American Journal of Plant Sciences
Vol.5 No.9(2014), Article ID:44558,10 pages DOI:10.4236/ajps.2014.59137
Chemical Composition and Allelopathic Activity of Parthenium hysterophorus and Ambrosia polystachya Weeds Essential Oils
Cíntia Alvarenga S. F. de Miranda1, Maria das G. Cardoso1*, Maria Laene M. de Carvalho2, Ana Cristina S. Figueiredo3, David L. Nelson4, Christiane M. de Oliveira1, Marcos de S. Gomes1, Juliana de Andrade1, Josefina A. de Souza1, Luiz R. Marques de Albuquerque1
1Department of Chemistry, Federal University of Lavras, Lavras, Brazil
2Department of Agricuture, Federal University of Lavras, Lavras, Brazil
3Universidade de Lisboa, Faculdade de Ciências de Lisboa, DBV, Instituto de Biotecnologia e Bioengenharia, Centro de Biotecnologia Vegetal, C2, Campo Grande, Lisboa, Portugal
4Federal University of the Jequitinhonha and Mucuri Valleys, Diamantina, Brazil
Email: *mcardoso@dqi.ufla.br
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 1 February 2014; revised 10 March 2014; accepted 24 March 2014
ABSTRACT
The volatile constituents of the essential oils from leaves from the weed plants Parthenium hysterophorus and Ambrosia polystachya were identified and quantified by GLC-MS and GLC. Allelopathic activities were determined by methods that evaluated the volatile effects and the direct application of these essential oils on the seed germination and seedling vigor of lettuce. We identified 27 compounds in the essential oil from P. hysterophorus, and the main constituents were germacrene-D (35.9%), trans-β-ocimene (8.5%) and β-myrcene (7.6%). In the essential oil from A. polystachya, 40 constituents were identified and the principal compounds were germacrene-D (29.3%), trans-β-ocimene (13.6%) and β-caryophyllene (9.8%). In both methods, the essential oil from A. polystachya presented a greater potential for reducing seed germination and seedling vigor in lettuce than the essential oil from P. hysterophorus, and this activity might be attributed to its higher content of monoterpenes.
Keywords: Volatile Oils; Allelopathy; Asteraceae

1. Introduction
Weeds are harmful to agriculture because they decrease the productivity and quality of agricultural products. In recent decades, invasive plants have mostly been eliminated by applying synthetic herbicides, which may cause environmental damage with appreciable toxic effects to living organisms, including human beings [1] . The abuse of these synthetic herbicides has increased the resistance of invasive herbs. This fact makes the use of bioherbicides a promising, economically and environmentally sustainable alternative [2] .
These allelochemicals of natural origin can be produced by all types of plant tissues. They act by different mechanisms such as volatilization, root exudation and decomposition of residues [3] . The weeds are noted for their allelopathic potential. They compete for resources with adjacent plants, with consequent inhibition of growth and development of cultures [4] .
Among these weeds, some species of Asteraceae, such as Parthenium hysterophorus and Ambrosia polystachya, stand out for their high allelopathic potential for crops. P. hysterophorus, commonly known as ragweed, is a plant native to the Americas and is one of the ten worst invasive species for agriculture, causing huge losses in many parts of world [5] . The Ambrosia genus includes over thirty weed species found mainly in the Americas. These species invade cultivated fields and reduce seed production. A. polystachya, known as ambrosia or cravorona americana, is one of these species [4] . Given the potential phytotoxicity of these invasive species, extracts and volatile compounds of P. hysterophorus and Ambrosia species have recently had their allelopathic potentials demonstrated [4] -[9] .
Some weeds can produce essential oils, which are complex mixtures of different secondary metabolites derived from various plant tissues (leaves, flowers, seeds, rhizomes, bark), among which we highlight terpenoids that may have biological functions, such as allelopathy, that are essential for survival and adaptation of the plant to the environment [10] . Given the fact that herbicides present substantial problems for agriculture, human health, and the environment and the fact that weeds stand out as a species with allelopathic activity, as well as a potential source of essential oils, the aim of this work was to chemically characterize the essential oils extracted from fresh leaves of P. hysterophorus and A. polystachya and to determine their potential allelopathic activities by assessing the effect of direct contact and contact with the volatile components on seed germination and the seedling vigor of lettuce (Lactuca sativa L.) by in vitro screening assays.
2. Material and Methods
2.1. Collection of Plant Material
The young leaves (rib and limb) of adult Parthenium hysterophorus and Ambrosia polystachya plants in the flowering stage were collected on the Campus of the Universidade Federal de Lavras (UFLA) in Lavras, MG, Brazil (21˚14'S, longitude 45˚00'W Gr and 918 m altitude). The species were collected in the early morning of days without precipitation in February 2012, the species identification was kindly performed by Doctor Mariana Esteves Mansanares, Department of Biology of UFLA and exsiccates were deposited in the ESAL-UFLA Herbarium under records numbering 26944 and 26948, respectively.
2.2. Extraction of the Essential Oils
The essential oils from fresh leaves were extracted by hydrodistillation using a modified Clevenger apparatus adapted to a round-bottom flask with a capacity of 4 L over a period of 2 hours [11] -[14] . The hidrolact was centrifuged for 5 minutes at 965.36g, and the oils were separated with a Pasteur pipette, transferred to amber glass bottles and stored at 4˚C.
2.3. Determination of Moisture and Yields of the Extractions
The determination of the moisture content of the fresh leaves was performed in parallel with the extraction of the essential oils [15] . In a 250 mL, round-bottom flask coupled to a Dean-Stark trap, was placed 5 g of chopped fresh leaves in 70 ml of cyclohexane. The flask was heated on a heating mantle for 2 h at a temperature of 81 ± 1˚C, and the quantity of water distilled was measured. The yields of the essential oils were expressed on a dry weight basis (DWB).
2.4. Identification of Essential Oil Constituents
The gas-liquid chromatography-mass spectrometric (GLC-MS) analyses were performed using a Perkin Elmer Autosystem XL gas chromatograph equipped with a fused-silica DB-1 column (30 m x 0.25 mm ID; film thickness, 0.25 m; J & W Scientific Inc.) coupled to a Perkin Elmer Turbomass mass spectrometer (software version 4.1). The oven temperature was programmed from 45 to 175˚C at 3˚C/minute and then at 15˚C/minute to 300˚C, where the temperature was maintained for 10 minutes. The temperature of the transfer line was 280˚C; the temperature of the ionization chamber was 220˚C; the carrier gas was helium, adjusted to a linear velocity of 30 cm/second; and the split ratio was 1:40. The identity of the compounds was determined by comparison of their retention indices relative to C9-C21 n-alkanes, along with the comparison of the mass spectra with those of standard commercial and reference compounds present in oils existing in the laboratory and with a library of mass spectra developed in the laboratory of the Centre for Departamento de Biologia Vegetal, Faculdade de Ciências, Universidade de Lisboa, Lisboa, Portugal [16] .
2.5. Quantification of Constituents of the Essential Oils
The essential oils were analyzed by gas-liquid chromatography (GLC) on a Perkin Elmer 8700 gas chromatograph equipped with two flame ionization detectors (FID), a system for data processing and an injector. The chromatograph possessed two columns of different polarity, with the following characteristics: a fused silica capillary column containing the DB-1 methylsilicone stationary phase (30 m × 0.25 mm ID; film thickness, 0.25 m; J & W Scientific Inc.) and a fused silica capillary column containing the DB-17HT phenylmethylsilicone stationary phase (30 m × 0.25 mm id). The oven temperature was programmed from 45˚C to 175˚C at 3˚C/minute, followed by an increase at 15˚C/minute to 300˚C, where the temperature was held for 10 minutes. The temperatures of the injector and detector were 290˚C and 280˚C, respectively. Hydrogen was used as the carrier gas with a flow rate of 30 cm/second, and the split ratio was 1:50. The percentage composition of the oils was determined by integration of peak areas without using correction factors. The values given represent the average of two injections.
2.6. Allelopathic Activity of the Essential Oils
Two bioassays were conducted to evaluate the allelopathic potential of the essential oils: the first assessed the effect of the volatile components and the second assessed the direct contact of the oil with the seeds on the germination of lettuce seeds (Regina SF 3500 cultivar). The 1% stock solutions were prepared with 0.5 mL of each essential oil emulsified in Tween 80 in a 1:1 (v/v) ratio and dissolved in distilled water. The remaining concentrations (0.1 and 0 01% v/v) were prepared by dilution. A solution of 1.0% v/v Tween 80 in water was used as a control [10] . The solutions of the essential oils were added at the beginning of the bioassay. During the experiments, only distilled water was added when necessary [17] . In both bioassays, seeds were packed in gerbox-type acrylic boxes (dimensions 11 × 11 × 4 cm). Two sheets of sterilized blotting paper were employed as substrates, where 50 seeds were distributed, totaling 200 seeds per treatment. The seeds were maintained in a growth chamber at a temperature of 20 ± 1˚C and a photoperiod of 12 h. In the direct contact method, the blotting paper was soaked with equivalent amounts of solutions of the different concentrations of the essential oil up to 2.5 times its dry weight [18] . The evaluation of the effect of the volatile components was determined by adding distilled water to the paper substrate in amounts equivalent to 2.5 times its dry weight [18] . Three-milliliter portions of the solutions of essential oils were placed on two sheets of filter paper affixed to the incubator lid, thereby avoiding direct contact of the solutions with the seeds [17] .
Germination was monitored for seven days, starting from the implementation of the test, with daily counts of lettuce seedlings, and the results were expressed in percentage of normal seedlings [18] . Seed vigor was determined from the following variables: first count of germination, which consisted of the evaluation of normal seedlings on the fourth day after sowing [18] ; Germination Speed Index (GSI) [19] ; average lengths of root and seedling shoots on the seventh day, which were measured with the aid of a millimeter-scale ruler and the means were expressed in centimeters [18] ; determination of the dry mass of seedlings in an incubator at 60˚C in Kraft paper bags to constant weight. After this period, the weighing and determination of the means by repetition were preformed, the results being expressed in grams [20] .
2.7. Statistical Analysis
The experimental design for both methods was completely randomized in a 4x2 factorial scheme (four concentrations and two essential oils), with four replications. The essential oils were compared, because these species were of the same botanical family, the Asteraceae. The different methods were not compared because they are known to have different chemical mechanisms. Significant factors from the F test (p < 0.05) were submitted to the Scott-Knott test of means (5%) for the determination of the models. The data were analyzed using the Statistical Analysis System Variance for Balanced Data—Sisvar [21] . The results were plotted in bar graphs with the values of the variables versus the concentrations analyzed using the GraphPad Prism software version 5.0.
3. Results and Discussion
The yields of essential oils obtained by hydrodistillation of the leaves from P. hysterophorus and A. polystachya were 0.04 and 0.33% (w/w), respectively. In the essential oil extracted from P. hysterophorus, 31 components were identified, amounting to 76% of its constitution, and 58 compounds (97%, Table 1) were identified in the essential oil extracted from A. polystachya. The identified components and their relative proportions are listed in Table 1 in order of elution on a DB-1 column.
Despite the large differences in the chemical compositions of the essential oils isolated from the two species, the sesquiterpene hydrocarbon fraction was the principal fraction in both cases, reaching 47% of the essential oil from P. hysterophorus and 49% of that from A. polystachya. Similarly, the monoterpene hydrocarbon fraction was the second most important fraction in these essential oils (19% of the essential oil from P. hysterophorus and 33% of that from A. polystachya). Germacrene (36%), trans-β-ocimene (9%) and β-myrcene (8%) were the dominant components isolated from the essential oil of P. hysterophorus. Germacrene (29%) and trans-β-ocimene (14%), followed by β-caryophyllene (10%), were also the principal compounds in the essential oil from A. polystachya.
Few studies on the chemical composition of these essential oils have previously been described in the literature. Bornyl acetate (9.15%), geraniol (7.53%) and phenylacetonitrile were identified as the major constituents of the essential oil extracted from the leaves of P. hysterophorus collected in India [22] . The authors identified 78% of the constituents of the essential oil, a total of 63 chemical compounds. With respect to A. polystachya, the essential oil from the flowers of this species was evaluated and determined that it is probably produced in the secretory ducts of the male and female flowers [23] . They identified 14 chemical compounds. The variation in the chemical compositions of the essential oils from different botanical species and the essential oils extracted from the same botanical species from different countries is related to the collection site, the vegetative cycle and edaphoclimatic factors [24] .
The effect of volatile components of the essential oils from P. hysterophorus and A. polystachya on seed germination and seedling vigor of lettuce was evaluated. An increase in the concentration of both essential oils did not significantly affect the first germination count or the total germination of the lettuce, whose mean values were 88.5% and 95% for the essential oil from A. polystachya and 94% and 96.5% for the oil from P. hysterophorus, respectively. The concentration of the essential oil from P. hysterophorus did not influence the GSI, the dry weight and the lengths of the aerial parts and roots. These variables had values of 75.6, 0.0370 g, 17.05 mm and 38.13 mm, respectively. The volatile components of the essential oil from P. hysterophorus did not have any allelopathic effect, unlike the essential oil from A. polystachya, which presented dose-dependent effects for the GSI, the dry matter and the measurements of the aerial parts and roots of lettuce seedlings. A 1% concentration of the essential oil from A. polystachya produced the largest reductions in the variables, with mean values of 47.4 for the GSI; 0.0328 g of dry matter, 12.48 mm for the length of the shoots and 30.85 mm for the root. The control presented values of 75.7, 0.0363 g, 17.88 mm and 38.43 mm, respectively, for the same variables. The responses of the GSI and lengths of the aerial parts and roots of seedlings to essential oil concentrations of 0.01% and 0.1% did not differ statistically. There was no statistical difference between the concentrations with regard to dry weight, although the weights were different from that of the control (Figure 1).
The essential oils from A. polystachya and P. hysterophorus in direct contact with the lettuce seeds did not affect the first count (fourth day) and total germination (seventh day) responses at the concentrations of 0.01% and 0.1%, with mean responses of 94% and 90.5% for A. polystachya and 92.5% and 94.5% for P. hysterophorus. Only the 1% concentration differed statistically from the other concentrations, with significantly lower mean values for these variables. The use of the essential oil from A. polystachya reduced them to 85% and 89%, while that of P. hysterophorus caused a reduction of 82.5% and 75.5%, respectively. This same response pattern
Table 1. Percentage composition of the essential oils obtained from the leaves of P. hysterophorus and A. polystachya harvested during flowering.
RI: Retention Indices relative to a series of n-alkanes C9-C21 on a DB-1 column. t: trace (<0.05%). *Compounds identified only on the basis of the mass spectra.
was observed when the influence of direct contact with the essential oil from P. hysterophorus was analyzed, wherein application of the 1% concentration also caused a reduction in the responses of the GSI variable from 76.51 to 42.76, the dry matter was reduced from 0.0375 g to 0.0265 g, the length of the shoots from 15.55 mm to 11.58 mm and that of the root from 42.95 mm to 21.85 mm. The direct contact with the essential oil from A. polystachya also caused allelopathic effects at concentrations lower than that of the essential oil from P. hysterophorus. There were no significant differences from the control variables for GSI, dry matter and length of the aerial parts and roots of lettuce seedlings. The application of the 1% concentrations of the essential oil from A. polystachya significantly decreased the mean values of the GSI from 76.28 to 49.64, the dry weight from 0.0338 g to 0.0373 g, the length of the shoots from 15.98 mm to 10.28 mm and the length of the roots from 42.35 mm to 31.6 mm (Figure 2).
The similar response profiles observed for the essential oil from A. polystachya in both methods can be explained by the fact that it delayed the initial germination of lettuce seeds (up to the third day of the experiment), which explains the constant values for the first germination and final germination and the decrease in the responses of the other variables so that higher concentrations of this oil were required. When the volatile components of the essential oil from P. hysterophorus were evaluated, no phytotoxicity was observed. Direct contact of this oil with the substrate affected the germination and seedling vigor only at the highest concentration tested (1%).
The effects of the volatile compounds from fresh flowers and leaves of P. hysterophorus on the growth of the Echinochloa crus-galli (L.) Beauv. and Digitaria sanguinalis L. grass species in transparent boxes were evaluated [25] . The volatiles were extracted from the headspace, and the constituents were identified by GC-MS. The principal volatile constituents identified from the flowers were the monoterpenes myrcene (56.67%) and

Figure 1. Mean values for the first germination count, total germination, GSI, seedling dry mass, and lengths of shoots and roots as a function of the concentrations of the essential oils from A. polystachya and P. hysterophorus, evaluated according to the effect of the volatile components. Means followed by the same uppercase letter for the comparison between the essential oils analyzed and the same lowercase letter for the comparison between concentrations of each essential oil do not differ significantly at 5% probability by the Scott-Knott test.
ocimene (26.28%), while those from the leaves were myrcene (28.07%) and α-pinene (14.52%). The volatile components from the flowers inhibited the growth of E. crus-galli and D. sanguinalis seedlings, while those from the leaves only affected the development of D. sanguinalis seedlings. The inhibition of growth of these species was justified by the higher content of volatile monoterpenes in the oil from the flowers.
The allelopathic activity of the essential oil from basil (Ocimum basilicum) on seed germination and the initial growth of lettuce, tomato and lemon balm seedlings was evaluated [26] . The major compounds found in this oil were the monoterpenes linalool and geraniol. The phytotoxic effects of the essential oil from basil were
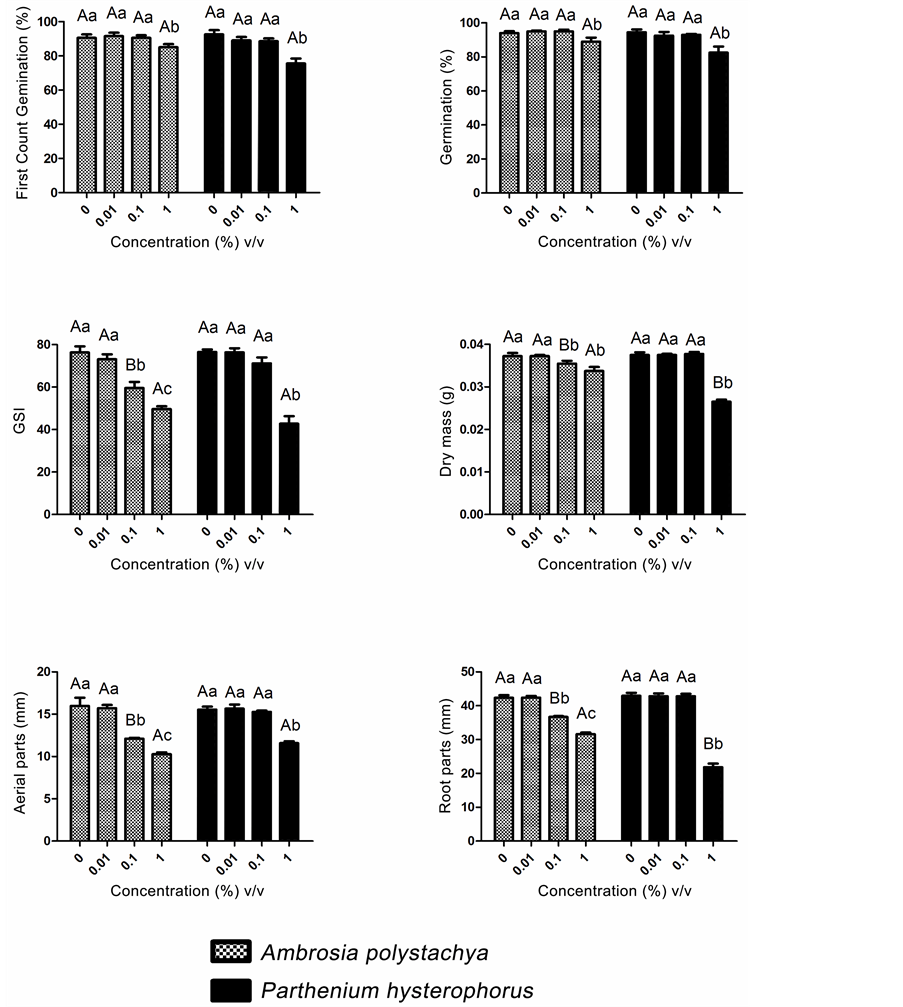
Figure 2. Mean values for the first germination count, total germination, GSI, seedling dry mass, length of shoot and length of root versus the concentrations of the essential oils from A. polystachya and P. hysterophorus, evaluated by the direct contact method. Means followed by the same uppercase letter for comparison between the essential oils analyzed and the same lowercase letter for comparison between concentrations of each essential oil do not differ significantly at 5% probability by the Scott-Knott test.
attributed to the monoterpene content, a fact that corroborated the findings of our experimento. This ability of monoterpenes to affect the germination and growth of plants can be explained by the ability of these compounds to cause morphological and physiological changes in plants, including the inhibition of the respiratory chain in the isolated mitochondria and mitosis, alteration of the integrity of cell membranes, deterioration of cuticular waxes, increased persperation, and lipid peroxidative damage to microtubes [26] .
The essential oils were extracted from invasive species belonging to the same botanical family, the Asteraceae; both oils contained germacrene-D as the major constituent (29.3% and 35.9% for A. polystachya and P. hysterophorus, respectively) and had different profiles for allelopathic activities. The allelopathic activity is rarely a result of a single constituent, generally being associated with a group of compounds, so the potential difference between allelopathic essential oils of these weeds can be associated with the presence of monoterpenes, a group of chemical compounds that stand out for their allelopathic potential by influencing seed germination and the vigor of seedlings [10] . This fact explains the different patterns of phytotoxic responses to the essential oils in the present study because, in both methods, the essential oil from A. polystachya produced more significant allelopathic effects than those of the essential oil from P. hysterophorus. This difference can probably be explained by the higher concentration of monoterpenes present in the essential oil from A. polystachya (34.2%) than in the oil from P. hysterophorus (20.1%).
4. Conclusion
The principal constituents found in the essential oils from A. polystachya were germacrene D (29.3%), trans- β-ocimene (13.6%) and β-caryophyllene (9.8%), whereas germacrene-D (35.9%), trans-β-ocimene (8.5%) and β-myrcene (7.6%) were found in the oil from P. hysterophorus. In both methods used in evaluating the allelopathic activity of the essential oils, that from A. polystachya presented a greater potential for reducing seed germination and seedling vigor of lettuce than the essential oil from P. hysterophorus. This difference can possibly be attributed to the higher content of monoterpenes in the essential oil from A. polystachya.
Acknowledgements
The authors acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Pest‑OE/EQB/LA0023/2011 for financial support and the scholarships awarded. D.L. Nelson was the recipient of a PVNS fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES).
References
- Singh, H.P., Batish, D.R., Setia, N. and Kohli, R.K. (2005) Herbicidal Activity of Volatile Oils from Eucalyptus citriodora against Parthenium hysterophorus. Annals of Applied Biology, 146, 89-94. http://dx.doi.org/10.1111/j.1744-7348.2005.04018.x
- Batish, D.R., Singh, H.P., Kohli, R.K. and Kaur, S. (2008) Eucalyptus Essential Oil as a Natural Pesticide. Forest Ecology and Management, 256, 2166-2174. http://dx.doi.org/10.1016/j.foreco.2008.08.008
- Weston, L.A. and Duke, S.O. (2003) Weed and Crop Allelopathy. Critical Reviews in Plant Sciences, 22, 367-389. http://dx.doi.org/10.1080/713610861
- Kong, C.H., Wang, P. and Xu, X.H. (2007) Allelopathic Interference of Ambrosia trifida with Wheat (Triticum aestivum). Agriculture, Ecosystems & Environment, 19, 416-420. http://dx.doi.org/10.1016/j.agee.2006.07.014
- Srivastava, J., Raghava, N. and Raghava, R.P. (2001) Allelopathic Potential of Parthenium to Reduce Water Absorption in Germinating Cowpea Seeds. Indian Journal Science Research, 2, 59-65.
- Chen, Y., Wang, J., Wu, X., Sun, J. and Yang, N. (2011) Allelopathic Effects of Parthenium hysterophorus L. Volatiles and Its Chemical Components. Allelopathy Journal, 27, 217-223.
- Khan, N., Naveed, K., Hussain, Z. and Khan, S.A. (2012) Assessment of Allelopathic Effects of Parthenium (Parthenium hysterophorus L.) Plant Parts on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.) Cultivars. Pakistan Journal of Weed Science Research, 18, 39-50.
- Patracchini, C., Vidotto, F. and Ferrero, A. (2011) Common Ragweed (Ambrosia artemisiifolia) Growth as Affected by Plant Density and Clipping. Weed Technology, 25, 268-276. http://dx.doi.org/10.1614/WT-D-09-00070.1
- Wakjira, M., Berecha, G. and Tulu, S. (2009) Allelopathic Effects of an Invasive Alien Weed Parthenium hysterophorus L. Compost on Lettuce Germination and Growth. African Journal of Agricultural Research, 4, 1325-1330.
- Silva, C.B., et al. (2009) Composição química e atividade alelopática do óleo volátil de Hydrocotyle bonariensis Lam (Araliaceae). Quimica Nova, 32, 2373-2376. http://dx.doi.org/10.1590/S0100-40422009000900026
- Agencia Nacional de Vigilancia Sanitaria (2000) Farmacopéia Brasileira.4th Edition, Parte I, Ateneu, São Paulo, 2-7.
- Gomes, M.S., et al. (2013) Multivariate Analysis of the Essential Oil Components of the Genus Citrus and Their Antifungal Activity. Científica, Jaboticabal, 41, 111-121.
- Teixeira, M.L., et al. (2012) Citrumelo Swingle: Caracterização química, atividade antioxidante e antifúngica dos óleos essenciais das cascas frescas e secas. Magistra, 24, 194-203.
- Gomes, M.S., et al. (2014) Use of Essential Oils of the Genus Citrus as Biocidal Agents. American Journal of Plant Sciences, 5, 299-305. http://dx.doi.org/ 10.4236/ajps.2014.53041
- Pimentel, F.A., et al. (2006) A Convenient Method for the Determination of Moisture in Aromatic Plants. Quimica Nova, 29, 373-375. http://dx.doi.org/10.1590/S0100-40422006000200031
- Mendes, M.D., et al. (2011) ISSR Molecular Characterization and Leaf Volatiles Analysis of Pittosporum undulatum Vent. Naturalized in the Azores Archipelago (Portugal). Industrial Crops and Products, 33, 710-719. http://dx.doi.org/10.1016/j.indcrop.2011.01.010
- Souza Filho, A.P.S., Guilhon, G.M.S.P., Zoghbi, M.G.B. and Cunha, R.L. (2009) Análise comparativa do potencial alelopático do extrato hidroalcoólico e do óleo essencial de folhas de cipó-d’alho (Bignoniaceae). Planta Daninha, 27, 647-653. http://dx.doi.org/10.1590/S0100-83582009000400002
- Brasil, Ministério da Agricultura e da Reforma Agrária (2009) Regras para Análise de Sementes. SNDA/DNDU/CLU, Brasília.
- Maguire, J.D. (1962) Speed of Germination Aid in Selection and Evaluation for Seedling Emergence and Vigor. Crop Science, 2, 176-177. http://dx.doi.org/10.2135/cropsci1962.0011183X000200020033x
- Krzyzanowski, F.C., Vieira, R.D. and França Neto, J.B. (1999) Vigor de sementes: Conceitos e testes. ABRATES, Londrina.
- Ferreira, D.F. (2011) Sisvar: A Computer Statistical Analysis System. Ciência e Agrotecnologia, 35, 1039-1042. http://dx.doi.org/10.1590/S1413-70542011000600001
- Chowdhury, A.R. (2002) Chemical Examination of Essential Oil from Parthenium hysterophorus L. Leaves. Indian Perfumer, 46, 45-48.
- Bonzani, J.S. and Grotta, A.S. (1970) Anatomy and Essential Oil of Parts of Ambrosia polystachya. Revista de Farmácia e Bioquímica da Universidade São Paulo, 8, 47-52.
- Gobbo-Neto, L. and Lopes, N.P. (2007) Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quimica Nova, 30, 374-381. http://dx.doi.org/10.1590/S0100-40422007000200026
- Rosado, L.D.S., et al. (2009) Alelopatia do extrato aquoso e do óleo essencial de folhas do manjericão “Maria Bonita” na germinação de alface, tomate e melissa. Revista Brasileira de Plantas Medicinais, 11, 422-428. http://dx.doi.org/10.1590/S1516-05722009000400010
NOTES

*Corresponding author.


