Agricultural Sciences
Vol.5 No.10(2014), Article
ID:49304,9
pages
DOI:10.4236/as.2014.510097
Potyvirus Affecting Uchuva (Physalis peruviana L.) in Centro Agropecuario Marengo, Colombia
William Aguirre-Ráquira1,2, Daniel Borda3, Lilliana Hoyos-Carvajal4
1Technische Universität München (TUM), Munich, Germany
2Grupo de Investigación “Horticultura”, Universidad Nacional de Colombia, Bogotá, Colombia
3Universidad Pontificia Javeriana, Bogotá, Colombia
4Universidad Nacional de Colombia, Medellín, Colombia
Email: wd.aguirre@tum.de, wdaguirrer@unal.edu.co
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 12 June 2014; revised 18 July 2014; accepted 19 August 2014
ABSTRACT
Fruit production and especially fresh tropical fruit trade, has an important relevance on world economy. Refining knowledge on virus diseases affecting tropical fruits is required to improve the understanding of these diseases, their dynamics and consequently, the ability to manage them. In this paper, samples of “uchuva” plants (Physalis peruviana L.) obtained from Centro Agropecuario Marengo (CAM) Municipality of Mosquera, Cundinamarca region of Colombia were analyzed after expressing symptoms of leaf chlorosis, leaf malformation, mosaic patterns and dwarfing. Electron microscopy revealed the presence of two different viral particles congruent with Potyvirus and Tobamovirus genus morphology. The presence of Potyvirus affecting the P. peruviana L. culture was confirmed in the samples analyzed by means of electronic microscopy images and serology. Similarly, the existence of viral particles with coherent characteristics of a putative Tobamovirus was observed. However, its presence could not be confirmed by means of serological tests. Nevertheless, its incidence should not be neglected. The mechanism of Potyvirus disease transmission in P. peruviana L. remains unknown, as well as the vectors associated with this disease. Therefore, complementary work and research should be considered. In addition to serology and electron microscopy, the use of indicator plants for diagnosis is suggested. Finally, a complete molecular characterization of the Potyvirus is recommended for a better understanding of the characteristics of its association with P. peruviana L.
Keywords:Plant-Virus, Solanaceae, Mixed-Infection, Vectors, Host-Plant, South America

1. Introduction
Physalis peruviana L., a member of the Solanaceae family, is a worldwide known exotic fruit and one of the most promising fruit crops in Colombia [1] , a country that actually stands with the highest production on the largest acreage of the world [2] [3] . This plant is grown as an annual or perennial culture according to the local growth conditions [4] [5] . Its fruits are characterized for being protected inside the “capacho”, a common name given to the widespread calyx that facilitates its transport and also allow it to easily be sold as a fresh fruit product [6] [7] . Further potential uses include dehydrated products [8] , juices, flesh pulp and sugar containing derivatives like jam [9] , chocolate and ice cream [6] , due to its excellent nutritional properties [10] [11] . In addition, P. peruviana L. is the object of diverse studies in order to take advantage of its secondary metabolites [12] , which exhibit wide biological properties that could have potential pharmacological, medicinal [4] [13] , and insecticidal use [10] .
In Colombia, Fusarium oxysporum is the pathogen which causes the main problems in P. peruviana L. fields. [14] . At production stage, most phytopathological problems are caused by fungal pathogens like Alternaria spp., Cladosporium spp., Phytoptora infestans [15] , Cercospora spp., Phoma spp. and bacteria as Ralstonia solanacearum [16] [17] . Other phytosanitary problems in Solanaceae are caused by several viruses. In case of P. peruviana L., the occurrence of the genus Alfamovirus, Bigeminivirus, Comovirus, Cucumovirus, Fabavirus, Fomovirus, Furovirus, Hybrigeminivirus, Ilarvirus, Luteovirus, Nepovirus, Potexvirus, Potyvirus, Tobamovirus, Tospovirus, Tymovirus [18] -[23] has been reported. In addition, the viroid Potato spindle tuber viroid (PSTVd) was reported to affect plants of P. peruviana L. in Turkey, New Zealand [24] and also in materials of a producer in Germany [25] . In Brazil, the presence of a Tospovirus was reported affecting 100% of a commercial plantation [18] , which turned out to be, apparently, the first report on the occurrence in natural conditions of Tomato Cholorotic spot virus (TCSV). In Colombia, reports include Cucumovirus, Potyvirus and Tobamovirus genus [26] -[28] as being individually identified. In case of mixed infections, inclusion bodies similar in morphology to Potyvirus/ Cucumovirus genus have been reported [26] . The variation of information—though consistent—regarding the production and area sowed with P. peruviana L. [29] , gives an important idea of the particular characteristics of Colombia’s productive system and its migratory character [14] . However, as occurs with many viral diseases, it is difficult to correlate sanitary and economic information. Concretely in this case, no data were found available to associate viral diseases with economic losses in P. peruviana L.
This article shows evidence of the presence of Potyvirus associated with P. peruviana L. plants. The increased scientific and economic relevance of this crop worldwide imply further research and analysis on this topic in order to understand and manage virus diseases on P. peruviana L.
2. Materials and Methods
2.1. Biological Samples
Leaf samples from P. peruviana L. plants (“Colombia” ecotype) of 18 months after transplant—which showed symptoms of chlorosis, severe defoliation, mosaic and dwarfing—were collected at two different times of the year 2011 from field number five at the Centro Agropecuario Marengo (CAM) (Figure 1), located at 2354 m a.s.l (above sea level). During the first collection (February), samples of approximately five to eight leaves per plant were randomly collected through the field, from those expressing the symptoms above mentioned. In the case of the second collection (March), a systematic procedure was followed on plants previously selected— based on the first collection results—including only the upper and mid-section of them, with the same amount of leaves. Samples were processed by means of serological tests and electron microscopy. In order to avoid tissue damage due to natural oxidation processes all samples were preserved in cold storage (4˚C) between the different analyses, which also facilitate the description of the symptoms observed on field. The plant suspension used in the different processes was obtained only from the leaves collected and ground in the presence of phosphate buffer.
2.2. Electron Microscopy
The suspension extracted from infected leaf tissue was processed by means of negative staining using copper grids covered with the polymer “formvar”. The grids were placed on the leaf extract for five minutes and later washed three times with distilled sterile water. Next, the grids were then stained in a watery solution of uranyl acetate for five minutes and finally desiccated for further manipulation under the electron microscope (JEOL JEM 1010), using the software Analysis 3.0 for the measurement procedures.
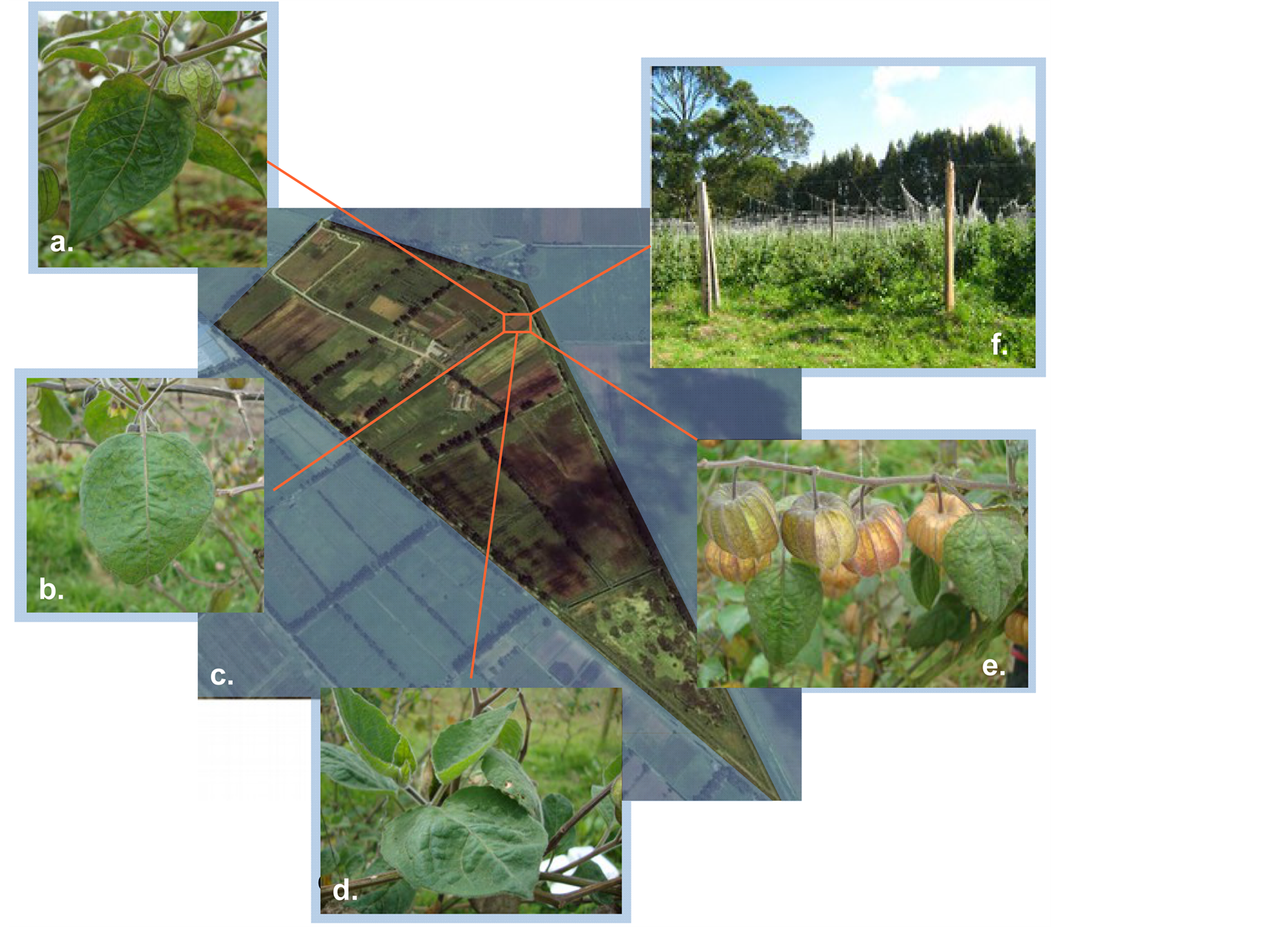
Figure 1. (a). Hyponasty (upward growth) observed in P. peruviana L. affected by Potyvirus disease. (b). Typical mosaic and hypertrophy observed on a leaf affected of P. peruviana L. (c). Centro Agropecuario Marengo (CAM). Km 14, vereda San José vía Mosquera-Bogotá, Cundinamarca. Exact location of the field sampled (+4˚40'57.31", −74˚12'49.08" Garmin etrex 30) (Google Maps, 2013). (d). Abnormal leaf tissue growth on symptomatic P. peruviana L. plants. Some leafs conserved their typical “heart shape” form even after been affected by virus diseases. (e). Fruit bearing on a virus affected plant of P. peruviana L. (f). Overview of the initial growing conditions of the P. peruviana L. field sampled.
2.3. Immunostrips
Leaves as a source of plant tissue were ground in the presence of phosphate buffer. The homogenate was transferred to 2.0 ml tubes and analyzed with immunostrips specific for Potyvirus and Tobamovirus (Kit Immunostrip Agdia®). After an incubation of 15 minutes to allow the reaction of the antigen and the antibody to take place, the immunostrips were evaluated.
2.4. PTA-ELISA
In this detection test, the antigen (sample) was covered with a sodium carbonate buffer. A specific monoclonal antibody for Potyvirus was used (Agdia®) and the reaction was observed at the absorbance wavelength of 405 nm (nano meter) after 30 minutes (samples first collection) and 60 minutes of incubation (samples second collection), using a ELISA Dynex-MRX reader (Table 1). In all tests a positive control was included for Potyvirus (infected leaf plant tissue), healthy leaf plant tissue was used as a negative control and sodium carbonate buffer without plant tissue was used as a blank control.
3. Results and Discussions
The obtained results confirmed the presence of Potyvirus associated with P. peruviana L. in Colombia. The images (Figure 2(a) and Figure 2(b)) showed inclusion bodies of flexuous type with a length of more than 500 nm, congruent with the characteristics of this genus [30] . Serology tests by the use of immunostrips and PTA-
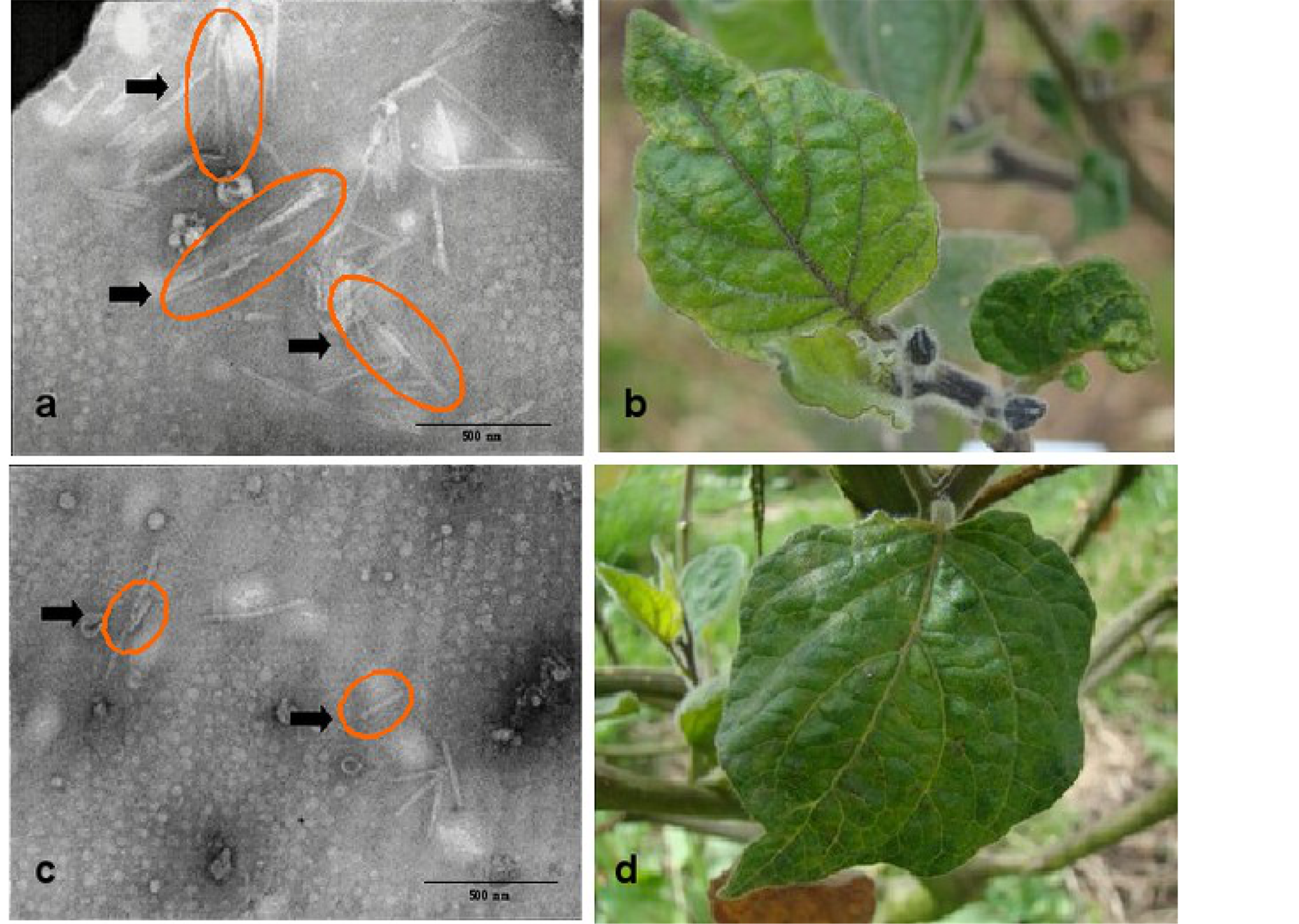
Figure 2. (a). Inclusion bodies observed and associated with the morphological characteristics of Potyvirus (orange ovals); (b). Chlorotic mosaic and leaf malformation as result of virus diseases on P. peruviana L; (c). Inclusion bodies observed and associated with the morphology of Tobamovirus (orange ovals); (d). Leaf malformation on P. peruviana L. plant infected by virus diseases.
Table 1. Results obtained by means of PTA-ELISA from samples collected, analyzed and discussed in this paper.
aAbsorbance at 405 nm after 30 minutes of reaction; bAbsorbance at 405 nm after 60 minutes of reaction. The first collection includes random materials from the Universidad Nacional de Colombia (UN) and the Centro Agropecuario Marengo (CAM). The second collection considered only materials at CAM. In the last case the letter and number used to label the plants correspond respectively to the row and number of each of the plants sampled on field. Plus sign (+) indicates a positive reactions while minus sign (−) indicate negative reactions.
ELISA (Table 1) confirmed also the presence of Potyvirus. In addition, another viral particle (Figure 2(c) and Figure 2(d)) was observed suggesting a mix-infection affecting the plant. In this case, given the rigid characteristics and size between 250 and 300 nm [30] we presumed that it corresponds to Tobamovirus genus, which has been reported to affect members of the Solanaceae family and particularly the genus Physalis [23] . Nevertheless, the presence of Tobamovirus could not be confirmed by means of serology tests.
It is assumed that many Potyvirus isolates detected in different parts of the world come basically from infected materials of South American origin [31] . This region is considered to be the center of origin of several species of the Solanaceae family, which coexist simultaneously with wild species closely related. Therefore it is assumed that such viral diversity is a result of the co-evolution and adaptation process [32] [33] .
Mixed infections are common and they impede an accurate detection based on the biological characteristics of the pathogen [34] , establishing a big challenge in the diagnosis process. This is because of the synergies established with the host plant, given as a result a wide range of diverse symptoms [35] . In the case of Tobamovirus, despite the fact that its presence could only be assumed by means of electronic microscopy but not by serology tests, its presence should not be neglected since the high diversity of both plants and viruses in South America. Negative results of PTA-ELISA for Tobamovirus could be explained either to a low sensitivity of the protocol or due to a low specificity of the antibody used for the Tobamovirus presented in the P. peruviana L. samples [33] . Tobamovirus mobility characteristics—cell to cell process—vary and change during the development of the infection and are still not completely known [36] . In this case, the use of indicator plants is a helpful option used in the detection of viral diseases, which is usually a result of multiple methods in order to improve the diagnosis process.
Only a few reports indicate problems with viral diseases in P. peruviana L. cultivation. Symptoms due to virus infections can appear or disappear depending on the environmental conditions [37] or behave as conditioning agents to other diseases [38] -[40] . Therefore, virus detection is required to avoid or minimize eventual losses due to unnoticed aspects that could lead to mistakes when planning the different management strategies required for this crop [24] . A correct identification of virus diseases is needed, since it has been proven that plant susceptibility to other pathogens is higher in those affected by viral diseases [41] .
Virus symptoms on plants include leaf deformations, color changes in specific patterns [42] [32] , local or systemic necrosis, with changes in tissue structure which finally can result in plant death. In some cases symptoms can be absent or masked [37] although the virus is present in the plant tissue. Consequently, the problem can be associated by mistake with nutrient deficiency, physiological disorders, xenobiotic agents or entomological damages [43] [40] . Regarding all these aspects it is very difficult to correlate viral diseases with economic losses suffered by the farmers [39] . The symptoms associated with the viral disease observed on P. peruviana L.— commonly expressed as a chlorotic mosaic—can vary their intensity from profound (Figure 3(a) and Figure 3(b)) to mild (Figure 3(d) and Figure 3(e)) as a result of the expression of the physiological disorder of the plant. These symptoms can be observed on whole leaves or can be restricted to the primary site of infection depending on the level of resistance expressed by the host [37] . The abnormal growth of plant cells (Figure 3(f)) causing hypertrophy—abnormal stretching of cells—and consistent malformations (Figure 2(b) and Figure 2(d)) was observed as well. Both external and internal quality parameters of fruits decrease due to the effect of the virus disease resulting in a shortened shelf life [41] . However, this last aspect is not completely proven in the specific case of P. peruviana L.
Nonetheless, it is necessary to point out that diagnosis should not be limited to symptom expression [37] . In this respect, isothermal methods for nucleic acid amplification [44] [45] are available nowadays for the diagnosis of viral diseases, in replacement of the traditional PCR (Polymerase chain reaction). The advantages of these techniques are based on their versatility, a minimum of technical requirements (no thermocycler required), high specificity, high amplification rates, short reaction times [46] and the possibility of being observed with the naked eye [47] . Though at the beginning, these methods have been used in medicine [48] , some works show their potential use in Potyvirus detection in plum trees in Germany [47] .
Another aspect to be considered is the possibility of alternative host plants for the virus that affect the P. peruviana L. culture, among them the weeds population growing along the fields. For Cundinamarca region of Colombia, Galinsoga spp, Raphanus raphanistrum L., Veronica persica., Hypochoeris radicata L., Holcus lantus L., Rumex acetocella L., Polygonum nepalense M. and Rumex crispus L. are frequently found [49] . Special attention should be paid to the last two species mentioned, as they are known for their high frequency on P. peruviana L. fields in Cundinamarca region (28% and 16 % respectively). The observation of mosaic patterns and leaf
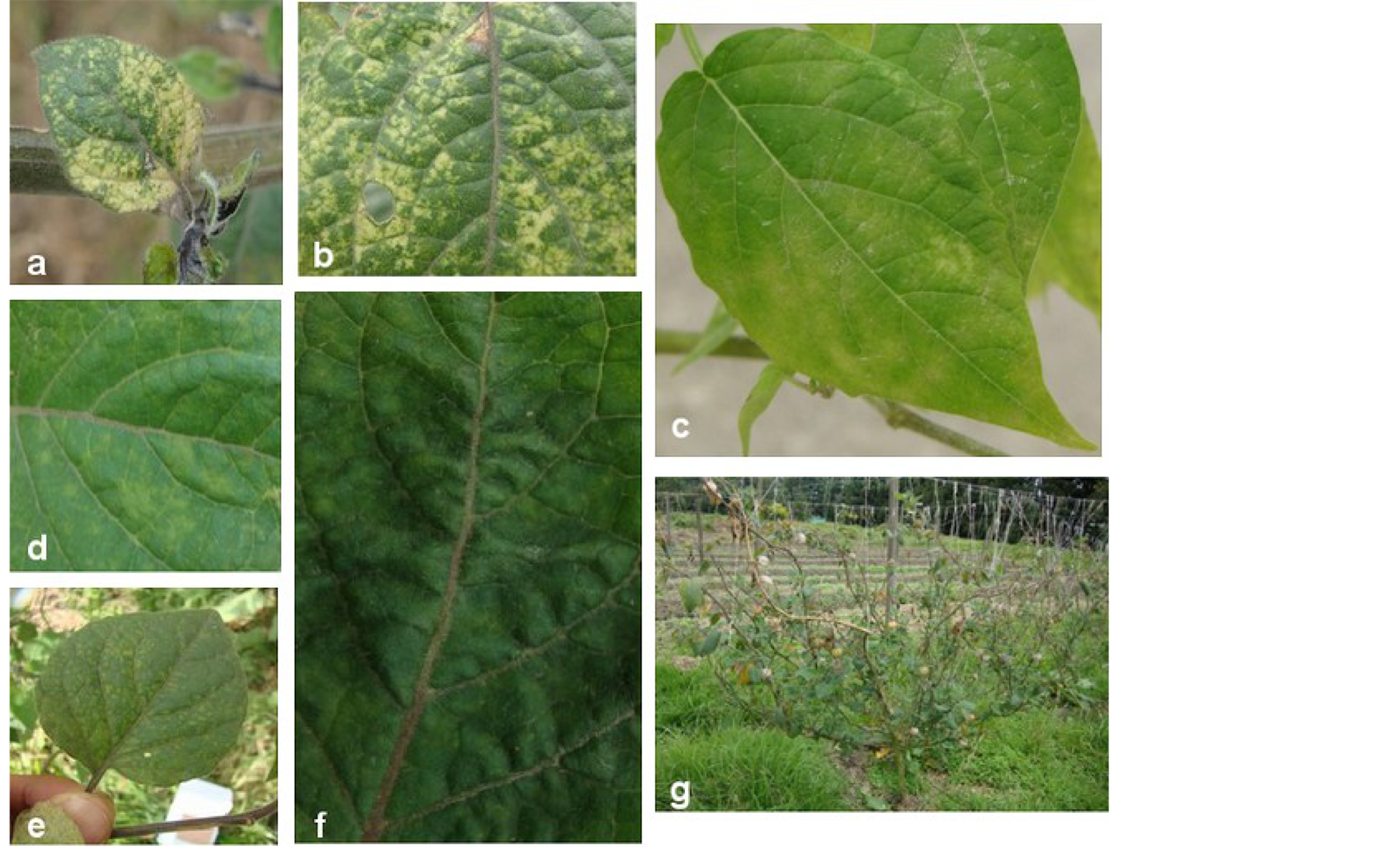
Figure 3. (a) and (b) Severe mosaic associated with symptoms generated from viral diseases in P. peruviana L. (c) P. peruviana L. used as indicator plant, expressing typical symptoms (chlorotic mosaic) caused by virus diseases; (d) and (e) Mild mosaic affecting P. peruviana L. (f) Typical hypertrophy observed on plants affected by viral diseases; (g) Severe defoliation on P. peruviana L. plant affected by viral diseases.
deformation similar to the ones observed in P. peruviana L. plants affected by virus (Rumex case) and the fact that they have been mentioned in previous reports as alternative host plants for Potyvirus [30] [50] supports this statement.
Within the possible virus vectors of the virus found in P. peruviana L., a wide range of insects and arthropods affecting different phenological states of the plant are present: Trialeurodes vaporariorum Westwood; Aphis spp., Myzuspersicae [26] ; Aculops lycopersici, Tarsonemus spp. (Mites) [51] [52] and Trips (Frankliniella spp.) [53] , Lepidoptera species of the Noctuidae family (Spodoptera frugiperda Smith and Copitarsia decolora Guenée) [54] .
Mechanical transmission of viral diseases of P. peruviana L. must be considered due to the regular practices and management procedures established for this crop [14] and due to reports about this mode of transmission of several species within Potyvirus and Tobamovirus genus [19] [41] [30] [50] . Additionally, although vegetative propagation methods are not common in Colombia for P. peruviana L., they should be considered in the case of virus spreading due to the common pruning practices within this crop. On the other hand, seed transmissible viruses and known viroids affecting [55] P. peruviana L. [5] are also distributed in this way [25] [56] . In Colombia, seeds—seed selection, seedlings production and sowing of these seedlings—constitute the main method used by growers for the establishment of their P. peruviana L. fields [2] [14] [57] . Thus, the possibility of viral transmission by means of physical methods should not be overlooked. Furthermore, even the identification and determination of promissory materials of P. peruviana L. [58] must be intended by complementary plant pathology criteria to avoid the spread of these virus diseases.
4. Conclusions
The molecular characterization of Potyvirus associated with P. peruviana L. is required to improve its detection and also to distinguish it from the presence of other potential virus diseases affecting this culture, especially given the intra-specific variability that they possess. In addition to this, there is not a clear identification of the transmission mechanism of this viral disease or its possible associated vectors, and the reason for which it is necessary to coordinate works that intend to clarify these aspects.
Acknowledgements
The authors express their gratitude to all the members of the virology unit of the Centro Internacional de Agricultura Tropical (CIAT), (symptoms description and electronic microscopy) especially to Mauricio Castaño and Cristian Olaya. Many thanks to Juan Carlos Rodríguez (CAM) and to the staff of the Clínica de plantas from the Universidad Nacional de Colombia (symptoms analyses). Finally a special thanks to Kostantin Wagner and Johannes Hadersdorfer at the Technische Universität München for their time and helpful suggestions.
This work was supported by the German Research Foundation (DFG) and the Technische Universität München within the funding program Open Access Publishing.
Dedicated to the memory of Roso Arsenio Ráquira Urián.
References
- Tafur Reyes, R. and Toro Mesa, J.C. (2007) Present and Future of Colombian Fruitgrowing. In: Carlos Rincón Humberto, H., Montaño de Mayolo, P. and Peñaloza Acosta, J., Eds., Colombian Tropical Fruits for the World: Production, Agroindustry, Marketing and Productive Chain, Produmedios, Bogotá D.C., 9-22. http://www.corpoica.org.co/sitioweb/archivos/publicaciones/memoriasfrutastropicales.pdf
- Herrera M., A.M., Ortiz A., J.D., Fischer, G. and Chacón S., M.I. (2011) Behavior in Yield and Quality of 54 Cape Gooseberry (Physalis peruviana L.) Accessions from North-Eastern Colombia Comportamiento en producción y calidad de 54 accesiones de uchuva. Agronomia Colombiana, 29, 361-371. http://www.revistas.unal.edu.co/index.php/agrocol/article/view/29027/37405.
- Peña, J.F., Ayala, J.D., Fischer, G., Chaves, B., Cardenas-Hernandez, J., et al. (2010) Relaciones semilla-fruto en tres ecotipos de uchuva (Physalis peruviana L.). Revista Colombiana de Ciencias Hortícolas, 4, 43-54. http://www.soccolhort.com/revista/pdf/magazin/Vol4/vol.4%20no.1/Vol.4.No.1.Art.4.pdf
- Rop, O., Mlcek, J., Jurikova, T. and Valsikova, M. (2012) Bioactive Content and Antioxidant Capacity of Cape Gooseberry Fruit. Central European Journal of Biology, 7, 672-679. http://dx.doi.org/10.2478/s11535-012-0063-y
- Verhoeven, J.T.J., Jansen, C.C.C., Botermans, M. and Roenhorst, J.W. (2010) Epidemiological Evidence That Vegetatively Propagated, Solanaceous Plant Species Act as Sources of Potato spindle tuber viroid Inoculum for Tomato. Plant Pathology, 59, 3-12. http://dx.doi.org/10.1111/j.1365-3059.2009.02173.x
- Puente, L.A., Pinto-Muñoz, C.A., Castro, E.S. and Cortés, M. (2011) Physalis peruviana Linnaeus, the Multiple Properties of a Highly Functional Fruit: A Review. Food Research International, 44, 1733-1740. http://dx.doi.org/10.1016/j.foodres.2010.09.034
- Guerrero, B., Velandia, M., Fischer, G. and Montenegro, H. (2007) Los ácidos carboxílicos y la humedad del suelo influyen en la producción y el rajado del fruto de uchuva (Physalis peruviana L.). Revista Colombiana de Ciencias Hortícolas, 1, 9-19. http://virtual.uptc.edu.co/revistas2013f/index.php/ciencias_horticolas/article/view/1141/1140.
- Mahecha Godoy, J.C. (2011) Simulación Matemática del Proceso de Deshidratación de la Uchuva (Physalis peruviana L.). M.Sc. Thesis, Universidad National de Colombia, Bogotá D.C. http://www.bdigital.unal.edu.co/4309/1/820048.2011.pdf.
- Fischer, G., Miranda, D., Piedrahita, W., Romero, J., (Eds.) (2006) Avances en Cultivo, Poscosecha y Exportación de la Uchuva (Physalis peruviana L.). Unibiblios, Bogotá D.C. http://www.asohofrucol.com.co/archivos/biblioteca/biblioteca_23_Avances%20cultivo%20uchuva.pdf.
- Ramadan, M.F. (2011) Bioactive Phytochemicals, Nutritional Value, and Functional Properties of Cape Gooseberry (Physalis peruviana): An Overview. Food Research International, 44, 1830-1836. http://dx.doi.org/10.1016/j.foodres.2010.12.042
- Valente, A., Albuquerque, T.G., Sanches-Silva, A. and Costa, H.S. (2011) Ascorbic Acid Content in Exotic Fruits: A Contribution to Produce Quality Data for Food Composition Databases. Food Research International, 44, 2237-2242. http://dx.doi.org/10.1016/j.foodres.2011.02.012
- Licodiedoff, S., Koslowski, L.A.D. and Ribani, R.H. (2013) Flavonols and Antioxidant Activity of Physalis peruviana L. Fruit at Two Maturity Stages. Acta Scientiarum Technology, 35, 393-399.http://dx.doi.org/10.4025/actascitechnol.v35i2.13265
- Lan, Y.-H., Chang, F.-R., Pan, M.-J., Wu, C.-C., Wu, S.-J., et al. (2009) New Cytotoxic Withanolides from Physalis peruviana. Food Chemistry, 116, 462-469. http://dx.doi.org/10.1016/j.foodchem.2009.02.061.
- Fischer, G. and Miranda, D. (2012) Uchuva (Physalis peruviana L.). In: Fischer, G., Ed., Manual para el cultivo de frutales en el trópico, Produmedios, Bogotá D.C., 851-853.
- Gilchrist, E., Jaramillo, S., Afanador, L. and Arango, R.E. (2009) Characterization of Phytophtora infestants Population in Antioquia, Colombia. Revista Facultad Nacional de Agronomía, Medellín, 62, 5013-5037.http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0304-28472009000200001
- Vargas, A.M., Quesada Ocampo, L.M., Céspedes, M.C., Carreño, N., González, A., et al. (2009) Characterization of Phytophthora infestans Populations in Colombia: First Report of the A2 Mating Type. Phytopathology, 99, 82-88.http://dx.doi.org/10.1094/PHYTO-99-1-0082
- Blanco, J. (2000) Manejo de enfermedades. In: Florez, V., Fischer, G. and Sora, A., Eds., Producción, Postcosecha y Exportación de la Uchuva (Physalis peruviana L.), Unibiblios, Bogotá D.C., 57-65.
- Eiras, M., Costa, I.F., Chaves, A.L., Colariccio, A., Harakava, R., et al. (2012) First Report of a Tospovirus in a Commercial Crop of Cape Gooseberry in Brazil. New Disease Reports, 25, 25.http://dx.doi.org/10.5197/j.2044-0588.2012.025.025
- King, A.M., Adams, M.J., Carstens, E. and Lefkowitz, E.J. (2013) Virus Taxonomy. Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses, Edinburgh. http://www.ictvonline.org/virusTaxonomy.asp?version
- Torres, R., Larenas, J., Fribourg, C. and Romero, J. (2012) Pepper necrotic spot virus, a New Tospovirus Infecting Solanaceous Crops in Peru. Archives of Virology, 157, 609-615. http://dx.doi.org/10.1007/s00705-011-1217-3
- Perea Dallos, M., Rodríguez, N.C., Fischer, G., Velásquez Lozano, M. and Micán Gutiérrez, Y. (2010) Uchuva: Physalis peruviana L. (Solanaceae). In: Perea Dallos, M., Matallan, L. and Tirado, A., Eds., Biotecnología aplicada al mejoramiento de los cultivos de frutas tropicales, Facultad de Ciencias, Bogotá D.C., 466-490.
- Trenado, H.P., Fortes, I.M., Louro, D. and Navas-Castillo, J. (2007) Physalis ixocarpa and P. peruviana, New Natural Hosts of Tomato chlorosis virus. European Journal of Plant Pathology, 118, 193-196.http://dx.doi.org/10.1007/s10658-007-9129-5
- Brunt, A., Crabtree, K., Gibbs, A., Watson, L., Dallwitz, M., et al. (1996) Known Susceptibilities of Solanaceae. Descriptions and Lists from the VIDE Database. http://sdb.im.ac.cn/vide/famly124.htm
- Ward, L.I., Tang, J., Veerakone, S., Quinn, B.D., Harper, J.S., Delmiglio, C., and Clover, G.R.G. (2010) First Report of Potato spindle tuber viroid in Cape Gooseberry (Physalis peruviana) in New Zealand. Plant Disease, 94, 479.http://dx.doi.org/10.1094/PDIS-94-4-0479A
- Verhoeven, J.T.J., Botermans, M., Roenhorst, J.W., Westerhof, J. and Meekes, E.T.M. (2009) First Report of Potato spindle tuber viroid in Cape Gooseberry (Physalis peruviana) from Turkey and Germany. Plant Disease, 93, 316.http://dx.doi.org/10.1094/PDIS-93-3-0316A
- Daza, P., Rodríguez, P. and Forero, M. (2011) Enfermedades de origen viral en cultivos de uchuva (Physalis peruviana L.) ubicados en el departamento de Cundinamarca. Fitopatologia Colombiana, 35, 128.
- Morales, F.J., Castaño, M., Arroyave, J.A., Olaya, C., Velasco, A.C., et al. (2006) Detección de virus en especies frutales cultivadas en Colombia. Fitopatologia Colombiana, 30, 39-49.
- Gómez, J.E., Morales, F.J. and Arroyave, J. (1997) El mosaico de la uchuva (Physalis peruviana L.) en Colombia. Ascolfi Informa, 23, 52.
- Legiscomex (2013) Inteligencia de mercados/Exportación de frutas exóticas colombianas. 1-20.http://www.legiscomex.com/BancoMedios/Documentos%20PDF/exportaciones-estudio-frutas-exoticas.pdf
- Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U. and Ball, L.A. (2005) Virus Taxonomy. Classification and nomenclature of viruses. Eighth Report of the International Committee on the Taxonomy of Viruses. Elsevier Academic Press, San Francisco.
- Stevenson, W.R., Rosemary, L., Franc, G.D. and Weingartner, D.P. (Eds.) (2001) Compendium of Potato Diseases. 2nd Edition, American Phytopathological Society, Saint Paul.
- Bukovinszki, á., Götz, R., Johansen, E., Maiss, E. and Balázs, E. (2007) The Role of the Coat Protein Region in Symptom Formation on Physalis floridiana Varies between PVY Strains. Virus Research, 127, 122-125.http://dx.doi.org/10.1016/j.virusres.2007.03.023
- Spetz, C. (2003) Molecular Resolution of a Complex of Potyviruses Infecting Solanaceous Crops at the Centre of Origin in Peru. Journal of General Virology, 84, 2565-2578. http://dx.doi.org/10.1099/vir.0.19208-0.
- Hull, R. (2001) Methods for Assay Detection and Diagnosis. Mattew’s Plant Pathology. Academic Press, San Diego, 627-674.
- Nie, X. and Singh, M. (2013) Response of Potato, Tobacco and Physalis floridana Plants to Mixed Infection with PVX, PVYNTN and PVY Strains. Canadian Journal of Plant Pathology, 35, 390-401.http://dx.doi.org/10.1080/07060661.2013.812581
- Tagami, Y. and Watanabe, Y. (2007) Effects of Brefeldin A on the Localization of Tobamovirus Movement Protein and Cell-to-Cell Movement of the Virus. Virology, 361, 133-140. http://dx.doi.org/10.1016/j.virol.2006.11.008.
- Dijkstra, J. and Khan, J.A. (2006) Symptomatology. In: Khan, J.A. and Dijkstra, J., Eds., Handbook of Plant Virology, Food Product Press, Binghampton, 23-32.
- Fischer, G., Aguirre-Ráquira, W. and Büttner, C. (2010) Conclusiones Taller: Producción de material de propagación libre de virus para cultivos hortícolas. Bogotá D.C., 4 October 2010, 1-4.
- Waterworth, H.E. and Hadidi, A. (2005) Economic Losses Due to Plant Viruses. In: Hadidi, A., Khetarpal, R.K. and Koganezawa, H., Eds., Plant Virus Disease Control, American Phytopathological Society, Saint Paul, 1-15.
- Buriticá, P. (1999) Directorio de patógenos y enfermedades de las plantas de importancia económica en Colombia. Instituto Colombiano Agropecuario, Medellín.
- Derks, A.F.L.M. (2006) Virus Diseases: Economic Importance and Control Strategy. In: Khan, J.A. and Dijkstra, J., Eds., Handbook of Plant Virology, Food Product Press, Binghamton, 235-251.
- Hu, X., Nie, X., He, C. and Xiong, X. (2011) Differential Pathogenicity of Two Different Recombinant PVYNTN Isolates in Physalis floridana Is Likely Determined by the Coat Protein Gene. Virology Journal, 8, 207.http://dx.doi.org/10.1186/1743-422X-8-207
- Morales, F.J. (2006) Detection and Identification of Plant Viruses and Disease Diagnosis. In: Khan, J.A. and Dijkstra, J., Eds., Handbook of Plant Virology, Food Product Press, Binghamton, 157-169.
- Zanoli, L. and Spoto, G. (2012) Isothermal Amplification Methods for the Detection of Nucleic Acids in Microfluidic Devices. Biosensors, 3, 18-43. http://dx.doi.org/10.3390/bios3010018
- Piepenburg, O., Williams, C.H., Stemple, D.L. and Armes, N. (2006) DNA Detection Using Recombination Proteins. PLoS Biology, 4, e204. http://dx.doi.org/10.1371/journal.pbio.0040204.
- Ling, K.-S., Li, R. and Bledsoe, M. (2013) Pepino mosaic virus Genotype Shift in North America and Development of a Loop-Mediated Isothermal Amplification for Rapid Genotype Identification. Virology Journal, 10, 1-12.http://dx.doi.org/10.1186/1743-422X-10-117
- Hadersdorfer, J., Neumüller, M., Treutter, D. and Fischer, T.C. (2011) Fast and Reliable Detection of Plum pox virus in Woody Host Plants Using the Blue LAMP Protocol. Annals of Applied Biology, 159, 456-466.http://dx.doi.org/10.1111/j.1744-7348.2011.00510.x
- Loo, J.F.C., Lau, P.M., Ho, H.P. and Kong, S.K. (2013) An Aptamer-Based Bio-Barcode Assay with Isothermal Recombinase Polymerase Amplification for Cytochrome-c Detection and Anti-Cancer Drug Screening. Talanta, 115, 159-165. http://dx.doi.org/10.1016/j.talanta.2013.04.051
- Plaza, G. and Pedraza, M. (2007) Reconocimiento y caracterización ecológica de la flora arvense asociada al cultivo de uchuva. Agronomía Colombiana, 25, 306-313. http://www.revistas.unal.edu.co/index.php/agrocol/article/view/14134
- Brunt, A., Crabtree, K., Gibbs, A., Watson, L., Dallwitz, M., et al. (1996) Index to Virus Genera. Descriptions and Lists from the VIDE Database. http://sdb.im.ac.cn/vide/genus039.htm
- Jerez, C. (2006) Reconocimiento de la entomofauna mayor presente en el cultivo de uchuva (Physalis peruviana L.) en el departamento de Cundinamarca. B.Sc. Thesis, Universidad Nacional de Colombia, Bogotá D.C.
- Saenz, A. and Getiva, J. (2003) ácaros asociados al cultivo de uchuva en municipios productores de Cundinamarca y Boyacá. Produmedios, Bogotá D.C.
- Hinestrosa, M. and Peláez, D. (2007) Manual fitosanitario para la protección de cultivos de fruta pequeña de clima frío moderado. Corporación PBA, Bogotá D.C.
- Díaz Niño, M.F. (2011) Eficiencia de tres especies del género Trichogramma (Hymenoptera: Trichogrammatidae) para el control de Spodoptera frugiperda Smith y Copitarsia decolora Guenée (Lepidoptera: Noctuidae) en el cultivo de uchuva. M.Sc. Thesis, Universidad Nacional de Colombia, Bogotá. http://www.bdigital.unal.edu.co/3984/
- Albrechtsen, S.E. (2006) Testing Methods for Seed-Transmitted Virus: Principles and Protocols. CABI Publishing, New York. http://dx.doi.org/10.1111/j.1365-3059.2006.01403.x
- Virscek Marn, M. and Mavric Plesko, I. (2012) First Report of Potato spindle tuber viroid in Cape Gooseberry in Slovenia. Plant Disease, 96, 150. http://dx.doi.org/10.1094/PDIS-08-11-0711
- Miranda, D., Ulrichs, C. and Fischer, G. (2010) Imbibition and Percentage of Germination of Cape Gooseberry (Physalis peruviana L.) Seeds under NaCl Stress Imbibición y porcentaje de germinación en semillas de uchuva (Physalis peruviana L.) bajo estrés por NaCl. Agronomia Colombiana, 28, 29-35.http://www.revistas.unal.edu.co/index.php/agrocol/article/view/17591/37341
- Herrera M., A.M., Fischer, G. and Chacón S., M.I. (2012) Agronomical Evaluation of Cape Gooseberries (Physalis peruviana L.) from Central and North-Eastern Colombia Evaluación agronómica de materiales de uchuva (Physalis peruviana L.). Agronomia Colombiana, 30, 15-24. http://www.revistas.unal.edu.co/index.php/agrocol/article/view/22440/135070


