Food and Nutrition Sciences
Vol. 4 No. 9 (2013) , Article ID: 36080 , 12 pages DOI:10.4236/fns.2013.49126
Anti-Obesity and Antihyperglycemic Effects of Crataegus aronia Extracts: In Vitro and in Vivo Evaluations
![]()
1Faculty of Science, The University of Jordan, Amman, Jordan; 2Faculty of Pharmacy, The University of Jordan, Amman, Jordan.
Email: *fatueafi@ju.edu.jo
Copyright © 2013 Entisar K. Al-Hallaq et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 9th, 2013; revised July 9th, 2013; accepted July 16th, 2013
Keywords: Crataegus aronia; Rosaceae; Pancreatic Lipase; Enzymatic Starch Digestion; High Cholesterol Diet; Flavonoids
ABSTRACT
Hypocholesterolemic activity of Crataegus aronia L. (Rosaceae) is therapeutically praised. Its potent antiobesity (P < 0.001, n = 6 - 8) as well as marked triacylglycerol-reducing efficacies (P < 0.001, n = 6 - 8) in 10 weeks-high cholesterol diet (HCD) fed rats are demonstrated. Pancreatic triacylglycerol lipase (PL), α-amylase and α-glucosidase are an interesting pharmacological target for the management of dyslipidemia, atherosclerosis, diabetes and obesity. Comparable to acarbose, acute starch induced postprandial hyperglycaemia as well glycemic excursions in normoglycemic overnight fasting rats was highly significantly (P < 0.001) dampened by C. aronia 100, 200 and 400 mg/Kg b.wt aqueous extracts (AE), but not acute glucose evoked postprandial hyperglycaemia increments, unlike diabetes pharmacotherapeutics metformin and glipizide. C. aronia aerial parts as well as fruits AEs (0.1 - 10 mg/mL) were identified as in vitro dual inhibitors of α-amylase and α-glucosidase with respective IC50 (mg/mL) of 2.1 ± 0.3 and 3.5 ± 0.7. Still, it lacked on in vitro hindrance of glucose movement, dissimilar to guar gum. Equivalent to orlistat (PL IC50 of 0.1 ± 0.0 μg/mL), C. aronia tested AEs and its purified bioactive phytoconstituents; quercetin and rutin, inhibited highly substantially in a dose dependent trend PL in vitro (n = 3), in an ascending order of obtained PL-IC50 (µg/mL): quercetin; 30.1 ± 2.8, rutin; 77.3 ± 11.7, C. aronia aerial parts; 225.2 ± 33.4 and C. aronia fruits; 286.1 ± 37.4. Flavonoid-rich C. aronia, as a functional food and a nutraceutical, modulating gastrointestinal carbohydrate and lipid digestion and absorption, maybe be advocated as an exquisite and potential candidate for combinatorial obesity-diabetes prevention and phytotherapy.
1. Introduction
With the rising prevalence of diabetes mellitus (DM) worldwide, several studies indicated that the incidence of type 2 DM (T2DM), impaired fasting glycaemia and obesity in Jordan is increasing [1-3]. These observations have been confirmed by the International Diabetes Federation (IDF) data stating that the current prevalence of DM in Jordan was at 10.1%. Among Middle Eastern and North Africa (MENA) countries; this percentage indicates the ninth highest prevalence [4]. Treatment goals of T2DM have centred on using oral agents that promote insulin secretion, improving tissues’ sensitivity to insulin, or reducing the rate of carbohydrate absorption from the gastrointestinal tract and retarding the development of diabetic complications [5]. Due to the good acceptance of herbal drugs among population, phytopharmaceuticals with demonstrated clinical efficacy could become a suitable alternative/complementary therapy to current medication for specific indications like the adjuvant treatment of diabetes [6]. There is still, however, an unmet need for the medicinal plants and phytopharmaceuticals with scientifically proven antidiabetic efficacy comparable to orthodox medicine [7-9]. In parallel realms, possible antiobesity therapeutics from nature were closely investigated as obesity was reaching alarming epidemic proportions globally [7-10]. Locally, ethnopharmacological studies and surveys confirmed the appreciable prevalence of herbal use among patients with diabetes in Jordan [11,12]. Comprehensively, multiple reports were investigating diverse biological efficacies, cardiovascular therapeutic benefits and phytochemical analyses of C. aronia (Rosaceae), among the other Crataegus spp. [13-25]. Diverse studies were conducted to explore medicinal plants as potential therapeutic agents for dual management of diabetes and hyperlipidemia via digestive enzymes’ inhibition namely pancreatic alphaamylase, intestinal alpha-glucosidase and pancreatic lipase [26,27]; hence, the purpose of present study was to investigate the inhibitory effects of crude aqueous extracts of C. aronia L. (Rosaceae) on these extrapancreatic digestive enzymes in vitro. Thus, more detailed investigations to elucidate C. aronia dual antidiabeticantiobesity pharmacotherapeutic effects on cell-free in vitro systems of carbohydrate and lipid extrapancreatic enzymatic digestion and absorption were undertaken. Additionally, acute and chronic in vivo effects were exhaustively investigated.
2. Materials and Methods
2.1. Chemicals and Biochemicals
Unless stated otherwise, all reference drugs (orlistat, metformin, glipizide, acarbose and atorvastatin with purities > 95%), reference flavonoids (rutin and quercetin with purities > 94%), reagents and chemicals were from Sigma (Dorset, UK). Dialysis tubing Spectra/Por® 7 Biotech Regenerated Cellulose (RC) membranes, MWCO 2000 was purchased from Spectrum Europe B.V, Breda, Netherlands. Shaking incubator was from LabTech®, Daihan LabTech Co., LTD. (Korea). Glucose GOD-PAP kit was obtained from BioLabo Reagents, France. In UV determinations; UV-VIS spectrophotometer from SpectroScan 80D (UK) was used. Sonicator (Bandelin Sonorex, Bandelin electronics, Germany) and rotary evaporator (Laborota 4000-efficient, Heidolph, Germany) were also used.
2.2. Plant Material
Dried whole flowers and leaves of C. aronia L. were purchased from herbalist shops in downtown, Amman; while fruits were collected during June-July from different places of the country (Ajloun, Al-Salt and Shafa Badran). The identification of the plants material was kindly confirmed by Professor D.M. Al-Eisawi (Department of Biological Sciences; The University of Jordan). Voucher specimens of the plant material were deposited at the Herbarium of the Department of Biological Sciences, and at the Department of Pharmaceutical Sciences, (Reference No. 27). All collected fresh plant samples were air dried at room temperature and coarsely powdered.
2.3. Preparation of the C. aronia Aqueous Extracts (AEs)
AEs were prepared by refluxing each 10 g of the dried coarsely powdered plant material with 100 mL tap water for 15 min. The overnight kept extracts were filtered twice through filter paper and the volume of the filtered solution was increased to 100 mL with tap water to obtain 10% (equivalent to 100 mg/1 mL) crude aqueous solutions. Sonication of stock crude extract or testing concentrations was performed before implementation of investigations. For pancreatic lipase experimentation; water was evaporated under vacuum at 40˚C using a rotary evaporator. The solid residues were collected and stored in dry conditions until analysis.
2.4. Preparation of C. aronia Crude Aqueous Extract (Aerial Parts as Well as Fruits) and Its Phytoconstituents for in Vitro Pancreatic Triacylglycerol Lipase Activity Assay
The tested aqueous extracts were initially dissolved in Tris-HCl buffer (2.5 mM (Promega, USA), pH 7.4 with 2.5 mM NaCl) to give five different stock solutions with a concentration range of 6.25 - 100 mg/mL (6.25, 12.5, 25, 50 and 100 mg/mL). Subsequently, 20 μL aliquot of each stock solution was used in the reaction mixture to give a final concentration range of 125 - 2000 μg/mL (125, 250, 500, 1000 and 2000 μg/mL). Extracts were prepared according to the traditional indications of use, thus DMSO or any other organic solvent, even to the minimum concentration was avoided [28]. Also, quercetin and rutin, isolated from C. aronia (dissolved in DMSO), were prepared into five different stock solutions with an initial concentration range of 0.625 - 10 mg/mL (0.625, 1.25, 2.5, 5 and 10 mg/mL) [13]. Thereafter, 20 μL aliquot of each stock solution was used in the reaction mixture to give a final concentration range of 12.5 - 200 µg/mL (12.5, 25, 50, 100 and 200 µg/mL). Finally, orlistat (Sigma, USA), the reference drug (dissolved in DMSO; 1 mg/mL), was prepared into six different stock solutions with a concentration range of 0.625 - 20 µg/ mL [29]. Thereafter, 20 μL aliquot of each stock solution was used in the reaction mixture to give a final concentration range of 0.0125 - 0.4 μg/mL.
2.5. Spectrophotometric Quantification of Pancreatic Lipase (PL) Activity and Assaying PL Inhibition by Test Extracts and Compounds
According to Bustanji et al., in vitro enzymatic PL activity was assayed. Subsequent determinations were undertaken for the tested extracts of different parts/phytoconstitutive compounds in comparison to control evaluations, to calculate the concentration required for PL 50% inhibition (IC50) [30].
2.6. In Vitro Enzymatic Starch Digestion Assay
In vitro enzymatic starch digestion was assayed with acarbose, as the reference drug [31]. The extent of polysaccharide breakdown into glucose was evaluated in a concentration range of different parts of plant aqueous extract 0.1, 0.5, 1.0, 1.25, 2.5, 5.0 and 10.0 mg/mL. The effects of acarbose at 100 μg/mL concentration were evaluated as well. Control (tap water only) samples contained neither acarbose nor plant extract.
2.7. Glucose Movement in Vitro Assay
In vitro glucose movement was assayed according to Kasabri et al. [32]. To imitate the viscosity-based diffusion hindrance of gel-forming dietary fibres, and hence, their postprandial glucose lowering efficacies in vitro, guar gum 50 mg/mL was used as a classical positive control, and 10, 20 and 40 mg/mL of C. aronia AEs in 0.22 M glucose in triplicates were dialysed against 0.15 M NaCl overnight at 37ºC with gentle shaking and a parallel plant-free (negative) control was included [33, 34].
2.8. In Vivo Confirmatory Studies
2.8.1. Oral Starch Tolerance Test (OSTT) and Oral Glucose Tolerance Test (OGTT)
With treatment plant administered in doses 100, 200 and 400 mg/Kg body weight (b.wt); OSTT and OGTT were conducted according to Kasabri et al. in the Experimental Animal Laboratory of the Faculty of Medicine, University of Jordan [31]. All animals were housed, fed and treated in accordance with the University of Jordan ethical guidelines for animal protection and experimental approval (registration number 218/2007-2008) was obtained from the Scientific Research Council at the Deanship of Academic Research and the Faculty of Pharmacy.
2.8.2. Body Weight and Triglycerides Determination
1) Experimental Animals The study was conducted at the Experimental Animal Laboratory of the Department of Biological Science, Faculty of Science; The University of Jordan. All animals were housed, fed and treated in accordance with the University of Jordan ethical guidelines for animal protection and experimental approval. Throughout the experimental period, animals were kept in single cages. Locally inbred male Wistar rats of 212.5 ± 1.9 g average body weight (b.wt) were used in the experiments. Rats were provided with normal diet chow (called basal diet hereafter) and water ad libitum for the duration of the experiment except during the 12 - 13 hrs fasting period preceding cholesterol administration and blood collection. Rats were divided into the followings groups (n = 6 - 8) as follows: Group 1: The control group was given only the standard diet ad libitum for 10 weeks. Group 2: The hypercholesterolemic control group was given the standard diet ad libitum and HCD once daily for 10 weeks [19]. Group 3: The preventive group was given the standard diet ad libitum. HCD and C. aronia AE (200 mg/Kg b.wt) were administered once daily for 10 weeks. Group 4: This group took standard diet + C. aronia AE (400 mg/Kg b.wt) for 10 weeks daily. Group 5: This group was given the standard diet ad libitum. HCD and atorvastatin (10 mg/Kg b.wt) were administered once daily for 10 weeks.
2) Crude Extract Administration C. aronia AE was administered to animals by gavage at 6:30 am daily. C. aronia AE doses for groups 3 and 4 were selected based on the preliminary LD50 screening The selected dose-400 mg/Kg/day-was 10× more diluted than the maximum soluble concentration of the extract, which did not cause any mortalities during LD50 experiments [13]. Blood samples were collected weekly from the retro orbital plexus of rats using a 10 μL glass capillary [35]. A blood sample was also collected just before the start of the experiment to measure the control values for each parameter for each animal group. All blood samples were collected after 12 - 13 h of fasting. Serum was collected from blood by centrifugation for 10 min at 3500 rpm and then stored at −80˚C until analysis. Analysis was conducted in duplicate and was completed within 1 - 3 days of collection. As study was on HFD rats, therefore quantitative determination of lipid profiles (cholesterol, HDL, LDL and VLDL) were achieved using enzymatically commercial available kits (Lab kits, Barcelona, Spain).
3) Body Weight Body weight was measured daily; doses of different treatment and plant extract were calculated and given according to body weight.
4) Triglycerides Serum triglyceride concentration was tested following the same above procedure.
5) Atherogenic Index Due to the fact that obesity is positively correlated with coronary heart diseases (CHD), it was valuable to check TC/HDL and HDL/LDL ratios as indicators as CHD predictors.
2.9. Statistical Analysis
The values are presented as mean ± S.E.M. (Standard Error of the Mean) of 3 - 4 independent experiments. Statistical differences between control and different treatment groups and A.U.Cs (incremental Area Under 24-h glucose Curve) were determined using Graphpad Prism one way analysis of variance (ANOVA) followed by Dunnett post test whenever appropriate (version 3.02 for windows; GraphPad Software, San Diego, CA, USA). Values were considered significantly different if P < 0.05 and highly significantly different if P < 0.01 and P < 0.001.
3. Results and Discussion
Pancreatic Lipase inhibition is one of the most widely studied mechanisms to determine the potential efficacy of natural products and ethnomedicinal botanicals as obesity modulating agents [29,36]. In this current study, the pancreatic triacylglycerol antilipase activity profiles of the crude aqueous extracts of C. aronia and their isolated quercetin and rutin are shown in Figure 1 [13]. Orlistat’s PL IC50 of 114.0 ± 4.0 ng/mL, equivalent to 0.2 ± 0.0 μM, is comparable to reported PL IC50 values elsewhere [29], thus promoting the pronounced sensitiveity and reliability of recruited PL activity assay (Table 1). Comparable to orlistat performance, a marked concentration-dependent PL inhibition trend was obtained for the tested extracts (the same figure) and their pure phytocomponents. PL IC50 values obtained for triple separate determinations are also illustrated (Table 1).
Most distinctively, Hawthorn flavonoids could modulate lipoprotein lipase expression in mice, comparable to therapeutic pioglitazone [37]. Pharmacological lipid lowering efficacies of Crataegus spp fruits are extensively elaborated elsewhere [38]. Additionally, Hawthorn combination with simvastatin exhibited a substantial lipid lowering effectiveness in hyperlipidemic albino rats [39]. These significant effects may be solidly related to the effect of the major compounds identified in the crude extract [29]. Unequivocally, Hawthorn fruit active principles, inhibiting synergistically HMG-CoA reductase and cholesterol absorption could manifest significant hypolipidemic benefits, further validating the hypocholesterolemic effect of C. aronia in high cholesterol diet fed rats [13,40-42]. In effect, the results indicate that the pancreatic triacylglycerol lipase inhibitory efficacy of C. aronia may be attributable to its multiple components acting additively or synergistically in optimal ratio. The discriminative inhibitory power of antilipolytic C. aronia for circulatory lipoprotein lipase, intracellular hormone sensitive lipase or pancreatic triacylglycerol lipase as separate pharmacological targets can be examined
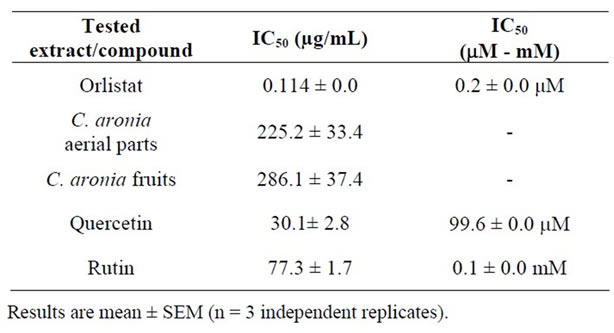
Table 1. In vitro pancreatic triacylglycerol lipase IC50 values for increasing concentrations of different parts of C. aronia AEs, its bioactive phytoconstituents; rutin and quercetin, and orlistat.
[30,43]. Further downstream studies into a plausible pivotal role in modulating adipogenic differentiation and accumulation maybe also conducted [44] in anticipation of in vivo tolerance, efficacy and safety [45-47]. All in all, pharmacological inhibition of dietary lipid digestion and absorption can induce favorable amelioration of dyslipidemia, atherosclerosis and obesity. Impressively, pancreatic triacylglycerol lipase natural inhibitors offer the utility for adjuvant or alternative treatment to statins or orlistat as likely synergies can exist between new and established lipid-lowering drugs [48]. Furthermore, the tested extracts and their isolated pure flavonoids were evaluated for their inhibitory potential of enzymatic starch digestion in comparison to acarbose therapeutic efficacy. Glucose liberation from starch was inhibited by 97.6% highly significantly with acarbose (0.1 mg/mL) as the reference drug, (P < 0.001, vs. drug-free control incubation, n = 3, Figure 2). Furthermore, Figure 2 demonstrates that C. aronia aerial parts AE concentrations 1 - 10 mg/mL had highly substantial dose-related reductions in aldohexose release from culinary polymeric cornstarch (P < 0.05 - 0.001 vs. plant-free control determinations, n = 3). With an IC50 of 2.1 ± 0.3 mg/mL, the highly significant dose related (P < 0.05 - 0.001) % decreases in enzymatic starch hydrolysis by tested extracts of C. aronia are summarized in Table 2. Additionally, C. aronia fruits AEs exerted highly significant (P < 0.001 vs. control determinations, n = 3) concentration-dependent inhibitions of enzymatic starch digestion, with an IC50 of 3.5 ± 0.7 mg/mL (Figure 3). Percent decreases in polysaccharide hydrolysis are tabulated in Table 2. The exquisite anti-α-glucosidase activity of Hawthorn leaf [49] can be strongly validated by comparable activity of its phytochemicals, mainly quercetin [50], and rutin [51]. Additively, quercetin and rutin exhibited an effective competitive inhibition of α-amylase. Taken together, the overall dual α-amylase and α-glucosidase inhibitory propensities of C. aronia could be the result of the combination of its several constituents performing in concert, in a holistic manner, thereby contributing to the restoration of homeostasis of energy consumption and utilization [52].
Using the glucose diffusion model; in vitro investigation of C. aronia extracts and its isolated flavonoids were undertaken in comparison to standard guar gum. Mean AUC (area under 24 h glucose curve) for the viscous water-soluble gel-forming guar gum (50 mg/mL) was decreased highly significantly by 30.8% ± 2.5% (P < 0.001) (n = 3, Figure 4) compared to overnight negative control. The efficacy of guar as a classical positive control has been elsewhere detailed [33]. Incomparable to guar gum, C. aronia extracts (10, 20 and 40 mg/mL) lacked any marked glucose diffusional hindrances into external solution across dialysis membrane (with res-
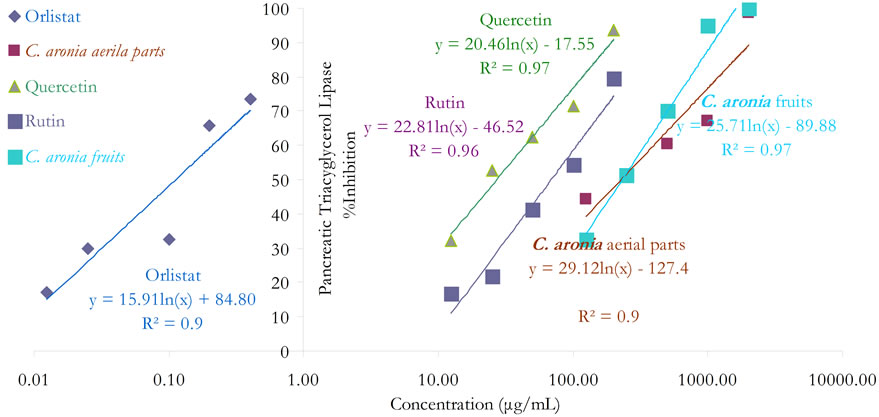
Figure 1. In vitro inhibitory effects of C. aronia (AE) different parts and its bioactive phytoconstituents concentrations in µg/mL on pancreatic triacylglycerol lipase activity.

Figure 2. In vitro inhibitory effects of C. aronia (AE) aerial parts concentrations in mg/mL on enzymatic starch digestion. Results are mean ± SEM (n = 3 independent replicates). *P < 0.05 and ***P < 0.001 compared to control (drug-free or plant-free) incubations, as determined by ANOVA followed by Dunnett post test.
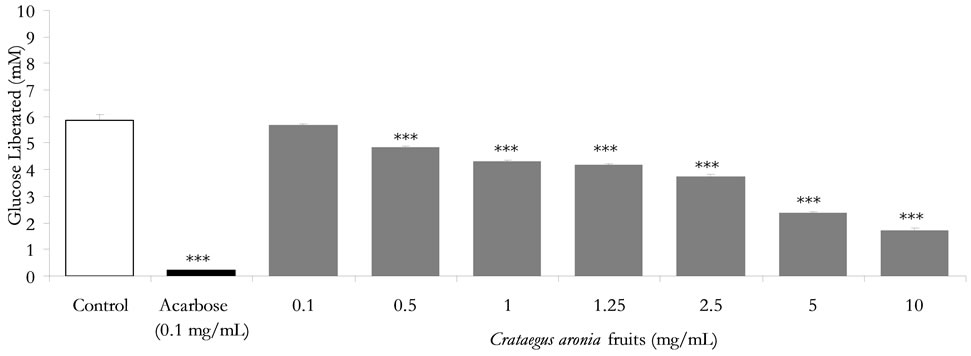
Figure 3. In vitro inhibitory effects of C. aronia (AE) fruits concentrations in mg/mL on enzymatic starch digestion. Results are mean ± SEM (n = 3 independent replicates). *P < 0.05 and ***P < 0.001 compared to control (drug-free or plant-free) incubations, as determined by ANOVA followed by Dunnett post test.
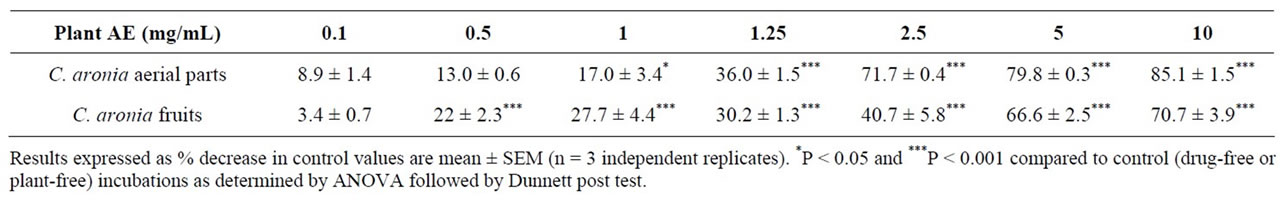
Table 2. Effects of ascending concentrations of different parts of C. aronia (AE) (mg/mL) on %reduction of enzymatic starch digestion in vitro.
pective 1.4% ± 0.8%, 1.0% ± 0.0% and 1.0% ± 0.1% AUC % reductions, P > 0.05, Figure 4).
Based on the optimal in vitro findings of the aqueous extract of C. aronia aerial parts, confirmatory acute in vivo studies (OSTT and OGTT) were conducted. At −30 min time point, the administration of acarbose 3 mg/Kg b.wt reduced highly significantly the starch induced postprandial hyperglycaemia at 45, 90 and 135 min post corn starch load at 0 min, thus evoking highly substantial reduction (P < 0.001 vs. untreated animals, n = 5 - 8) of the overall glycemic excursion AUC compared to controls (Figure 5).
Exceedingly superior to acarbose, C. aronia AE at concentrations 100, 200 and 400 mg/Kg b.wt diminished highly markedly (p < 0.001 vs. untreated rats, n = 5 - 8) AUC of overall glycemic excursions (Figure 5). Compared to control rats, Figure 5 mirrors the highly significant minimized increments in acute postprandial hyperglycemia evoked by C. aronia AE (100, 200 and 400 mg/Kg b.wt) at the determination time points 45 min (P < 0.001), 90 min (P < 0.001) and 135 min (P < 0.001) postculinary cornstarch oral intake. Most importantly and impressively, such pronounced C. aronia bioeffects on rats’ gastrointestinal starch digestion were comparable to those effectively obtained in vitro; a finding that can complement its claimed antihyperglycemic effects in STZ-diabetic rats [20]. 30 min pre-glucose-load in OGTTs; treatments with metformin (300 mg/Kg b.wt) or glipizide (0.6 mg/Kg b.wt) minimized highly significantly (P < 0.001 compared to control rats, n = 5 - 8) the overall glycemic excursions (Figure 6). The figure demonstrates the highly substantial antihyperglycemic efficacies of both oral antidiabetic therapeutics 45 (P < 0.001), 90 (P < 0.001) and 135 min (P < 0.001) following sugar load. Oral administration of C. aronia AEs did not evoke any marked improvement of glucose tolerance AUCs in comparison to control determinations respective AUCs, contrary to metformin and glipizide therapeutic propensities (Figure 6).
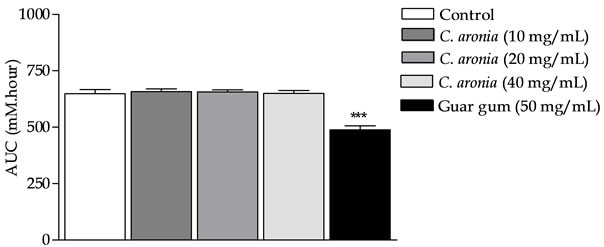
Figure 4. In vitro effects of C. aronia AE concentrations (mg/mL) in on the incremental AUC of 24 h glucose movement. ***P < 0.001 compared to control (drug-free or plantfree) incubations, as determined by ANOVA followed by Dunnett post test.
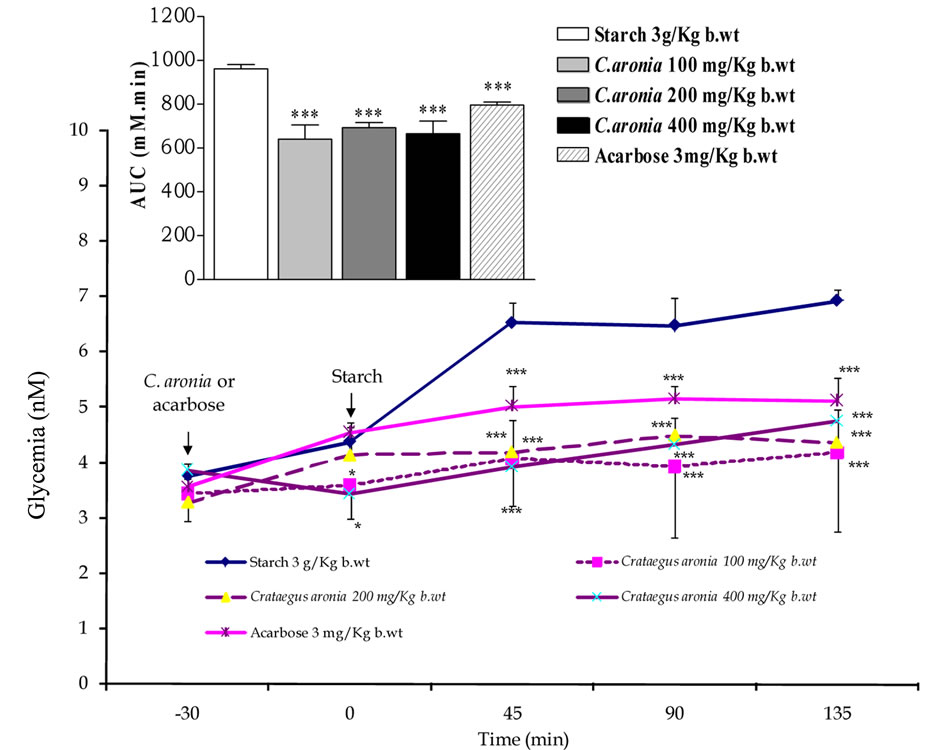
Figure 5. Modulatory postprandial antihyperglycemic effects of C. aronia (AE) concentrations in mg/Kg b.wt on oral starch tolerance over 165 min and AUC in normoglycemic overnight fasting rats. *P < 0.05 and ***P < 0.001 compared to control untreated animals, as determined by ANOVA followed by Dunnett post test.
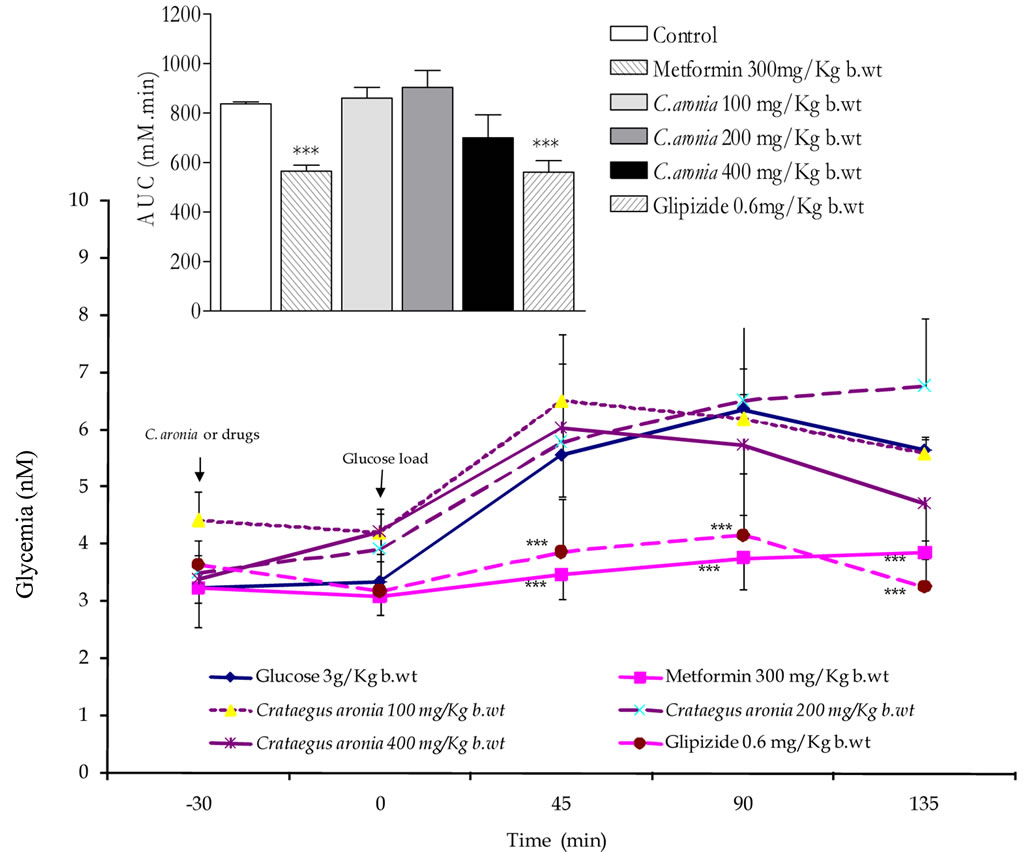
Figure 6. Effects of C. aronia (AE) concentrations in mg/Kg b.wt on oral glucose tolerance over 165 min and AUC in normoglycemic overnight fasting rats. *P < 0.05 and ***P < 0.001 compared to control untreated animals, as determined by ANOVA followed by Dunnett post test.
In parallel terms, none of C. aronia AEs exhibited any postprandial acute antihyperglycemic activity in glucose fed rats at any determination point (Figure 6), thus further ascertaining the lack of in vitro efficacies on gastrointestinal glucose movement. Suggestively, coadministration C. aronia with acarbose may reduce the therapeutic concentration required for its effective dual a-amylase and a-glucosidase inhibitions. Such anticipated synergistic interactions, at best, may impact/ substitute the clinical prescriptions of acarbose for type-2 diabetics’ postprandial glycaemia management [53,54]. As pancreatic enzymes elevation in type 2 diabetes has been marked; hence inhibition of these enzymes serves multiple pharmacotherapeutic targets in treatment of diabetes, obesity and hyperlipoproteinemia [55-57]. Starch blockers with dietary lipid blockers are successful antiobesity therapeutic perspectives [58-61].
In vivo studies were conducted to evaluate the effects of 10 weeks administration of C. aronia AE on body weight, triacylglycerol levels and atherogenic indices in HCD fed rats. Weights were standardized by considering the starting weight for each animal as 100%. Body weights of all groups increased with time. As shown in Figure 7, C. aronia 400 mg/Kg-group exhibited significant decrease in body weight compared to control (P < 0.05) group. Additionally, in the same figure, significant body weight decrease was obtained for the HCD + C. aronia 200 mg/Kg b.wt when compared to HCD alone (p < 0.01 - 0.001). Body weight AUC10weeks in HCD-rats was significantly increased compared to control animals (1192.5 ± 81.5 vs. 1032.4 ± 60.8, n = 6 - 8 rats/group, P < 0.05, Figure 7). Most impressively, body weightAUC10weeks in rat groups of HCD + C. aronia 200 mg/Kg b.wt and C. aronia 400 mg/Kg b.wt were normalized to control’s AUC (953.3 ± 29.7 and 962.7 ± 13.1 vs. 1032.4 ± 60.8, n = 6 - 8 rats/group, P > 0.05, respectively, Figure 7). Interestingly, body weight AUC10weeks in HCD + C. aronia 200 mg/Kg b.wt was highly significantly less than those of HCD rats (respective 953.3 ± 29.7 and 962.7 ± 13.1 vs. 1192.5 ± 81.5, n = 6 - 8 rats/group, P < 0.01, Figure 7). Unlike C. aronia effects, chronic atorvastatin oral treatment did not change markedly body weight AUC10weeks (1054.8 ± 26.9 vs. either 1032.4 ± 60.8 or 1192.5 ± 81.5, n = 6 - 8 rats/group, P > 0.05).
Figure 8 demonstrates the effect of C. aronia AE on triacylglycerol levels (TAG). TAG-AUC 10 weeks in HCD fed-animals is highly significantly greater than control’s
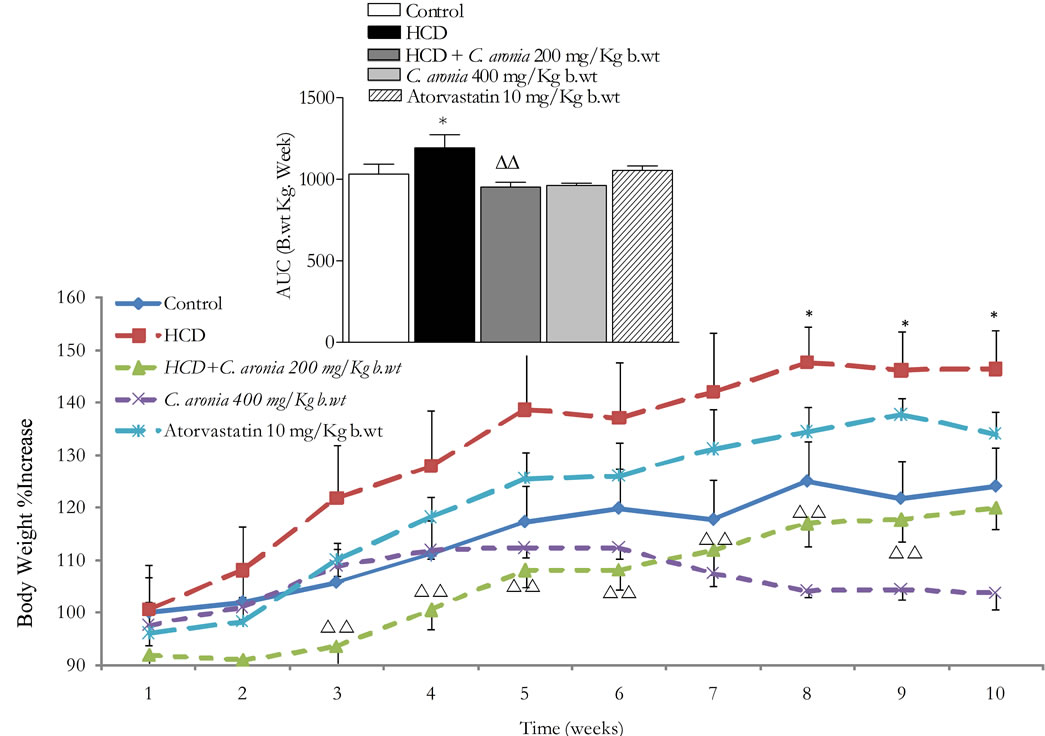
Figure 7. In vivo chronic study effects of C. aronia AE on the body weight and the incremental AUC of 10 weeks treatment. *P < 0.05 compared to control group and ∆∆P < 0.01 compared to HCD as determined by ANOVA followed by Dunnett post test.
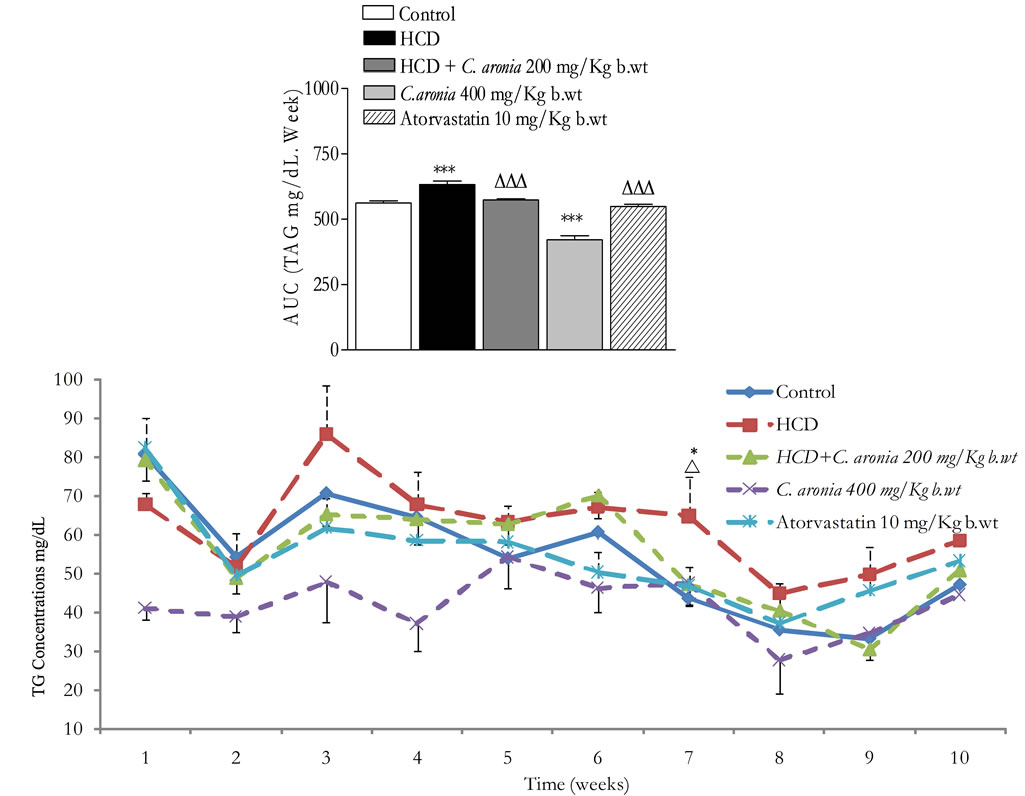
Figure 8. In vivo chronic study effects of C. aronia AE on the incremental AUC of 10 weeks treatment on triglycerides concentrations. ***P < 0.001 compared to control group, ∆∆P < 0.01 compared to HCD as determined by ANOVA followed by Dunnett post test.
AUC (633 ± 30.0 vs. 562 ± 20.4, n = 6 - 8 rats/group, P < 0.01). In the same figure, orally administrated atorvastatin 10 mg/Kg b.wt normalised TAG-AUC 10weeks in HCD fed-animals in comparison to HCD fed rats (548.6 ± 17.8 vs. 633 ± 30.0 and 548.6 ± 17.8 vs. 562 ± 20.4, n = 6 - 8 rats/group, P < 0.001 and P > 0.05 respectively). Likewise, TAG-AUC10weeks in HCD + C. aronia 200 mg/Kg b.wt-animals has been normalized as in that of control’s (573.6 ± 14.2 vs. 562 ± 20.4, n = 6 - 8 rats/group, P > 0.05, Figure 8) and is highly significantly less than HCD-fed animals’ AUC (573.6 ± 14.2 vs. 633 ± 30.1, n = 6 - 8 rats/group, P < 0.01, Figure 8). Interestingly, TAG-AUC10weeks in C. aronia 400 mg/Kg b.wtanimals is highly significantly less than control’s (421.3 ± 45.0 vs. 562 ± 20.4, n = 6 - 8 rats/group, P < 0.001) This finding confirms the in vitro PL inhibitory potential of C. aronia extracts.
The American Heart Association has classified obesity as one of the major risk factors of coronary heart diseases (CHD) [62]. Therefore, prevention of obesity can minimize the occurrence of CHD. Additionally, amelioration of the different ratios such as HDL/LDL is considered as target for preventing/reduction risk of CHD [63]. Table 3 shows the ratios of TC/HDL and HDL/LDL. HCD group has high TC/HDL ratio after 10 weeks of treatment and low HDL/LDL ratio when compared to preventive group that was treated similarly except that C. aronia AE (200 mg/Kg/day) was given along with HCD (4.1 ± 0.5 vs. 2.3 ± 0.2, and 0.9 ± 0.5 vs. 1.4 ± 0.3, respectively). On the other hand, both TC/HDL and HDL/LDL ratios for group (C. aronia 400 mg/dL/Kg) are close to those of control group, both groups took the same diet except that C. aronia AE was administered besides in treated group (C. aronia 400 mg/Kg) (1.9 ± 0.1 vs.1.7± 0.1 and 1.8 ± 0.3 vs. 3.3 ± 0.8, respectively). The above results are further indications of beneficial effects of C. aronia AE on atherogenic indices (predictors of CHD). As the occurrence of obesity is on the rise, various recent studies were accomplished on obesity treatment through suppression of triglycerides accumulation by inhibiting the digestion of dietary lipids and minimizing intestinal fat absorption [30].
In this study it was shown that C. aronia AE results in vivo regarding triglyceride and those of body weight were significantly lower when compared to normal diet. However, compared to the HCD group, C. aronia AE in preventive group was able to preserve lower percent of body weight and TG concentration throughout the whole period (10 weeks). These data pursue the same line of those in vitro results for pancreatic lipase when C. aronia and/or its pure phytocomponents compared to orlistat, potent drug known for PL inhibition. These results may suggest that C. aronia AE has the same mode of action of orlistat through inhibition of PL to digest dietary fats
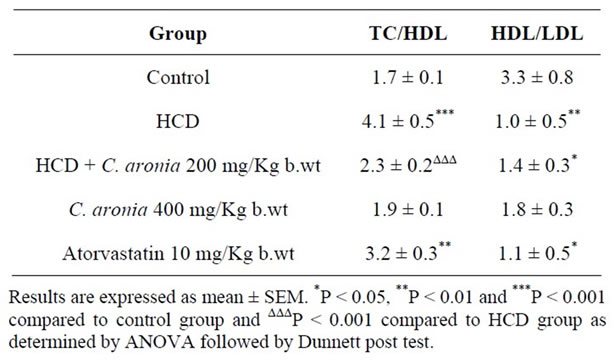
Table 3. In vivo effects of C. aronia extracts on TC/HDL and HDL/LDL ratios.
[30]. Moreover, our findings are in agreement with those recently reported by Mnafgui et al., indicating that PL inhibition leads to decreases in lipid profiles [64]. Additionally, previous studies found that flavonoids have an effective role in PL inhibition, so did our in vitro study when using C. aronia pure compounds [64].
Succinctly C. aronia phytochemicals in optimal ratio can inhibit crucial gastrointestinal enzymes involved in carbohydrate and lipid digestion and absorption thus advocating a dual-target phytotherapeutic/preventive strategy in glycaemia control of obesity-diabetes (diabesity) [65].
4. Acknowledgements
We are grateful for Deanship of Academic Research— The University of Jordan for funding this work [Grant number 29/2010-2011]. Technical assistance of Dana AlQudah, Esra Fodah, Haneen Ramadan, Hazar Shawash and Ismail Abaza is acknowledged.
REFERENCES
- K. Ajlouni, Y. S. Khader, A. Batieha, H. Ajloun and M. El-Khateeb, “An Increase of Diabetes Mellitus in Jordan over 10 Years,” Journal of Diabetes Complications, Vol. 22, No. 5, 2008, pp. 317-324.
- N. R. Bulatova, A. F. Yousef and S. M. AbuRuz, “Antiplatelet Therapy for Primary and Secondary Prevention in Jordanian Patients with Diabetes Mellitus,” Thrombosis Research, Vol. 121, No. 1, 2007, pp. 43-50. doi:10.1016/j.thromres.2007.03.006
- M. Zindah, A. Belbeisi, H. Walke and A. H. Mokdad, “Obesity and Diabetes in Jordan: Findings from the Behavioural Risk Factor Surveillance System, 2004,” Prevention of Chronic Disease, Vol. 5, No. 1, 2008, pp. 1-8.
- International Diabetes Federation (IDF), “Prevalence Estimates of Diabetes Mellitus (DM)-MENA,” IDF Diabetes Atlas, 4th Edition, 2009. http://www.diabetesatlas.org/content/prevalence-estimates-diabetes-mellitus-dm-2010
- Z. H. Israili, “Advances in the Treatment of Type 2 Diabetes Mellitus,” American Journal of Therapeutics, Vol. 18, No. 2, 2011, pp. 117-152. doi:10.1097/MJT.0b013e3181afbf51
- F. U. Afifi-Yazar, V. Kasabri and R. Abu Dahab, “Medicinal Plants from Jordan in the Treatment of Diabetes: Traditional Uses vs. in Vitro and in Vivo Evaluations. Mini-Review,” Planta Medica, Vol. 77, No. 11, 2011, pp. 1210-1220. doi:10.1055/s-0031-1279983
- C. Anderson, S. Rayalam, M. A. Della-Fera and C. A. Baile, “Phytochemicals and Adipogenesis,” Biofactors, Vol. 36, No. 6, 2010, pp. 415-422. doi:10.1002/biof.115
- M. Kazemipoor, C. W. W. M. Radzi, G. A. Cordell and I. Yaze, “Potential of Traditional Medicinal Plants for Treating Obesity: A Review,” 2010 International Conference on Nutrition and Food Sciences. IPCBEE, Singapore, Vol. 39, No. 1, 2012, pp. 1-6.
- WHO, “Obesity and Overweight 2012,” 2012. http://www.who.int/mediacentre/factsheets/fs311/en/
- J. W. Yun, “Possible Antiobesity Therapeutics from Nature—A Review,” Phytochemistry, Vol. 71, No. 14-15, 2010, pp. 1625-1641. doi:10.1016/j.phytochem.2010.07.011
- A. Al-Aboudi and F. U. Afifi, “Plants Used for the Treatment of Diabetes in Jordan: A Review of Scientific Evidence,” Pharmaceutical. Biology, Vol. 49, No. 3, 2011, pp. 221-239. doi:10.3109/13880209.2010.501802
- M. Wazaify, F. U. Afifi, M. El-Khateeb and K. Ajlouni, “Complementary and Alternative Medicine Use among Jordanian Diabetes Patients,” Complementary Therapies in Clinical Practice, Vol. 17, No. 2, 2011, pp. 71-75. doi:10.1016/j.ctcp.2011.02.002
- E. K. Al-Hallaq, F. U. Afifi and S. S. Abdalla, “Evaluation of the Hypocholesterolemic Effect and Phytochemical Screening of the Hydroethanolic Extract of Crataegus aronia from Jordan,” Natural Product Communications, Vol. 7, No. 1, 2012, pp. 35-38.
- R. Bahri-Sahloul, S. Ammar, R. B. Fredj, S. Saguem, S. Grec, F. Trotin and F. H. Skhiri, “Polyphenol Contents and Antioxidant Activities of Extracts from Flowers of Two Crataegus azarolus L. Varieties,” Pakistan Journal of Biological Sciences, Vol. 12, No. 9, 2009, pp. 660-668. doi:10.3923/pjbs.2009.660.668
- O. Calişkan, K. Gündüz, S. Serçe, C. Toplu, O. Kamiloğlu, M. Sengül and S. Ercişli, “Phytochemical Characterization of Several Hawthorn (Crataegus spp.) Species Sampled from the Eastern Mediterranean Region of Turkey,” Pharmacognosy Magazine, Vol. 8, No. 29, 2012, pp. 16-21. doi:10.4103/0973-1296.93305
- N. Keskin, R. Mammadov and P. Ili, “The Effects of Crataegus aronia var. dentata Browicz Extract on Biochemical Indices and Apoptosis in Partially Hepatectomized Liver in Rats,” Bosnian Journal of Basic Medical Sciences, Vol. 12, No. 3, 2012, pp. 177-181.
- R. Khalil, N. Abuharfeil and B. Shabsoug, “The Effect of Crataegus aronia Aqueous Extract in Rabbits Fed with High Cholesterol Diet,” European Journal of Scientific Research, Vol. 22, 2008, pp. 352-360.
- D. Kumar, V. Arya, Z. A. Bhat, N. A. Khan and D. N. Prasad, “The Genus Crataegus: Chemical and Pharmacological Perspectives,” Brazilian Journal of Pharmcognosy, Vol. 22, No. 5, 2012, pp. 1187-1200.
- P. Ljubuncic, I. Portnaya, U. Cogan, H. Azaizeh and A. Bomzon, “Antioxidant Activity of Crataegus aronia Aqueous Extract Used in Traditional Arab Medicine in Israel,” Journal of Ethnopharmacology, Vol. 101, No. 1-3, 2005, pp. 153-161. doi:10.1016/j.jep.2005.04.024
- P. Ljubuncic, H. Azaizeh, U. Cogan and A. Bomzon, “The Effects of a Decoction Prepared from the Leaves and Unripe Fruits of Crataegus aronia in StreptozotocinInduced Diabetic Rats,” Journal of Complementary and Integrative Medicine, Vol. 3, No. 1, 2006, in press. doi:10.2202/1553-3840.1027
- A. S. Shatoor, “Acute and Sub-Acute Toxicity of Crataegus aronia syn. azarolus (L.) Whole Plant Aqueous Extract in Wistar Rats,” American Journal of Pharmacology and Toxicology, Vol. 6, No. 2, 2011, pp. 37-45. doi:10.3844/ajptsp.2011.37.45
- A. S. Shatoor, “Cardio-Tonic Effect of the Aqueous Extract of Whole Plant of Crataegus aronia syn: azarolus (L) on Isolated Rabbit’s Heart,” African Journal of Pharmacy and Pharmacology, Vol. 6, No. 26, 2012, pp. 1901-1909.
- A. S. Shatoor, H. Soliman, F. Al-Hashem, B. E. Gamal, A. Othman and N. El-Menshawy, “Effect of Hawthorn (Crataegus aronia syn. azarolus (L)) on Platelet Function in Albino Wistar Rats,” Thrombosis Research, Vol. 130, No. 1, 2012, pp. 75-80.
- M. S. Shekha and O. A. M. AlHabib, “Vasorelaxant, Antispasmodic and Cardiotonic Effect of the Chloform Fraction of Crataegus aronia on Isolated Rat Muscles,” International Journal of Biological and Biomedical Sciences, Vol. 1, No. 1, 2012, pp. 6-11.
- M. C. Tassell, R. Kingston, D. Gilroy, M. Lehane and A. Furey, “Hawthorn (Crataegus spp.) in the Treatment of Cardiovascular Disease,” Pharmacognosy Reviews, Vol. 4, No. 7, 2010, pp. 32-41. doi:10.4103/0973-7847.65324
- S. Adisakwattana and B. Chanathong, “Alpha-Glucosidase Inhibitory Activity and Lipid-Lowering Mechanisms of Moringa oleifera Leaf Extract,” European Review for Medical and Pharmacological Sciences, Vol. 15, No. 7, 2011, pp. 803-808.
- C. B. Etoundi, D. Kuate, J. L. Ngondi and J. Oben, “AntiAmylase, Antilipase and Antioxidant Effects of Aqueous Extracts of Some Cameroonian Spices,” Journal of Natural Products, Vol. 3, 2010, pp. 165-171.
- I. Gurbuz, O. Ustun, E. Yesilada, E. Sezik and O. Kutsal, “Anti-Ulcerogenic Activity of Some Plants Used as Folk Remedy in Turkey,” Journal of Ethnopharmacology, Vol. 88, No. 1, 2003, pp. 93-97. doi:10.1016/S0378-8741(03)00174-0
- S. Habtemariam, “The Antiobesity Potential of Sigmoidin A,” Pharmaceutical Biology, Vol. 50, No. 12, 2012, pp. 1519-1522. doi:10.3109/13880209.2012.688838
- Y. Bustanji, I. AlMasri, M. Mohammad, M. Hudaib, K. Tawaha, H. Tarazi and H. AlKhatib, “Pancreatic Lipase Inhibition Activities of Trilactone Terpenes of Ginkgo biloba,” Journal of Enzyme Inhibition and Medicinal Chemistry, Vol. 26, No. 4, 2011, pp. 453-459. doi:10.3109/14756366.2010.525509
- V. Kasabri, F. U. Afifi and I. Hamdan, “In Vitro and in Vivo Antihyperglycemic Effects of Five Selected Indigenous Plants from Jordan Used in Traditional Medicine,” Journal of Ethnopharmacology, Vol. 133, No. 2, 2011, pp. 888-896. doi:10.1016/j.jep.2010.11.025
- V. Kasabri, R. Abu-Dahab, F. U. Afifi, R. Naffa and L. Majdalawi, “Modulation of Pancreatic MIN6 Insulin Secretion and Proliferation and Extrapancreatic Glucose Absorption with Achillea santolina, Eryngium creticum and Pistacia atlantica Extracts: In vitro Evaluation,” Journal of Experimental Integrative Medicine, Vol. 2, No. 3, 2012, pp. 245-254.
- M. S. Butt, A. Ahmad and M. K. Sharif, “Influence of Pectin and Guar Gum Composite Flour on Plasma Biochemical Profile of Streptozocin-Induced Diabetic Male Albino Rats,” International Journal of Food Properties, Vol. 10, No. 2, 2007, pp. 345-361. doi:10.1080/10942910601052707
- A. M. Gallagher, P. R. Flatt, G. Duggy and Y. H. A. Abdel-Wahab, “The Effects of Traditional Antidiabetic Plants on in Vitro Glucose Diffusion,” Nutrition Research, Vol. 23, No. 3, 2003, pp. 413-424. doi:10.1016/S0271-5317(02)00533-X
- S. Y. Pan, R. Yang, H. Dong, Z. L. Yu and K. M. Ko, “Bifendate Treatment Attenuates Hepatic Steatosis in Cholesterol/Bile Saltand High-Fat Diet Induced Hypercholesterolemia in Mice,” European Journal of Pharmacology, Vol. 552, No. 1-3, 2006, pp. 170-175. doi:10.1016/j.ejphar.2006.09.011
- C. D. Zheng, Y. Q. Duan, J. M. Gao and Z. G. Ruan, “Screening for Anti-Lipase Properties of 37 Traditional Chinese Medicinal Herbs,” Journal of the Chinese Medical Association, Vol. 73, No. 6, 2010, pp. 319-324. doi:10.1016/S1726-4901(10)70068-X
- C. Fan, J. Yan, Y. Qian, X. Wo and L. Gao, “Regulation of Lipoprotein Lipase Expression by Effects of Hawthorn Flavonoids on Peroxisome Proliferator Response Element Pathway,” Journal of Pharmaceutical Sciences, Vol. 100, No. 1, 2006, pp. 51-58. doi:10.1254/jphs.FP0050748
- T. Jurikova, J. Sochor, O. Rop, J. Mlcek, S. Balla, L. Szekeres, V. Adam and R. Kizek, “Polyphenolic Profile and Biological Activity of Chinese Hawthorn (Crataegus pinnatifida BUNGE) Fruits,” Molecules, Vol. 17, No. 12, 2012, pp. 14490-14509. doi:10.3390/molecules171214490
- S. Kausar, Z. Zaheer, M. Saqib and B. Zia, “The Effect of Crataegus (Hawthorn) Extract Alone and in Combination with Simvastatin on Serum Lipid Profile in Hyperlipidemic Albino Rats,” Biomedica, Vol. 27, 2011, pp. 140- 147.
- A. L. De le Garza, F. I. Milagro, N. Boque, J. Campion and J. A. Martinez, “Natural Inhibitors of Pancreatic Lipase as New Players in Obesity Treatment,” Planta Medica, Vol. 77, No. 8, 2011, pp. 773-785. doi:10.1055/s-0030-1270924
- Z. Zhang, W. K. K. Ho, Y. Huang, A. E. James, L. W. Lam and Z. Y. Chen, “Hawthorn Fruit Is Hypolipidemic in Rabbits Fed a High Cholesterol Diet,” Journal of Nutrition, Vol. 132, No. 1, 2002, pp. 5-10.
- W. Huang, X. Ye, Z. Zhao, P. Lan, L. Wang, M. Liu, Y. Gao, J. Zhu, P. Li and P. Feng, “The Inhibition Activity of Chemical Constituents in Hawthorn Fruit and Their Synergistic Action to HMG-CoA Reductase,” Zhongguo Zhong Yao Za Zhi, Vol. 35, No. 18, 2010, pp. 2428-2431.
- Y. Guo, G. Wu, X. Su, H. Yang and J. Zhang, “Antiobesity Action of a Daidzein Derivative on Male Obese Mice Induced by a High Fat Diet,” Nutrition Research, Vol. 29, No. 9, 2009, pp. 656-663. doi:10.1016/j.nutres.2009.09.005
- M. H. Moon, J. K. Jeong, Y. J. Lee, J. W. Seol, D. C. Ahn, I. S. Kim and S. Y. Park, “18β-Glycyrrhetinic Acid Inhibits Adipogenic Differentiation and Stimulates Lipolysis,” Biochemical and Biophysical Research Communications, Vol. 420, 2012, pp. 805-810. doi:10.1016/j.bbrc.2012.03.078
- A. Bryson, S. de la Motte and C. Dunk, “Reduction of Dietary Fat Absorption by the Novel Gastrointestinal Lipase Inhibitor Cetilistat in Healthy Volunteers,” British Journal of Clinical Pharmacology, Vol. 67, No. 3, 2009, pp. 309-315. doi:10.1111/j.1365-2125.2008.03311.x
- S. Ebdrup, H. H. Refsgaard, C. Fledelius and P. Jacobsen, “Synthesis and Structure-Activity Relationship for a Novel Class of Potent and Selective Carbamate-Based Inhibitors of Hormone Selective Lipase with Acute in Vivo Antilipolytic Effects,” Journal of Medicinal Chemistry, Vol. 50, No. 22, 2007, pp. 5449-5456. doi:10.1021/jm0607653
- J. Zhang, M. J. Kang, M. J. Kim, M. E. Kim, M. E. Song, J. H. Song, Y. M. Lee and J. I. Kim, “Pancreatic Lipase Inhibitory Activity of Taraxacum officinale in Vitro and in Vivo,’’ Nutrition Research and Practice, Vol. 2, No. 4, 2008, pp. 200-203. doi:10.4162/nrp.2008.2.4.200
- A. S. Wierzbicki, T. C. Hardman and A. Viljoen, “New Lipid-Lowering Drugs: An Update,” International Journal of Clinical Practice, Vol. 66, No. 3, 2012, pp. 270- 280. doi:10.1111/j.1742-1241.2011.02867.x
- H. Li, F. Song, J. Xing, R. Tsao, R. Liu and S. Liu, “Screening and Structural Characterization of α-Glucosidase Inhibitors from Hawthorn Leaf Flavonoids Extract by Ultrafiltration LC-DAD-MSn and SORI-CID FTICR MS,” Journal of American Society for Mass Spectrometry, Vol. 20, No. 8, 2009, pp.1496-1503. doi:10.1016/j.jasms.2009.04.003
- P. Fan, L. Terrier, A. E. Hay, A. Marston and K. Hostettmann, “Antioxidant and Enzyme Inhibition Activities and Chemical Profiles of Polygonum sachalinensis F. Schmidt ex Maxim (Polygonaceae),” Fitoterapia, Vol. 81, No. 2, 2010, pp. 124-131. doi:10.1016/j.fitote.2009.08.019
- Y. Q. Li, F. C. Zhou, F. Gao, J. S. Bian and F. Shan, “Comparative Evaluation of Quercetin, Isoquercetin and Rutin as Inhibitors of Alpha-Glucosidase,” Journal of Agriculture and Food Chemistry, Vol. 57, No. 24, 2009, pp. 11463-11468. doi:10.1021/jf903083h
- P. Bansal, P. Paul, G. Shankar, D. Munjal, P. G. Nayak, K. I. Priyadarsini and M. K. Unnikrishnan, “Flavonoid Rich Fraction of Pilea microphylla (L.) Attenuates Metabolic Abnormalities and Improves Pancreatic Function in C57BL/KsJ-db-db Mice,” Biomedicine and Preventive Nutrition, Vol. 1, No. 4, 2011, pp. 268-272. doi:10.1016/j.bionut.2011.09.002
- S. Adisakwattana, O. Lerdsuwankij, U. Poputtachai, A. Minipun and C. Suparpprom, “Inhibitory Activity of Cinnamon Bark Species and Their Combination Effect with Acarbose against Intestinal α-Glucosidase and Pancreatic α-Amylase,” Plant Foods and Human Nutrition, Vol. 66, No. 2, 2011, pp. 143-148. doi:10.1007/s11130-011-0226-4
- D. Grussu, D. Stewart and G. J. McDoagall, “Berry Polyphenols Inhibit α-Amylase in Vitro: Identifying Active Components in Rowanberry and Raspberry,” Journal of Agriculture and Food Chemistry, Vol. 59, No. 6, 2011, pp. 2324-2331. doi:10.1021/jf1045359
- J. Maolly, K. Gurney, K. Shan, P. Yan and S. Chen, “Increased Variability and Abnormalities in Pancreatic Enzyme Concentrations in otherwise Asymptomatic Subjects with Type 2 Diabetes,” Diabetes Metabolic Syndrome and Obesity: Targets and Therapy, Vol. 5, 2012, pp. 419-429.
- N. Ikarashi, R. Takeda, K. Ito, W. Ochiai and K. Sugiyama, “The Inhibition of Lipase and Glucosidase Activities by Acacia Polyphenol,” Evidence-Based Complementary and Alternative Medicine, Vol. 2011, 2011, Article ID: 272075. doi:10.1093/ecam/neq043
- S. N. Raju, D. S. Kumar, D. Banji, A. Harani, P. Shankar, P. A. Kumar and V. R. Reddy, “Inhibitory Effects of Ethanolic Extract of Piper trioicum on Amylase, Lipase and α-Glucosidase,” Der Pharmacia Lettre Journal, Vol. 2, 2010, pp. 237-244.
- M. I. Kotowaroo, M. F. Mahomoodally, A. Gurib-Fakim and A. H. Subratty, “Screening of Traditional Antidiabetic Medicinal Plants of Mauritius for Possible α-Amylase Inhibitory Effects in Vitro,” Phytotherapy Research, Vol. 20, No. 3, 2006, pp. 228-231. doi:10.1002/ptr.1839
- W. C. Obiro, T. Zhang and B. Jiang, “The Nutraceutical Role of the Phaseolus vulgaris α-Amylase Inhibitor,” British Journal of Nutrition, Vol. 100, No. 1, 2008, pp. 1- 12. doi:10.1017/S0007114508879135
- [61] X. Wu, X. Xu, J. Shen, N. V. Perricone and H. G. Preuss, “Enhanced Weight Loss from a Dietary Supplement Containing Standardized Phaseolus vulgaris Extract in Overweight Man and Women,” Journal of Applied Research, Vol. 10, No. 2, 2010, pp. 73-79.
- [62] A. Gholamhoseinian, B. Shahouzehi and F. Sharifi-far, “Inhibitory Effect of Some Plant Extracts on Pancreatic Lipase,” International Journal of Pharmacology, Vol. 6, No. 1, 2010, pp. 18-24. doi:10.3923/ijp.2010.18.24
- [63] R. H. Eckel and R. M. Krauss, “American Heart Association Call to Action: Obesity as a Major Risk Factor for Coronary Heart Disease,” Circulation, Vol. 978, 1998, pp. 2099-2100. doi:10.1161/01.CIR.97.21.2099
- [64] B. L. McVeigh, B. L. Dillingham, J. W. Lampe and A. M. Duncan, “Effect of Soy Protein Varying in Isoflavone Content on Serum Lipids in Healthy Young Men,” American Journal of Clinical Nutrition, Vol. 83, No. 2, 2006, pp. 244-251.
- [65] K. Mnafgui, K. Hamden, H. Ben Salah, M. Kchaou, M. Nasri, S. Slama, F. Derbali, N. Allouche and A. Elfeki, “Inhibitory Activities of Zygophyllum album: A Natural Weight-Lowering Plant on Key Enzymes in High-Fat Diet Fed Rats,” Evidence Based Complementary Alternative Medicine, Vol. 2012, 2012, Article ID: 620384.
- [66] S. M. Jeong, M. J. Kang, H. N. Choi, J. H. Kim and J. I. Kim, “Quercetin Ameliorates Hyperglycemia and Dislipidemia and Improves Antioxidant Status in Type 2 Diabetic db/db Mice,” Nutrition Research and Practice, Vol. 6, No. 3, 2012, pp. 201-207.
NOTES
*Corresponding author.

