Food and Nutrition Sciences
Vol. 3 No. 5 (2012) , Article ID: 19083 , 8 pages DOI:10.4236/fns.2012.35093
Studying Some Physicochemical Characteristics of Crust Coated with White Egg and Chitosan Using a Deep-Fried Model System
![]()
Department of Food Science and Technology, Faculty of Agriculture, Ferdowsi University of Mashhad, Mashhad, Iran.
Email: *Ansarifar.elham@gmail.com
Received December 21st, 2011; revised April 5th, 2012; accepted April 13th, 2012
Keywords: Frying; Deep-Fried Crust Model System; chitosan; white egg
ABSTRACT
In this paper, the effects of frying time, white egg (0%, 5% and 10% w/w) and chitosan (0%, 0.5% and 1.5% w/w) addition to the batter formulation on the quality of simulated crispy deep-fried Kurdish cheese nugget crusts was studied by using a deep-fried crust model. Moisture content, oil content, color and hardness of the samples were determined. Crust models were fried at 190˚C for 60, 120 and 180 s. Batter formulations and frying time significantly (p < 0.01) affected moisture, oil content, color and hardness of Crust models. Batter formulation contain 10% white egg was found to be an effective ingredient in decreasing oil content of Crust models. The mean moisture and fat content of Crust models formed with batter contained 10% white egg, fried at 190˚C, for 180 s were 6.207 ± 0.447 and 5.649 ± 0.394. Batters containing 5% white egg and 1.5% chitosan showed the lowest moisture content and the highest oil content among all the formulations. Crust models containing combination of white egg and chitosan were the darkest. Hardness of samples containing chitosan were the highest, specially for ch1.5 The mean hardness in 60, 120 and 180 s of frying in this formulation were 21.518 ± 0.481, 36.871 ± 1.758 and 49.563 ± 1.847 respectively.
1. Introduction
Deep-fat frying can be defined as the process of cooking through contact with hot oil [1]. Fried foods constitute a primary choice in the diets and have remained ever popular among today’s consumers of all ages. Because of changes in life style of consumers in term of preparation and consumption of food, the relative importance of fried foods is apparent [2]. Batter used for fried foods can be defined as liquid dough, basically consisting of flours, starches, seasoning and water. Batters have become more sophisticated complex systems in which the nature of the ingredients is very wide ranging and their interaction affects the finished product [3]. One of the main problems associated with batter-coated food consumption is the considerable amount of oil absorbed during the prefrying and frying operations [4]. One approach would be using an edible film ingredient that will improve the coating performance and be served as a shield to control the diffusion of moisture and fat in battered and breading products. Various types of edible coatings and films have been reported on the application in fried food, including methylcellulose [5,6], corn zein [7], hydroxypropyl methylcellulose [8] dextrin and dried egg [9], chitosan [4,10]. The ability of these coatings to limit moisture transfer may enable fried food products to maintain their crispness by inhibiting moisture transfer from the food material to the crust, and by limiting moisture absorption from the environment into the crust. When the liquid batter comes into direct contact with the hot frying oil, it sets around the piece of food and becomes a crisp crust [1]. Chitosan is apoly-β-(1→4)-2-amine-deoxy-D-glucopyranose and a naturally occurring component in shells of crustaceans and the cell walls of fungi. Chitosan is currently a field of interest due to its functional characteristics. Special functional properties include antimicrobial activity [11], serum cholesterol lowering ability [12], antioxidative activity in muscle foods [13], emulsification [12]. In addition, chitosan cannot be digested and absorbed in human intestine and therefore contributes to the benefits of dietary fiber [10]. White Egg is a complex protein system made up of a solution of globular proteins containing ovomucin fibers [14]. Ovalbumin, which constitutes more than half of white egg protein by weight, is the only fraction that contains free sulfhydryl (SH) groups. Other proteins, such as ovotransferrin, ovomucoid, and lyzozyme contain disulfide (S-S) bonds [15]. The S-S bonds are considered important in film formation for proteins containing cysteine and cystine amino acids [16]. Variations in core properties (i.e., moisture content, porosity, surface roughness) and batter viscosity affect the amount of batter adhering to the product before frying which will have a pronounced effect on crust properties [3]. Also it is often difficult and time-consuming to separate the crust/batter from the product core, making rapid analysis of the batter/crust a difficult task. Finally, the fried product often shows poor reproducibility. To avoid this problem a deep-fried crust model (DFCM) system was used for making deep-fried crusts [17,18]. The thickness of the batter before deep frying can be controlled independently of the batter viscosity and therefore the effects of the thickness on the crust properties can also be controlled. The aim of this study was to design a deep-fried model (DFM) system allowing the production of reproducible crusts with the flexibility of adapting to different batter and core types. Since the presence of a core is essential to simulate the crust formation of a real product, in the study from moisture content of Kurdish cheese nugget was used to adjust the humidity of silica as core. There have been no previous studies in Kurdish cheese nugget. Also studying the effects of white egg and chitosan addition on the quality properties of CRUST MODELs have been studied.
2. Materials and Methods
2.1. Batter Preparation
Wheat flour was purchased from local market to prepare the batter. According to the manufacturer, the composition of the wheat flour was 8.5% (w.b.) protein, 0.6% (w.b.) ash content and 14.2% moisture. A control batter was prepared by mixing wheat flour (90.8%), baking powder (3.1%), flavor (pepper) (0.6%), and salt (5.5%). In addition, chitosan (Sigma chemical com, low molecular weight) (0%, 0.5% and 1.5%) and white egg (0%, 5% and 10%) was added, replacing the same amount of wheat flour. The water/dry mix proportion was always 1.2:1 (w/w) in batter containing chitosan and 1% acetic acid. The temperature of the water before mixing was 15˚C. The ingredients were mixed in a mixer (Moulinex, type BM4) for 2 min.
2.2. Crust Model Preparation
Deep-fried crusts were prepared using a model system [17]. Batters were deep-fried using a stainless steel device that holds two aluminum cups (64 mm diameter, 28 mL volume). Each cup was covered by gauze with a mesh diameter of 0.18 mm and a wire diameter of 0.14 mm. The gauze was coated with Teflon to prevent the crust from caking to the gauze during frying, in which 2 samples can be fried simultaneously. Each crust model was prepared by depositing first 4 mL of batter on Teflon-coated gauze that was placed above a cup containing 5 g of silica gel. Finally breading (Pars Bryank, Mashhad) was added to the batter. The presence of a core is essential to simulate the crust formation of a real product. Water evaporating from the core during frying plays an important role in crust formation. This vapor will have an effect on crust temperature. Conditioned silica powder provided an ideal core, moisture content was easily adjusted, and properties of the powder were highly reproducible. Silica is inert, thus, apart from the moisture evaporating, no other reactions are taking place during frying. Khorasan Kurdish cheese is a traditional Iranian white cheese made from raw ewes’ milk. It moisture content is almost 65%. The moisture content of the silica powder was set by storing the powder on a flat surface in a germinator (Altus 400-Iran, 375litet) to simulate the wet core characteristic of deep-fried battered Kurdish cheese nugget, in 22˚C for at least two days. Crust models were prepared by frying in refined sunflower oil (Nina, Iran) due to its high smoking point, in a thermostatically temperature controlled fryer (Black & Decker, Type 01) for 60, 120 and 180 seconds at 190˚C. Crust models were separated from the gauze. After frying, the crusts could be separated easily from the gauze for further analysis. All examinations of the crust models were measured 30 min after frying.
2.3. Moisture Content Analysis
Moisture content analysis was performed according to the procedure described in the AOAC (1984) [19]. The crust models were dried in a conventional oven (Memmert, 154 Beschickung loading, model 100 - 800) at 105˚C for 24 h. The samples were cooled in desiccators and moisture contents were determined by difference in weight in terms of dry basis.
2.4. Fat Extraction
Fat content determination was carried out by soxhlet extraction technique using AOAC (1990) [20]. The dried samples used for moisture content determination were subsequently ground using a blender. The ground sample (2 - 4 g) was weighed with an electronic balance (TR- 4102D, Denver Instrument Co., Denver, CO) and placed in a thimble. Fat was extracted in solvent extractor using petroleum ether (Extra pure, ET0091) during a period of 6 h. The thimbles were further dried at 105˚C for 60 min to remove residue solvent and moisture. Then the thimbles were cooled in a desicator and subsequently weighed. The oil contents were obtained in terms of dry basis.
2.5. Color
Color of crust models was measured using an image processing technique as following:
1) A computer vision system generally consists of four basic components: illumination, a camera, computer hardware, and software. In this research, sample illumination was achieved with four fluorescent lights (Opple, 8 W, model: MX396-Y82, 60 cm in length) with a color index (Ra) close to 95%. The illuminating lights were placed in a wooden box, 45 cm above the sample and at the angle of 45˚ with sample plane to give a uniform light intensity over the crust models sample [21]. The interior walls of the wooden box were painted black to minimize background light. So that stabilization the lighting system, it was switched on for about 30 min prior to acquiring images. A color digital camera (Canon EOS 1000D, Taiwan) with lens focal length 35 mm was located vertically at a distance of 25 cm from the sample. The angle between the camera lens axis and lightening sources was around 45˚. The iris was operated in manual mode, with the lens aperture of 4, ISO 200 and speed 1/80 s (no zoom, no flash) to achieve high uniformity and repeatability. Images were captured with the mentioned digital camera at 2592 × 3888 pixels and connected to the USB port of a computer. Canon Digital Camera Solution Software (Canon Utilities Zoom Browser EX Version 6.1.1) was used to acquire the images in the computer in JPEG format.
2) Image preprocessing: Improvement of background’s contrast of images and segmentation (to separate the true images of the bell peppers from background) was performed using Adobe Photoshop (Adobe, v.8.0).
3) Alteration of RGB chromatic space into L*a*b* units: Since the L*a*b* color is device independent and providing consistent color regardless of the input or output, the images taken were converted into L*a*b* units. In the L*a*b* space, the color perception is uniform, and therefore, the Euclidean distance between two colors is almost in agreement with the color difference perceived by the human eye [22]. The net color difference (ΔE) was calculated with the relation:
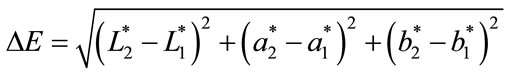
where L* is referred to as the lightness or luminance, while a* is defined along the axis of red-green, and b* is defined along the axis of yellow-blue. A positive value of a* indicates that red is the dominant color, while a negative value suggests the dominance of green. The same applies the b* component on the yellow-blue axis, a positive value indicates that yellow is dominant, while a negative value suggests the dominance of blue [23]. Where, 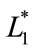 ,
, 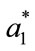 and
and 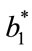 represented the readings before frying (batter), and
represented the readings before frying (batter), and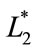 ,
, 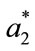 and
and 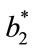 represented the individual readings at any frying time. In this study, the image analysis was managed using ImageJ software (National Institutes Health, Bethesda, Md, USA) version 1.40 g.
represented the individual readings at any frying time. In this study, the image analysis was managed using ImageJ software (National Institutes Health, Bethesda, Md, USA) version 1.40 g.
2.6. Hardness
Texture of the samples was determined in terms of hardness. Hardness of the crust models were measured 30 min after frying, using a texture analyzer (QTS25 CNS Farnell, UK) interfaced to a personal computer. A Probe ball was attached to the instrument for the penetration test. The test settings were: test speed 120 mm/min, trigger force 5 g, travel distance of the probe 7 mm. The crusts were placed on a horizontal flat plate with 60 mm slot aperture below the place. Hardness was defined as the peak force for this penetration.
2.7. Statistical Analysis
Frying experiments were replicated three times under each experimental condition. Data obtained from analysis were assessed by ANOVA (Analysis of Variance) to determine the significant differences between the effects of frying time, chitosan and white egg on quality parameters of the crust models by using the statistical software program Minitab for Windows (Version 14) to find out which parameters are significant for the specified quality parameter. Duncan’s multiple comparison tests were applied to determine the difference among the means. The level of statistical significance was determined at 95% probability.
3. Results and Discussion
3.1. Moisture Content
There was a significant difference (p < 0.05) between moisture content of crust models 30 min after frying (table 1). The moisture content of treated and control with increasing frying time decreases. Similar pattern was reported by other authors [24,25]. According to the Figure 1, Moisture content W10, w5 and control were 29.607 ± 3.800, 21.908 ± 0.480 and 20.526 ± 4.168 in 60 s frying time respectively. Reduced moisture loss could be related to thermal gelation and the film-forming ability of white egg [26]. Ovalbumin which constitutes more than half of white egg protein by weight is the only fraction that contains free sulfhydryl (SH) groups. By heating, white egg film-forming solutions had greater concentrations of surface SH groups than unheated solutions [27].
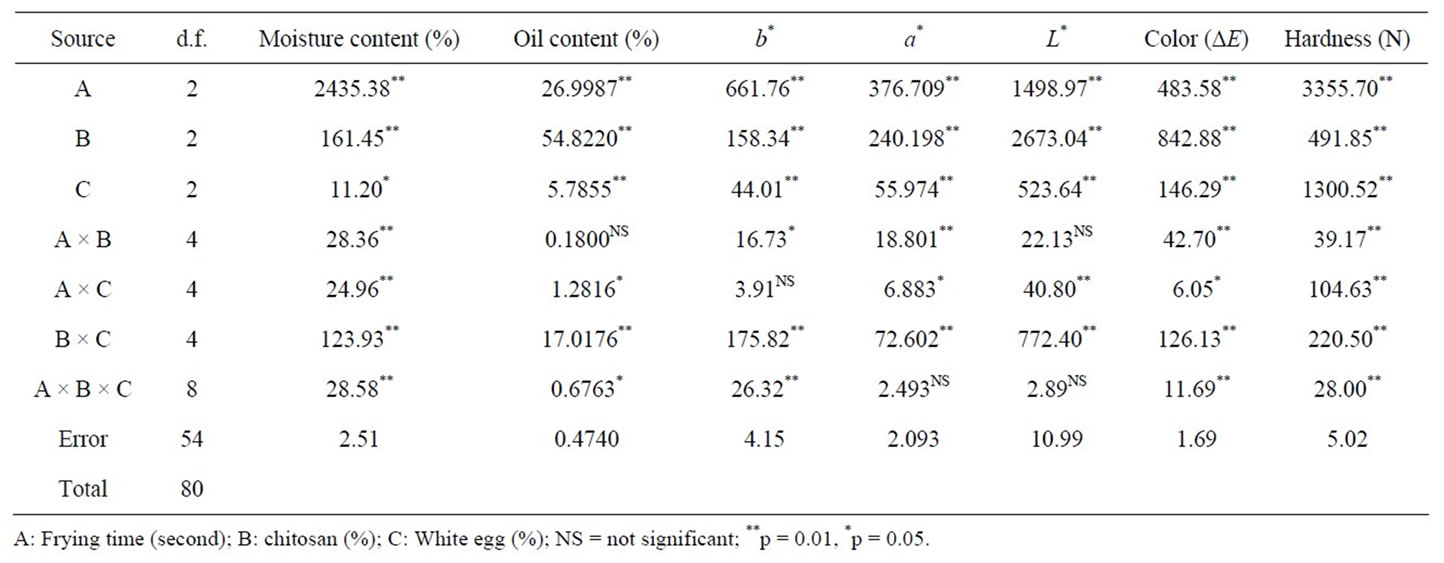
Table 1. Analysis of variance results (mean squares values).
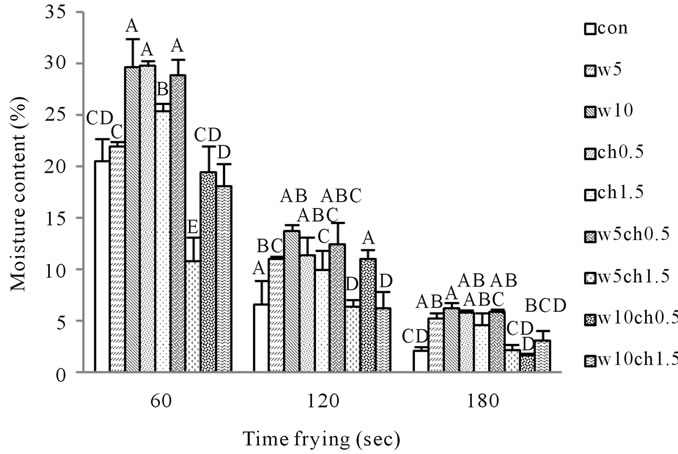
Figure 1. Effect of frying time on moisture content of crust models at 190˚C contain Different percentages of chitosan and white egg, and both. Con: control batter; w5: batter contain 5% egg white; w10: batter contain 10% egg white; ch0.5: batter contain 0.5% chitosan; ch1.5: batter contain 1.5% chitosan; w5ch0.5: batter contain 5% egg white and 0.5% chitosan; w5ch1.5: batter contain 5% egg white and 1.5% chitosan; w10ch0.5: batter contain 10% egg white and 0.5% chitosan; w10ch1.5: batter contain 10% egg white and 1.5% chitosan.
The increased surface SH concentration is important for white egg film formation, because, upon exposure to air, the SH groups are converted to intermolecular S-S bonds through oxidation and sulfhydryl-disulfide interchange reactions [15,28]. Therefore formed film make the hard crust that prevents water loss. The results showed that crust models with chitosan had less moisture content compared to crust models including white egg (Figure 1). Moisture content of ch0.5, ch1.5 and control were 29.734 ± 0.514, 25.296 ± 0.785 and 20.526 ± 4.168 in 60 s time of frying respectively. In the study carried out by Wu et al. (2000), they used CH coating (2%) as a barrier to control moisture loss and lipid oxidation in precooked beef patties and found that CH coating reduced the moisture loss but was not effective in controlling lipid oxidation [29]. Batter formulations Contain both white egg and chitosan, their ability to retain moisture of less than or equal to the control sample (Figure 1). Because of interaction between chitosan and white egg, reduced their ability to forming films.
3.2. Fat Content
The fat content was dependent on the frying time. Breading portion seemed to absorb more of the frying oil with longer residence time in the fryer of the frying temperature. Other authors also reported higher oil uptake with increasing frying time [30]. This could be attributed to the porous structure which might have facilitated the transport of oil and moisture simultaneously. Moisture content is an important factor in determining oil uptake during deep fat frying. Water evaporation creates cavities during frying. These capillary pores work as pathways in the food and subsequently filled by oil [24]. There was an opposite relationship between moisture loss and oil uptake, so higher oil content corresponded with lower moisture content [24,31]. The effect of frying time, chitosan and white egg were significant (p < 0.05) in controlling oil content during deep fat frying (Table 1).The fat content of crust models of ch0.5, ch1.5 and control were 5.611 ± 0.419, 6.674 ± 0.040 and 6.915 ± 1.515 respectively (Figure 2) and also w10, w5 and control samples had 3.647 ± 1.103, 4.236 ± 0.254 and 6.915 ± 1.515 fat content after 60 s of frying respectively (Figure 2). Egg albumen reduced the oil content significantly, but yielded softer products [32]. All combinations of chitosan and white egg resulted in significantly lower fat content of crust models as compared to the control except for samples ch1.5w10 and ch1.5w5 (Figure 2).
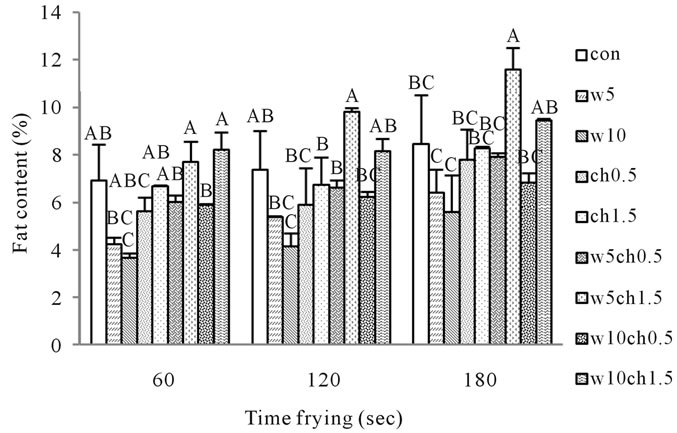
Figure 2. Effect of frying time on fat content of crust models at 190˚C contain Different percentages of chitosan and white egg, and both. Con: control batter; w5: batter contain 5% egg white; w10: batter contain 10% egg white; ch0.5: batter contain 0.5% chitosan; ch1.5: batter contain 1.5% chitosan; w5ch0.5: batter contain 5% egg white and 0.5% chitosan; w5ch1.5: batter contain 5% egg white and 1.5% chitosan; w10ch0.5: batter contain 10% egg white and 0.5% chitosan; W10ch1.5: batter contain 10% egg white and 1.5% chitosan.
3.3. Color
Coated foods develop a golden color, between yellow and brown, when they are fried. This is a characteristic that consumers normally consider very attractive. During frying, the appearance of dark notes tends to be avoided. A light golden tone is the benchmark color for determining the end of the final frying process. The lightness (L*) and yellowness (b*) of the crust models decreased with frying time as observed in Table 2. While redness (a*) increased significantly with frying time. Krokida et al. (2001) reported different results on fried potatoes [33]. They found that the lightness of potato strips increased during frying. The color of deep-fat fried chicken nuggets was studied by Dogan et al. (2005a), they found that as frying time increased, L* values decreased and the a* value increased [34].
Redness is not a desirable color in fried food products in general [33] while yellowness is a desirable color in such products. Batter formulations containing white egg were lightest. (Table 2). Mohamed et al. (1988) observed that the addition of ovalbumin to a batter improved crispness and color owing to the amine groups present in proteins participating in the Maillard reaction. Chitosan content in coating solutions increased, the brownish color increased in coated samples. These may be due to browning reaction of polysaccharide with protein complex when it was heated at high temperature [35]. Crust models containing combination both of white egg and chitosan had the darkest color. This could be attributed to the browning occurring due to the presence of reducing Polysaccharide in the breading portion .These Polysaccharide combine with amines from the protein ingredients of the batter system to from a combination that undergoes non-enzymatic browning known as the Maillard reaction. As shown in table 2 Color difference (ΔE) increased during frying time. Results showed that crust models treated with ch0.5w5 had the highest ΔE in 60, 120 and 180 s of frying, 19.436 ± 1.466, 30.497 ± 0.335 and 35.488 ± 1.359 respectively. As expected, highest ΔE were observed at higher frying time.
3.4. Hardness
In breaded or battered products, one of the main texture characteristics is crispness/crunchiness. It is a primary criterion of sensory quality because it largely determines their acceptability. The crispness that the external layer acquires during frying depends on its initial composition and on the frying temperature and time. During the frying process, it is dehydrated until it provides a crisp texture in the outer part while protecting the tenderness of the inner part [36]. Crispness of the product was represented by the maximum force values encountered during a puncture test. The effect of temperature on the maximum force values was significant (p < 0.01) (Table 1). The obtained results for hardness and the mean separation are given in table 2. Hardness of samples containing white egg was equal or lower than control. A significant decrease in the peak force of the fried coating pieces was obtained when dextrin or dried egg was added to the batter mixes, indicating reduced hardness of the fried samples [22] and also [37] explained that egg contains albumin, which helps to bind the breading/batter to the product contributing to batter stability. But hardness of samples containing chitosan wer the highest (table 2). Hardness increased significantly with frying time. Ateba & Mittal (1994) found that the hardness in the texture increased linearly with frying time like the observations made in this study. Moisture loss, protein denaturation and starch gelatininzation have been reported as the major factors influencing texture changes in the batter and crust [37].
4. Conclusion
The major advantages of working with the crust model are the better reproducibility of the crusts, the ease by which crusts can be separated from the core, and the possibility of varying batter/core ratio independent of batter viscosity. This greatly facilitates studying the effect of ingredient and processing variations on the resulting snacks with a moist interior and dry crust. In the study addition of different percentage of chitosan and white egg to the batter formulations was examined during deep
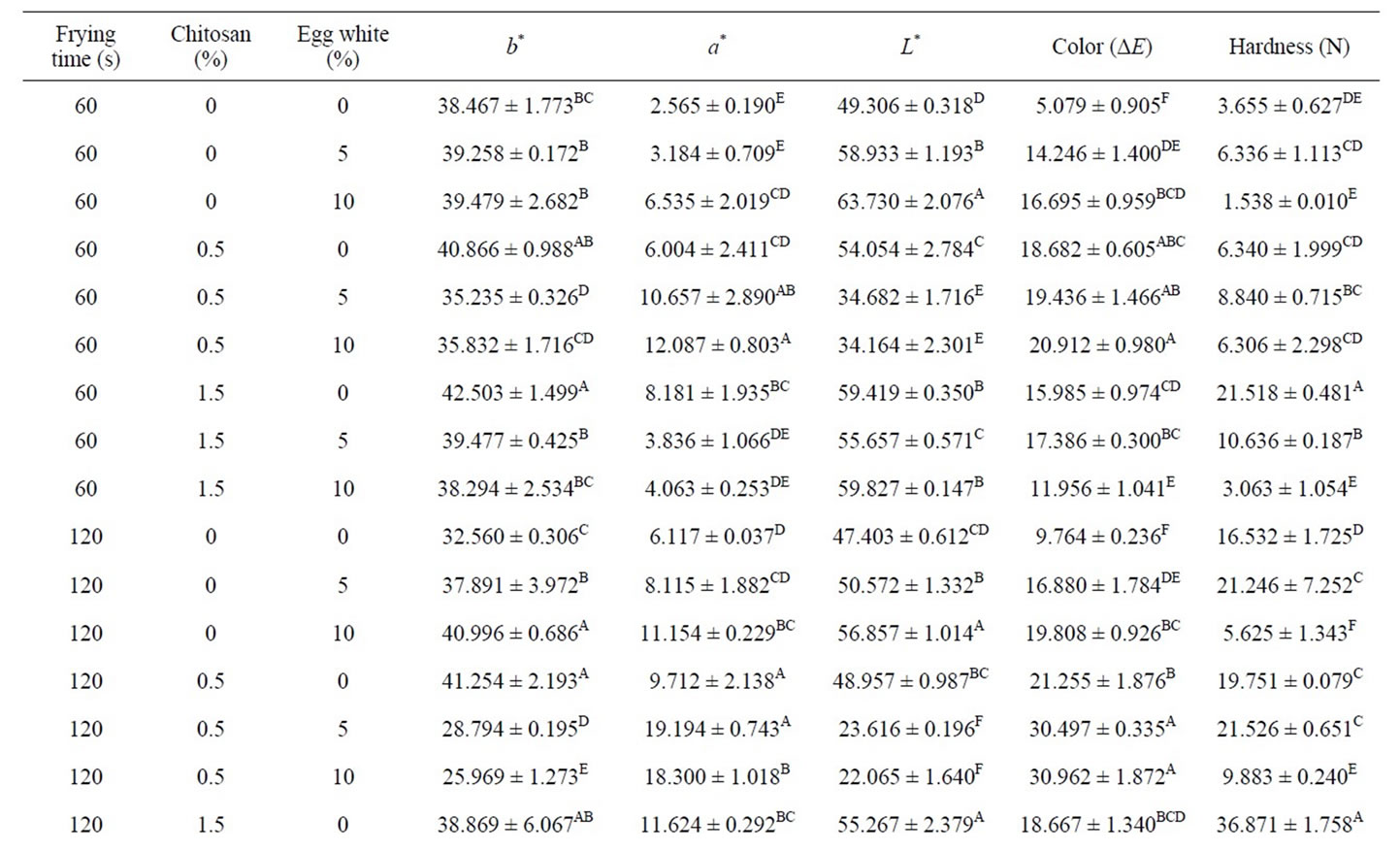
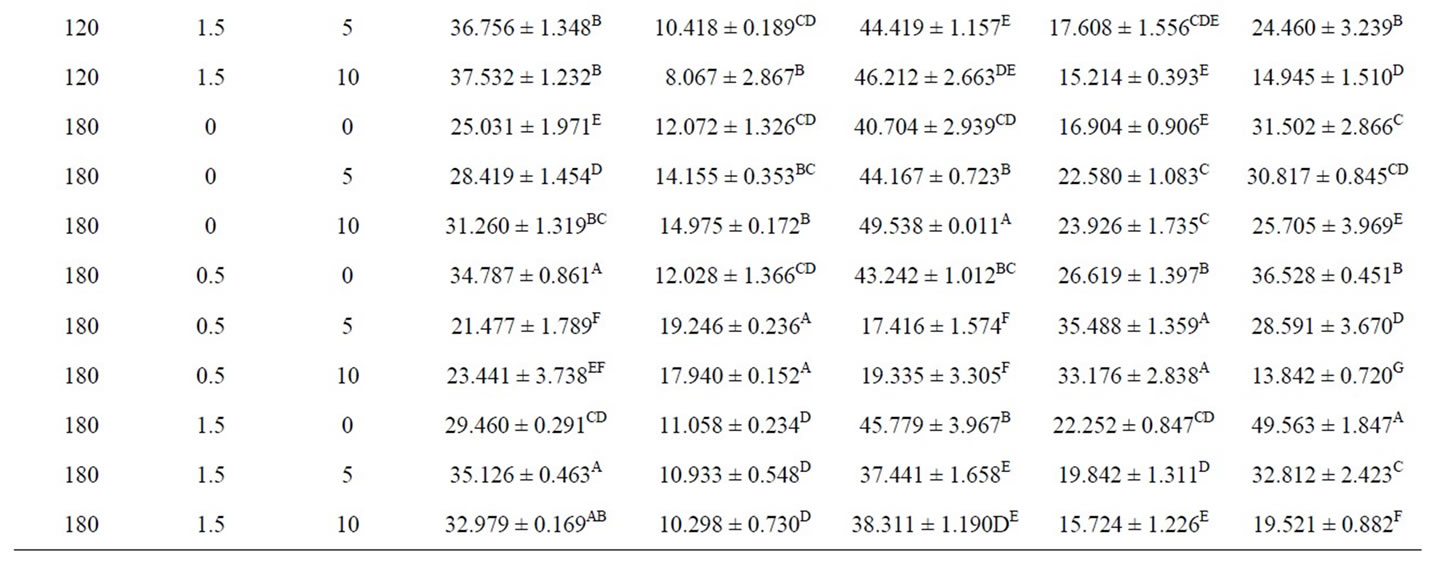
Table 2. Average values color and hardness of fried at different processing conditions.
fat frying of crusts model. White egg provided lighter colored samples with higher L* and lower a* values in frying. It retained more moisture. Samples containing chitosan had the highest hardness. The result showed that batter formulation had a significant effect on moisture and fat content, color and hardness.
REFERENCES
- S. Chen, H. Chen, Y. Chao and R. Lin, “Effect of Batter Formula on Qualities of Deep-Fat and Microwave Fried Fish Nuggets,” Journal of Food Engineering, Vol. 95, No. 2, 2009, pp. 359-364. doi:10.1016/j.jfoodeng.2009.05.016
- W. Usawakesmanee, M. S. Chinnan, P. Wuttijumnong, A. Jangchud and N. Raksakulthai, “Effect of Edible Coating Ingredients Incorporated into Predusting Mix on Moisture Content, Fat Content and Consumer Acceptability of Fried Breaded Product,” Songklanakarin Journal of Science and Technology, Vol. 30, 2008, pp. 25-34.
- S. M. Fiszman and A. Salvador, “Recent Developments in Coating Batters,” Trends in Food Science and Technology, Vol. 14, No. 10, 2003, pp. 399-407. doi:10.1016/S0924-2244(03)00153-5
- W. Usawakesmanee, P. Wuttijumnong and M. Chinnan, “The Effects of Edible Coating Ingredient as a Barrier to Moisture and Fat of Fried Breaded Potato,” Kasetsart Jurnal, Nutural Science, Vol. 39, 2005, pp. 98-108.
- S. Albert and G. S. Mittal, “Comparative Evaluation of Edible Coating to Reduce Fat Uptake in a Deep-Fried Cereal Product,” Food Research International, Vol. 35, No. 5, 2002, pp. 445-458. doi:10.1016/S0963-9969(01)00139-9
- F. D. Nasiri, M. Mohebbi, F. T. Yazdi and M. H. Khodaparast, “Effects of Soy and Corn Flour Addition on Batter Rheology and Quality of Deep Fat-Fried Shrimp Nuggets,” Food and Bioprocess Technology, 2010.
- P. Mallikarjunan, M. S. Chinnan, V. M. Balasbramaniam and R. D. Phillip, “Edible Coating for Deep Fat Frying of Starchy Products,” Lebensmittel-Wissenshaft und-Technolgie, Vol. 30, No. 7, 1997, pp. 709-714
- K. I. Holownia, M. S. Chinnan, M. C. Erickson and P. Mallikarjunan, “Quality Evaluation of Edible Film Coated Chicken Strips and Frying Oils,” Journal of Food Science, Vol. 65, No. 6, 2000, pp. 1087-1090. doi:10.1111/j.1365-2621.2000.tb09423.x
- R. Baixauli, T. Sanz, A. Salvador and S. M. Fiszman, “Effect of the Addition of Dextrin or Dried Egg on the Rheological and Textural Properties of Batters for Fried Foods,” Food Hydrocolloids, Vol. 17, No. 3, 2003, pp. 305-310. doi:10.1016/S0268-005X(02)00091-7
- K. W. Lin and J. Y. Chao, “Quality Characteristics of Reduced-Fat Chinese-Style Sausage as Related to Chitosan’s Molecular Weight,” Meat Science, Vol. 59, No. 4, 2001, pp. 343-351. doi:10.1016/S0309-1740(01)00084-5
- G. J. Tsai and W. H. Su, “Antibacterial Activity of Shrimp Chitosan against Escherichia coli,” Journal of Food Protection, Vol. 62, No. 3, 1999, pp. 239-243.
- S. H. Lee, “Effect of Chitosan on Emulsifying Capacity of Egg Yolk,” Journal of Korean Society of Food and Nutrition, Vol. 25, No. 1, 1996, pp. 118-122.
- J. St. Angelo and J. R. Vercellotti, “Inhibition of Warmed over Flavour and Preserving of Uncured Meat Containing Materials,” US Patent, Vol. 4, 1989, pp. 556, 871.
- S. A. Woodward, “Egg Protein Gels. In Food Gels,” In: P. Harris, Ed., Elsevier Science Publishers Ltd., Essex, 1990, pp. 175-199.
- Y. Mine, “Sulfhydryl Groups Changes in Heat-Induced Soluble Egg White Aggregates in Relation to Molecular Size,” Journal of Food Science, Vol. 58, 1992, pp. 254- 255. doi:10.1111/j.1365-2621.1992.tb05468.x
- T. H. Gennadios, C. L. M. Weller and J. M. Krochta, “Edible Coatings and Films Based on Proteins,” Edible Coatings and Films to Improve Food Quality, 1994.
- J. E. Visser, H. de Beukelaer, R. J. Hamer and T. A. Van Vliet, “New Device for Studying the Deep-Frying Behavior of Batters and Resulting Crust Properties,” Cereal Chemistry, Vol. 85, No. 3, 2008, pp. 417-424. doi:10.1094/CCHEM-85-3-0417
- T. Primo-Martín, D. W. Sanz, A. Steringa, S. M. Salvador, A. Fiszman and T. Van Vliet, “Performance of Cellulose Derivatives in Deep-Fried Battered Snacks: Oil Barrier and Crispy Properties,” Journal of Food Hydrocolloids, Vol. 24, No. 8, 2010, pp. 702-708. doi:10.1016/j.foodhyd.2010.04.013
- “AOAC Official Methods of Analysis,” Association of Official Analytical Chemists, Washington, 1990.
- “AOAC Official Methods of Analysis,” 14th Edition, Association of Official Analytical Chemists, Washington, 1984.
- R. Quevedo, J. Aguilera and F. Pedreschi, “Color of Salmon Fillets by Computer Vision and Sensory Panel,” Food and Bioprocess Technology, Vol. 3, 2009, pp. 637- 643. doi:10.1007/s11947-008-0106-6
- F. Pedreschi, J. León, D. Mery, P. Moyano, R. Pedreschi and K. Kaack, “Granby K Color Development and Acrylamide Content of Pre-Dried Potato Chips,” Journal of Food Engineering, Vol. 79, No. 3, 2007, pp. 786-793. doi:10.1016/j.jfoodeng.2006.03.001
- D. W. Sun, “Computer Vision Technology for Food Quality Evaluation,” Elsevier Inc., New York, 2008.
- M. Ngadi, Y. Li and S. Oluka, “Quality Changes in Chicken Nuggets Fried in Oils with Different Degrees of Hydrogenatation,” Lebensmittel-Wissenshaft und-Technology, Vol. 40, 2007, pp. 1784-1791.
- M. Mariscal and P. Bouchon, “Comparison between Atmospheric and Vacuum Frying of Apple Slices,” Food Chemistry, Vol. 107, No. 4, 2008, pp. 1561-1569. doi:10.1016/j.foodchem.2007.09.031
- S. Mohamed, N. A. Hamid and M. A. Hamid, “Food Components Affecting the Oil Absorption and Crispness of Fried Batter,” Journal of Science and Food Agriculture, Vol. 78, 1988, pp. 39-45. doi:10.1002/(SICI)1097-0010(199809)78:1<39::AID-JSFA82>3.0.CO;2-G
- A. Handa, T. H. Gennadios, G. W. Froning, N. Kuroda and M. A. Hanna, “Tensile, Solubility, and Electrophoretic Properties of Egg White Films as Affected by Surface Sulfhydryl Groups,” Journal of Food Science, Vol. 64, No. 1, 1999, pp. 82-85. doi:10.1111/j.1365-2621.1999.tb09865.x
- S. Hayakawa and S. Nakai, “Contribution of Hydrophobicity, Net Charge and Sulfhydryl Groups to Thermal Properties of Ovalbumin,” Canadian Institute of Food Science and Technology Journal, Vol. 18, 1985, pp. 290- 295.
- Y. Wu, J. W. Rhim, C. L. Weller, F. Hamouz, S. Cuppett and M. Schnepf, “Moisture Loss and Lipid Oxidation for Precooked Beef Patties Stored in Edible Coatings and Films,” Journal of Food Science, Vol. 65, No. 2, 2000, pp. 300-304. doi:10.1111/j.1365-2621.2000.tb15997.x
- L. S. Kassama and M. O. Ngadi, “Development of Pores and Pore Size Distribution in Chiken Meat during Deep Fat Frying,” Canadian Society of Agricultural Engineers, Mansonville, Vol. 1, 2000, p. 322.
- N. Akdeniz, S. Sahin and G. F. Sumnu, “Unctionality of Batters Containing Different Gums for Deep-Fat Frying of Carrot Slices,” Journal of Food Engineering, Vol. 75, No. 4, 2006, pp. 522-526. doi:10.1016/j.jfoodeng.2005.04.035
- S. F. Dogan, S. Sahin and G. Sumnu, “Effect of Containing Different Protein Types on the Quality of deep Fat-Fried Chicken Nuggets,” European Food Research and Technology, Vol. 220, 2005, pp. 502-508. doi:10.1007/s00217-004-1099-7
- M. K. Krokida, V. Oreopoulou, Z. B. Maroulis and D. Marinos-Kouris, “Effects of Pre-Drying on Quality of French Fries,” Journal of Food Engineering, Vol. 49, No. 4, 2001, pp. 347-354. doi:10.1016/S0260-8774(00)00233-8
- S. F. Dogan, S. Sahin and G. Sumnu, “Effects of Soy and Rice Flour Addition on Batter Rheology and Quality of Deep Fat-Fried Chicken Nuggets,” Journal of Food Engineering, Vol. 71, 2005, pp. 127-132. doi:10.1016/j.jfoodeng.2004.10.028
- R. A. A. Muzzareli, “Encyclopaedia of Polymer Science and Engineering,” Wiley, New York, Vol. 3, 1985, pp. 430-440.
- B. Altunakar, S. Sahin and G. Sumnu, “Functionality of Batters Containing Different Starch Types for Deep-Fat Frying of Chicken Nuggets,” European Food Research and Technology, Vol. 218, No. 4, 2004, pp. 318-322. doi:10.1007/s00217-003-0854-5
- R. Loewe, “Role of Ingredients in Batter Systems,” Cereal Food World, Vol. 38, No. 9, 1993, pp. 673-677.
NOTES
*Corresponding author.

