de drainage sous l’impact des pollutions d’origines diffuses et ponctuelles: Le cas du Grand Canal de la plaine du Rhône. 180 p.
- Gominan, O.S.A. (1999) Contribution de l’étude écologique et de la biologie des espèces de poisson du genre Clarias dans la valée de l’Ouémé: Habitat, Alimentation, Croissance et Reproduction. Thèse de Doctorat d’Ingénieur Agronome, FSA/UAC, 110 p.
- Abouzid, H. and Outair, A. (1991) Les Nitrates dans les eaux. 7ème Congres Mondiale des ressources en eau, Vol. 2, Rabat, 13-18 Mai 1991.
- Wedmeyer (1997) In Melard Ch Bases biologiques de l’aquaculture. Note de cours. DES Aquaculture, CEFRA, Université de Liège, Liège, 213 p.
- Chapman, D. and Kimstach, V. (1996) Selection of Water Quality Variable. W of Biota, Sediments and Water Quality Assessments: A Guide to of the Use of Biota. In: Sediments and Water in Environment Monitoring, 2nd Edition, Chapmam Edition, E& FN Spon, London, 59-126.
- Huber, G. and Schaub, C. (2011) Guide des fertilisations Azotés utilisables en Bio, Paris, 14 p.
- IBGE (2005) Qualité physico-chimique et chimique des eaux de surface: Cadre général. 4.
- Derwich, E., Beziane, Z., Benaabidate, L. and Belghyti, D. (2008) Evaluation de la qualité des eaux de surface des Oueds Fes et Sebou utilisées en agriculture maraichère au Maroc. Larhyss Journal, 7, 59-77.
- El Hafiane, F., Rami, A. and El Hamouri, B. (2003) Mécanismes d'élimination de l'azote et du phosphore dans un chenal algal à haut Rendement. Revue des sciences de l'eau/Journal of Water Science, 16, 157-172.
- Dovonou, F., Aina, M., Boukari, M. and Alassane, A. (2011) Pollution physico-chimique et bactériologique d’un écosystème aquatique et ses risques écotoxicologiques: Cas du lac Nokoue au Sud Benin. International Journal of Biological and Chemical Sciences, 5, 1590-1602.
- Voermans, J.A.M., Verdoes, N. and Hartog, L.A. (1994) Environmental Impact of Pig Farming. Pig News and Information, 15, 51-54.
- Moal, J.F., Martinez, J., Guiziou, F. and Coste, C.M. (1995) Ammonia Volatilization Following Surface-Applied Pig and Cattle Slurry in France. Journal of Agricultural Science, 125, 245-252. http://dx.doi.org/10.1017/S0021859600084380
- Kermarrec, C. and Robin, P. (2002) Emissions de gaz azotés en élevage de porcs sur litière de sciure. Journées de la Recherche Porcine, 34, 155-160.
- Hassan, R., Khadija, E., Belghyti, D. and Hadji, M. (2013) Physico-Chemical Waste Water Unit of Sugar SUNABEL Mechraa Belksiri. ScienceLib Editions Mersenne, 5.
- Rodier (2012) Analyse de l’eau. 20225 p.
- Laferriere, M.(1996) L'industrie porcine et les risques reliés à la santé humaine. Vecteur Environnement, 29, 27-31.
- Salanitro, J.P., Blake, I.G. and Muirhead, P.A. (1977) Isolation and Identification of Fecal Bacteria from Adult Swine. Applied and Environmental Microbiology, 33, 79-84.
NOTES
*Corresponding author.