American Journal of Plant Sciences
Vol.4 No.8(2013), Article ID:35467,12 pages DOI:10.4236/ajps.2013.48198
Antioxidant Activity and Phytochemical Screening of Crude Endophytes Extracts of Tabebuia argentea Bur. & K. Sch.

1Department of Biotechnology, Shridevi Institute of Engineering & Technology, Tumkur, India; 2Department of Studies in Biochemistry, Mangalore University, PG Center, Cauvery Campus, Madikeri, India.
Email: *dravidateja07@gmail.com
Copyright © 2013 M. Govindappa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 8th, 2013; revised June 9th, 2013; accepted July 11th, 2013
Keywords: Tabebuia argentea; Endophytes; Antioxidant Activity; Phytochemicals
ABSTRACT
The aim of this study is to investigate in vitro antioxidant activities and the phytochemical screening endophytes. Seven different endophytic fungi were isolated from different parts of the plant and their extracts subjected to know antioxidant properties and phytochemical screening. Phytochemical analysis revealed the presence of tannins, flavonoids, steroids, alkaloids, phenols and proteins from different solvents extracts of different endophytes. The antioxidant activity was evaluated by six separated methods: scavenging of free radical DPPH, FRAP, TBA, superoxide radical, FTC and iron methods. All seven different endophytes yielded almost all phytochemicals in methanol extracts which were tested. The endophytes A. niger, Penicillium sp. and Trichoderma sp. have shown potential in vitro antioxidant activities. Further work is needful to isolate the exact compound which is responsible for antioxidant activity and biophysical characterization will be done in the future.
1. Introduction
Tabebuia argentea (Bignoniacae) is a large and yellow flowering tree. Tabebuia sp. has proven to be rich source of many organic compounds, especially, of phenolic and poliophenolic nature. Such substances have been classified as cytotoxic, antimicrobial and antifungal [1,2] by the presence of anthroquinone compounds. Many natural naphthoquinone and lapachol have extensively been used to control cancer and they have ability to interfere with topoisomerase enzymes, which are critical for DNA replication in cells [3]. The antitumor activity of lapachol may be due to its interaction with nucleic acids and the interaction of the naphthoquinone moiety between base pairs of the DNA helix occurs with subsequent inhibition of DNA replication and RNA synthesis [4]. Other biological activities of lapachol are antimetastatic activity [5], antimicrobial and antifungal activity [6], antiviral activity [7], anti-inflammatory [8], antiparasitic activity [4], leishmanicidal activity [9] and molluscicidal activity [10].
Increasing evidence indicates that Reactive Oxygen Species (ROS), (for example O2 and OH) and free radical mediated reactions can cause oxidative damage to biomolecules (for example lipids, proteins and DNA), eventually contributing to aging, cancer, atherosclerosis and other neurodegenerative disorders [11]. Antioxidants are thought to be highly effective in the management of ROS mediated tissue impairments. Many antioxidant compounds possess anti-athrosclerosis, antitumor, antimutagenic, antibacterial, or antiviral activities to lesser or greater extent [12,13]. Many endopytic fungi have shown antioxidant activity. The present study was aimed to isolate different endophytes, identity phytochemicals from different endophytes and different endophytic extracts were used for antioxidant properties. The present investigation data was different from earlier works [14], we used other endophytes what they haven’t noticed and we tried six different types of antioxidant activity methods for comparison.
2. Materials and Methods
2.1. Plant Material
Plant materials were collected from different places of Karnataka, India during April, 2011. The collected plant was authenticated from the Department of Botany, ManasaGangotri, University of Mysore, Mysore, Karnataka, India and Government Ayurvedic College, Mysore, and herbarium was prepared.
2.2. Isolation and Identification of Endophytic Fungi
The protocol for isolation follow methods used in other endophytestudy [15] with slight modifications. The plant tissues were washed in running tap water for one hour. Fifty segments of leaves from each plant were cut into 5 mm 2 pieces, including a vein (25 samples) and intervein (25 samples). 25 segments of branches were then cut randomly to a length of 5 mm. Endophytic fungi were isolated from the bark of the plant (25 segments). Twenty five segments (5 mm long) were cut from the stems and the roots. The total 150 segments of plant material were treated by triple surface sterilization techniques. Each piece was then placed on malt extract agar (malt extract 20 g/l), rose Bengal (0.033 g/l), chloromphenicol (50 mg/l; agar 15 g/l). All plates were incubated at 26˚C ± 2˚C until mycelium grew out; hyphal tips were cut and transferred to potato dextrose agar (PDA). Half strength PDA was used for subculture and stock culture. Identification was based on colony, hyphal morphology of the fungal cultures and characteristics of the spores [16,17].
2.3. Fungal Cultivation and Extraction of Metabolites
The fungal endophytes were cultivated on Potato Dextrose Broth by placing agar blocks of actively growing pure culture (3 mm diameter) in 250 ml Erlenmeyer flasks containing 100 ml of the medium. The flasks were incubated at 26˚C ± 2˚C for 1 week, with periodical shaking at 150 rpm. After the incubation period, the cultures were taken out and filtered through sterile cheesecloth to remove the mycelia mats. The fungal metabolites from different endophytic mycelial mats were extracted by using ethyl acetate solvent extraction. Equal volume of the filtrate and solvents were taken in a separating funnel and was shaken vigorously for 10 min. The solution was then allowed to stand, the cell mass got separated and the solvent so obtained, was collected. All solvents were evaporated and the resultant compound was dried in vacuum evaporator using MgSO4 to yield the crude extract [14].
2.4. Phytochemical Screening
Investigation on the phytochemical screening of Tabebuiaargentea extract revealed the presence of saponins, steroids, terpenoids, glycosides, alkaloids, tannins, anthraquinoneand flavonoids.
2.4.1. Test for Saponins
1 ml aliquots of the various plant extracts were combined with 5 ml water which is 60˚C, then, shaken for 2 min, as saponins are known to posses frothing activity, the volume of froth produced in this experiments was observed and recorded every 10 min for a period of 30 min [18].
2.4.2. Test for Steroids
1 ml of the respective plant extract was treated with three drops of acetic anhydride and one drop of concentrated sulphuric acid. A colour change from deep green, turning to brown indicated the presence of sterols [18].
2.4.3. Test for Anthraquinones
The Borntrager test was used for the detection of anthraquinones. 2 ml of the test sample was shaken with 4 ml of hexane. The upper lypophilic layer was separated and treated with 4 ml of dilute ammonia. If the lower layer changed to violet pink, it indicates the presence of anthraquinones [18].
2.4.4. Test for Cardiac Glycosides
1 ml of the sample solution was mixed with 1 ml of glacial acetic acid then treated with one drop of 5% methanolic ferric choride solution. 1 ml of concentrated sulphuric acid was carefully poured down the sides of test tube. The appearance of brownish ring between the two layers with lower layer turning blue green upon standing indicates the presence of cardiac glycosides.
2.4.5. Test for Tannins
1 ml of the sample solution was mixed with 1 ml vanillin hydrochloride reagent. Appearance of purple colour indicates the presence of tannins.
2.4.6. Test for Flavonoids (Magnesium Hydrochloride Reduction Test)
To the methanol extract, added 5 ml of 95% ethanol and few drops of conc. HCl. To this solution 0.5 g of magnesium turnings were added. Observance of pink coloration indicated the presence of flavonoids.
2.4.7. Test for Lapachol Identification
Dried endophytic extract and flower extract of T. argentea was extracted with ethyl acetate. 1 g of the endophytic and flower extract was re-crystallized in petroleum ether and benzene (80:60) and heated at 139˚C to 140˚C for 5 min. 2 ml of ferric chloride solution was added and observed for the color change [19].
2.5. Antioxidant Activities of the Endophytic Extracts
2.5.1. FRAP Assay
FRAP reagents was freshly prepared by mixing 25 ml acetate buffer (300 mM, pH 3.6), 0.5 mL 2,4,6-tris(2- pyridyl)-S-triazine (TPTZ) solution (10 Mm TPTZ in 40 Mm/I HCL) and 2.5 mL FeCl3 (2 Mm) water solution. Each sample (150 µl) (0.5 mg/ml) dissolved in methanol was added to 4.5 Ml of freshly prepared FRAP reagent and stirred. After 5 min, absorbance was measured at 593 nm, using FRAP working solution as blank [20]. A calibration curve of ferrous sulphate (100 to 1000 umol/l) was used and results were expressed in µmol Fe2+/mg dry weight extract. The relative activity of the sample was compared to L-ascorbic acid.
2.5.2. DPPH Radical Assay
The hydrogen atom or electron donation ability of the corresponding extracts and some pure compounds was measured from the bleaching of purple coloured methanol solution of DPPH. This spectrophotometric assay uses stable radical diphenylpicrylhydrazyl (DPPH) as a reagent [21] (Gulluce et al., 2006). Fifty microlitres of various concentrations of the extracts in methanol was added to 5 ml of a 0.004% methanol solution of DPPH. After a 30 min incubation period at room temperature the absorbance was read against s blank at 517 nm. Inhibition free radical DPPH in percent (I%) was calculated in the following way:
 where A blank is the absorbance of the control reaction (containing all reagents except the test compound), and A sample is the absorbance of the test compound. Extract concentration form the graph plotted inhibition (IC50) was calculated from the graph plotted against extract concentration. Synthetic antioxidant reagent butylatedhyroxytoulene (BHT) was used in triplicate.
where A blank is the absorbance of the control reaction (containing all reagents except the test compound), and A sample is the absorbance of the test compound. Extract concentration form the graph plotted inhibition (IC50) was calculated from the graph plotted against extract concentration. Synthetic antioxidant reagent butylatedhyroxytoulene (BHT) was used in triplicate.
2.6. Superoxide Radical Scavenging Activity
Superoxide radical 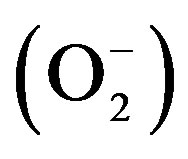 was generated from the photoreduction of riboflavin and was deducted by nitro blue tetrazolium dye (NBT) reduction method. Measurement of superoxide anion scavenging activity [22]. The assay mixture contained sample with 0.1 ml of Nitro blue tetrazolium (1.5 mM NBT) solution, 0.2 ml of EDTA (0.1 M EDTA), 0.05 ml riboflavin (0.12 mM) and 2.55 ml of phosphate buffer (0.067 M phosphate buffer). The control tubes were also set up where in DMSO was added instead of sample. The reaction mixture was illuminated for 30 min and the absorbance at 560 nm was measured against the control samples. Ascorbate was used as the reference compound. All the tests were performed in triplicate and the results averaged. The percentage inhibition was calculated by comparing the results of control and test samples.
was generated from the photoreduction of riboflavin and was deducted by nitro blue tetrazolium dye (NBT) reduction method. Measurement of superoxide anion scavenging activity [22]. The assay mixture contained sample with 0.1 ml of Nitro blue tetrazolium (1.5 mM NBT) solution, 0.2 ml of EDTA (0.1 M EDTA), 0.05 ml riboflavin (0.12 mM) and 2.55 ml of phosphate buffer (0.067 M phosphate buffer). The control tubes were also set up where in DMSO was added instead of sample. The reaction mixture was illuminated for 30 min and the absorbance at 560 nm was measured against the control samples. Ascorbate was used as the reference compound. All the tests were performed in triplicate and the results averaged. The percentage inhibition was calculated by comparing the results of control and test samples.
2.7. Iron Chelating Activity
The principle is based on the formation of O-Phenanthroline-Fe2+ complex and its disruption in the presence of chelating agents. The reaction mixture containing 1 ml of 0.05% O-Phenanthroline in methanol, 2 ml ferric chloride (200 μM) and 2 ml of various concentrations ranging from 10 to 1000 μg was incubated at room temperature for 10 min and the absorbance of the same wasmeasured at 510 nm. EDTA was used as a classical metal chelator [23]. The experiment was performed in triplicates.
2.8. Ferric Thiocyanate (FTC) Method
Different extracts (4 mg) and standards (4 mg; BHT, vitamin C and vitamin E) were mixed with 4 ml of absolute ethanol, 4.1 ml of 2.52% linoleic acid in absolute ethanol, 8 ml of 0.02 M phosphate buffer (pH 7.0) and 3.9 ml of distilled water. The mixture was placed at 40˚C (0.1 ml) and was then mixed with 9.7 ml of 75% (v/v) ethanol and 0.1 ml 30% ammonium thiocyanate. Three minutes after adding ferrous chloride (0.1 ml of 2 × 10−2 M ferrous chloride), the absorbance was measured at 500 nm in a spectrophotometer. This step was repeated every 24 h until the control reached its maximal absorbance value. The mixture without added sample was used as a control [24]. The inhibition of lipid peroxidation (%) was estimated by the following formula:
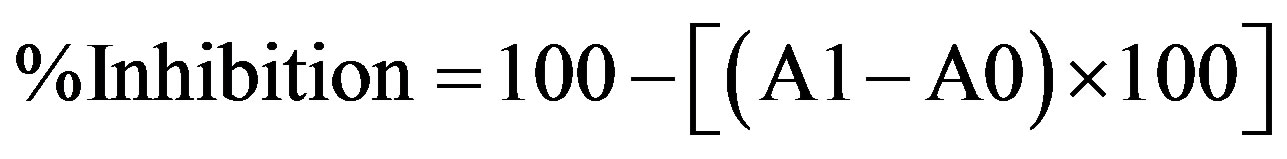 where A0 is the absorbance of the control and A1 is the absorbance of the sample extracts [25].
where A0 is the absorbance of the control and A1 is the absorbance of the sample extracts [25].
2.9. Thiobarbituric Acid (TBA) Method
Extracts (2 ml) and standard solutions (2 ml) on the final day (day 8) of the FTC assay were added to 1 ml of 20% aqueous trichloroacetic acid and 2 ml of 0.67% aqueous thiobarbituric acid. After boiling for 10 min, the samples were cooled. The tubes were centrifuged at 3000 rpm for 30 min. Absorbance of the supernatant was evaluated at 532 nm in a spectrophotometer [26]. The antioxidant activity was calculated by percentage of inhibition in this method as follows:
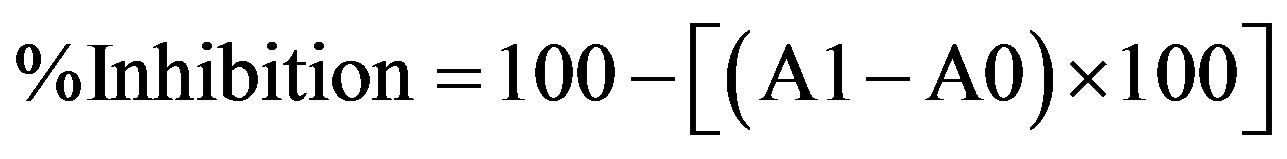
where A0 is the absorbance of the control and A1 is the absorbance of the sample extracts [25].
2.10. Free Radical Scavenging Activity
The ability of methanol and chloroform extracts of MC to scavenge 1,1-diphenyl-2-picryl-hydrazyl (DPPH) free radicals was estimated [27]. MC extracts (3 ml) with six different concentrations (15.62, 31.25, 62.5, 125, 250 and 500 μg/ml) were mixed with 1 ml of a 0.1 mM methanol solution of DPPH. The absorbance was measured by a spectrophotometer at 517 nm at 30 min intervals against a blank (pure ethanol). The percentage of radical scavenging activity was calculated using the following formula:

where A0 is the absorbance of the control and A1 is the absorbance of the sample extracts [25].
Lower absorbance values show higher free radical scavenging activity. Ascorbic acid was used as a reference standard in different concentrations (1.56, 3.12, 6.25, 12.5, 25 and 50 μg/ml). The 50% inhibitory concentration value (IC50) is indicated as the effective concentration of the sample that is required to scavenge 50% of the DPPH free radicals [27].
2.11. Determination of Total Phenolic Content
Total Phenolic Content (TPC) in endophytic extracts was determined using Folin-Ciocalteu’s colorimetric method. To 5 ml of 0.3% HCl in methanol/deionised water (60:40, v/v), 100 mg of the ethanol extract was added. From the resulting mixture (100 μl) was added to 2 ml of 2% aqueous sodium carbonate. The mixture was incubated for 2 min. To that 100 μl of 50% Folin-Ciocalteu’s reagent was added and incubated for 30 min, absorbance was measured at 750 nm against blank. The content of total phenol was calculated on the basis of the calibration curve of gallic acid and the results were expressed as mg of gallic acid equivalents (GAEs) per g of extract [28].
2.12. Flavonoid Determination
The fungal extract (250 μl) was mixed with distilled water (1.25 ml) and NaNO2 solution (5%, 75 μl). After 5 min theAlCl3 H2O solution (10%, 150 μl) was added. After 6 min, NaOH (1M, 500 μl) and distilled water (275 μl) were added to the mixture. The solution was mixed well and the intensityof the pink color was measured at 510 nm against blank. The content of flavonoid was calculated on the basis of the calibration curve of quercetin and the results were expressed as mg of quercetin equivalents per g of extract [29].
2.13. Statistical Analysis
Analysis of variance (ANOVA) was used to determine the significance of difference between treatment groups (p < 0.05). Means between treatment groups were compared for significance using Duncan’s new Multiple Range post test.
3. Results
3.1. Identification of Endophytes
The plant materials were collected from six different places of Tumkur district of Karnataka, India (Table 1). Seven different fungal species were reported from all incubated parts (leaves, bark, stem and root). A. niger, A. flavus, Penicillium sp., Rhizopus sp. and Fusarium sp. were presented in sample 1, 2, 3, 4, 5 and 6, where Rhizopus sp. was not reported from sample 5 and 6. The Alternaria sp. has reported from sample 3, Trichoderma sp. has showed presence in sample 4 and 5 (Table 2).
3.2. Phytochemical Screening
Phytochemical analysis of methanol solvent extracts of endophytes revealed the presence of saponins, phenol compounds, anthraquinones, flavonoids, steroids, cardiac glycosides and tannins. Extracts from cultures of all (seven different fungal species) endophytic fungi gave a wide variety of biological activities. Endophytes, A. niger, Penicillium sp., Trichoderma sp. and plant extracts has showed presence of all compounds (of saponins, phenolic compounds, anthraquinones, steroids, cardiac glycosides and tannins), whereas other endophytesalso have all the phytochemical except tannins. The presence of anthraquinones was observed in only threeendophytes (A. niger, Penicillium sp. and Trichoderma sp.) and flower extract (Table 3).

Table 1. Collection of plant material from different place.

Table 2. Isolation of endophytes from Tabebuia argentea on PDA media.
+ = Presence, − =Absent, Experiments were repeated thrice for each sample.
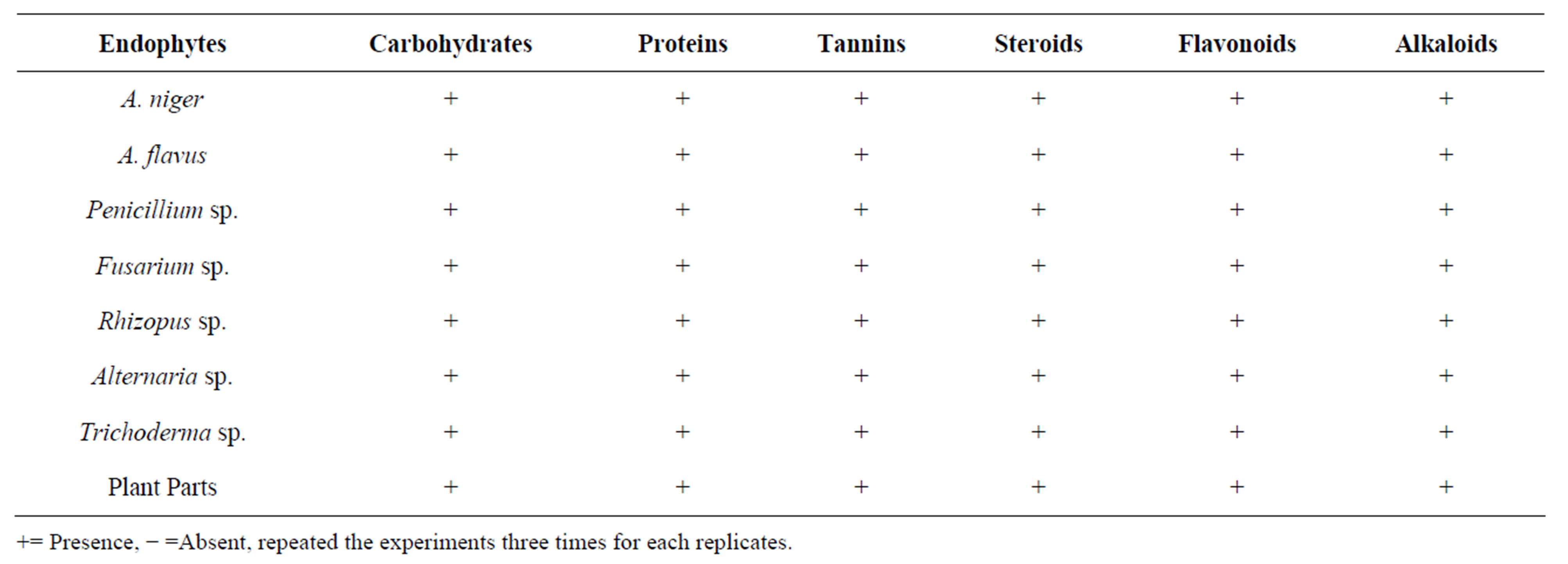
Table 3. Phytochemical analysis of endophytic extracts.
+= Presence, − =Absent, repeated the experiments three times for each replicates.
3.3. Lapachol Identification Test
1 g of the endophyte and flower extract was recrystallized in petroleum ether and benzene (80:60) and heated at 139˚C to 140˚C for 5 min. 2 ml of ferric chloride solution was added and observed for colour change. Yellow colour confirmed the presence of quinoned compounds (Naphthoquinone). Naphthoquinone was identified in A. niger, Penicillium sp. and Trichoderma sp. and in plant extract. Other endophytes did not show the presence of naphthoquinone presence (Table 4).
3.4. In Vitro Antioxidant Activity
Table 5 shows the absorbance and percentage of inhibition of all endophytic samples. The absorbance values of methanol extracts of A. niger, Penicillium sp. and Trichoderma sp. were significantly (P < 0.05) higher than the absorbance value of BHT and vitamin E. The percentage inhibition of methanolic extracts of A. niger, Penicillium sp. and Trichoderma sp. were significantly (P < 0/05) lower than BHT and Vitamin E.
Superoxide is highly reactive molecule that reacts with various substances produced through metabolic processes. Superoxidase dismutase enzymes present in aerobic and anaerobic organisms catalyses the breakdown of superoxide radical. Percentage scavenging of superoxide anion examined at different concentrations of methanol extract of three endophytes (125, 250, 500, and 1000) was depicted in Table 6. The percentage scavenging of suoeroxide radical surged with the enhanced concentration of endophytic extract. The maximum scavenging activity of endophytes extract and quercetin at 1000 µg/ml was found to be 96.48 ± 0.014 (A. niger), 83.84 ± 0.009 (Penicillium sp.) and 80.97 ± 0.022 (Trichoderma sp.) respectively. Superoxide scavenging ability of plant extract might primarily be due to the presence of flavonoids.
Iron is essential for life because it is required for oxygen transport, respiration and activity of many enzymes. However, iron is an extremely reactive metal and catalyzes oxidative changes in lipids, proteins and other cellular components. It causes lipid peroxidation through the reaction and decomposes the lipid hydroxide into peroxyl and alkoxyl radicals that can perpetuate the chain reactions. Iron biding capacity of methanol extracts three different endophytes and metal chelator EDTA at various concentrations (125, 250, 500, 1000 µg/ml) were examined and the values (Table 7). Maximum chelating of metal ions at 1000 µg/ml for endopytes extracts and
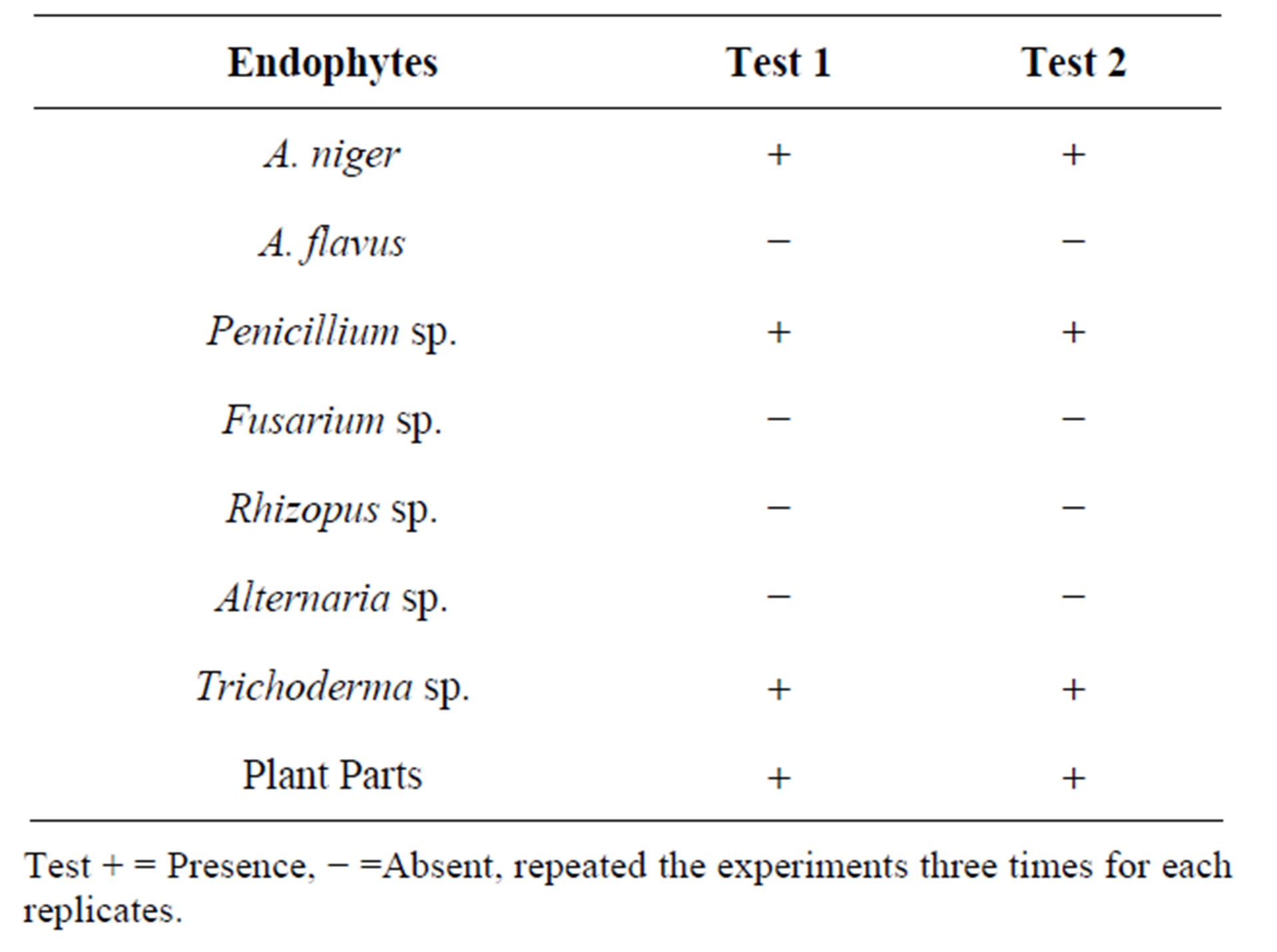
Table 4. Identification of lapachol from different endophytes using different procedures.
Test + = Presence, − =Absent, repeated the experiments three times for each replicates.
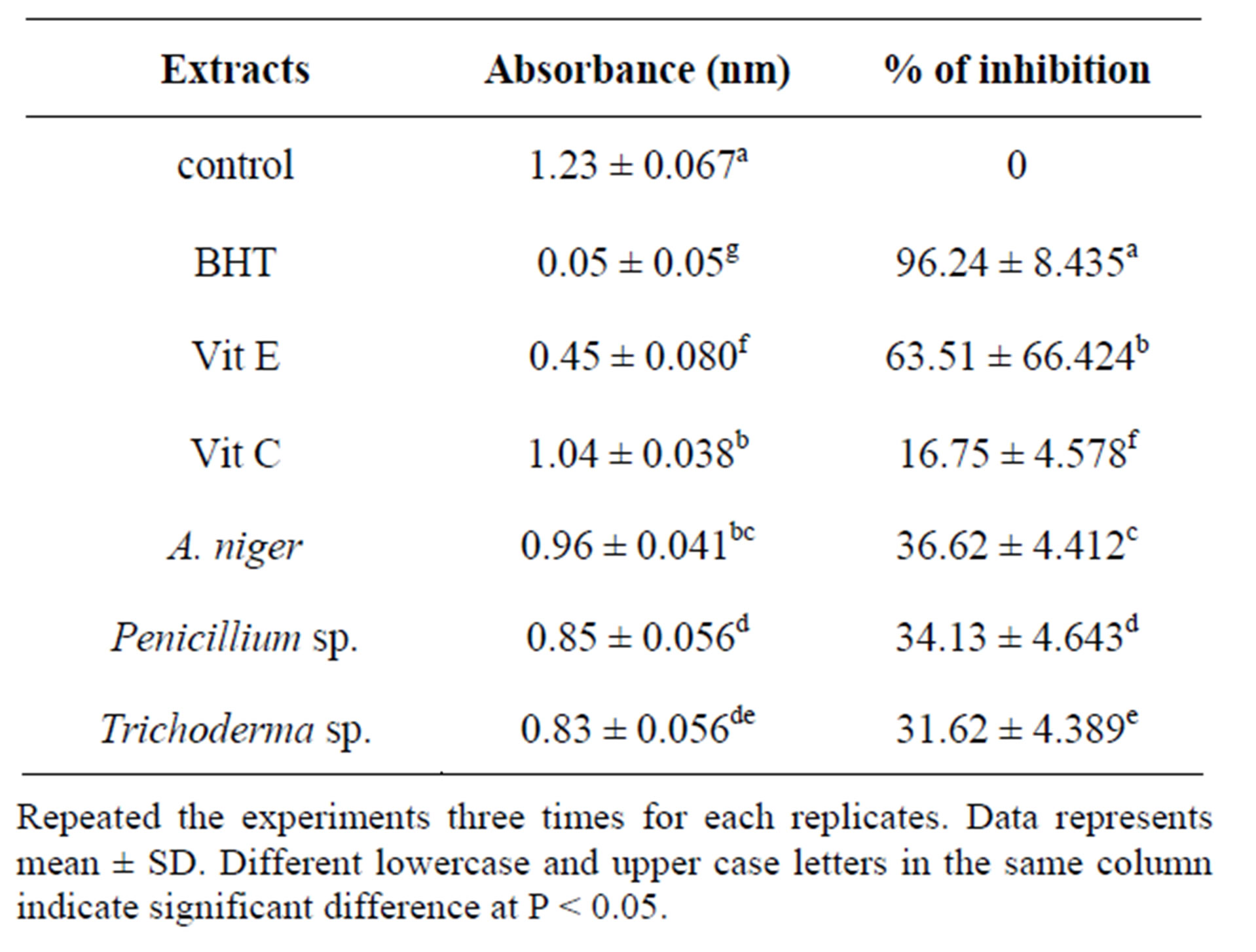
Table 5. Total antioxidant activity of endophytes by TBA method.
Repeated the experiments three times for each replicates. Data represents mean ± SD. Different lowercase and upper case letters in the same column indicate significant difference at P < 0.05.
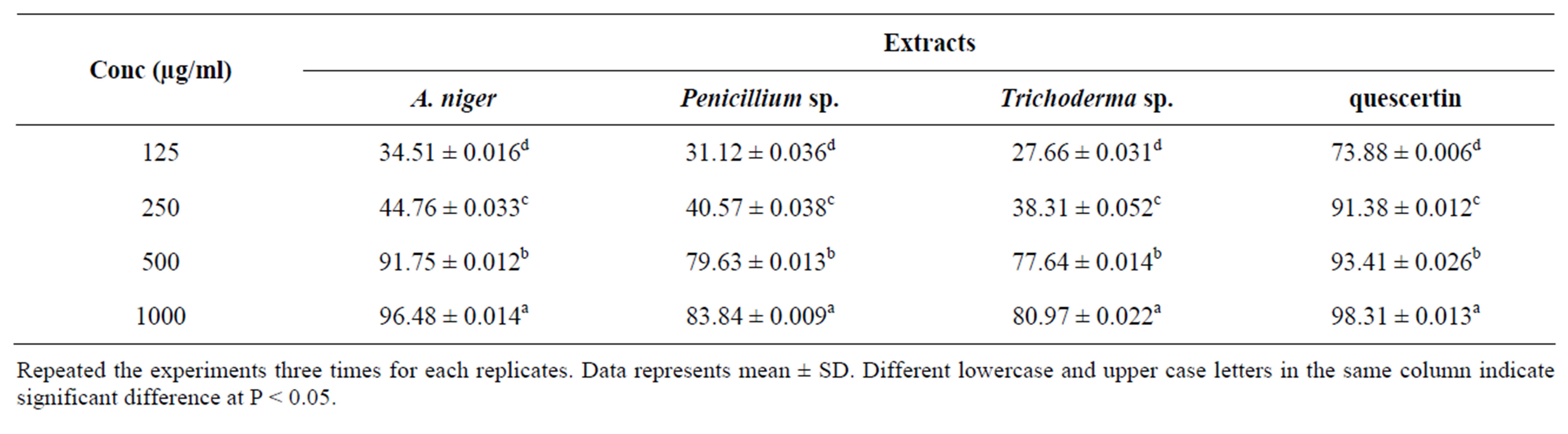
Table 6. Effect of endophytes extract on super oxide radical scavenging activity.
Repeated the experiments three times for each replicates. Data represents mean ± SD. Different lowercase and upper case letters in the same column indicate significant difference at P < 0.05.
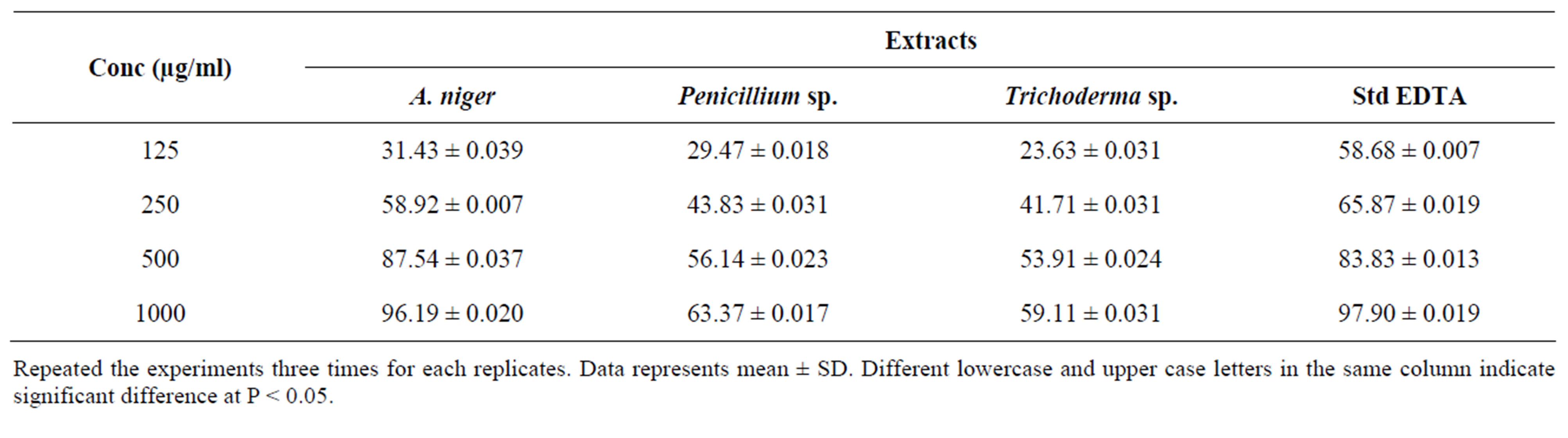
Table 7. Effect of endophytes extracts on iron chelation.
Repeated the experiments three times for each replicates. Data represents mean ± SD. Different lowercase and upper case letters in the same column indicate significant difference at P < 0.05.
EDTA was found to be 96.19 ± 0.020 (A. niger), 63.37 ± 0.017 (Penicillium sp.), 59.11 ± 0.022 (Trichoderma sp.) and 97.90 ± 0.019 (EDTA).
As shown in Table 8, the absorbance of methanol extracts of three endophytes at 7 days was significantly (P < 0.05) decreased when compared with absorption of extracts at 6 day. In contrast, endophyte A. niger extract had a significantly (P < 0.05) higher than Penicillium sp. and Trichoderma sp. The absorbance of endophytic methanol extracts at 7 and 8 day was markedly lower than the absorbance of vitamin C and higher than the vitamin E. In contrast, the A. niger methanol extract had a significant higher absorbance at 6 and 7 day when compared to vitamin C and vitamin E. The control had a highest value throughout the study. The highest absorbance value was recorded at 6 day, then dropped at 7 day due to malonialdehyde (MDA) compounds from linoleic acid oxidation, in which peroxidase reacts with ferrous chloride to form reddish ferric pigment (Table 9).
The percentage of inhibition of methanol extract of A. niger (74) followed by Trichoderma sp. (66) and Penicillium sp. (62) was significantly higher than the percentage

Table 8. Antioxidant activity of standards and different endophytes of T. argentea by FTC method.
Repeated the experiments three times for each replicates. Data represents mean ± SD. Different lowercase and upper case letters in the same column indicate significant difference at P < 0.05.
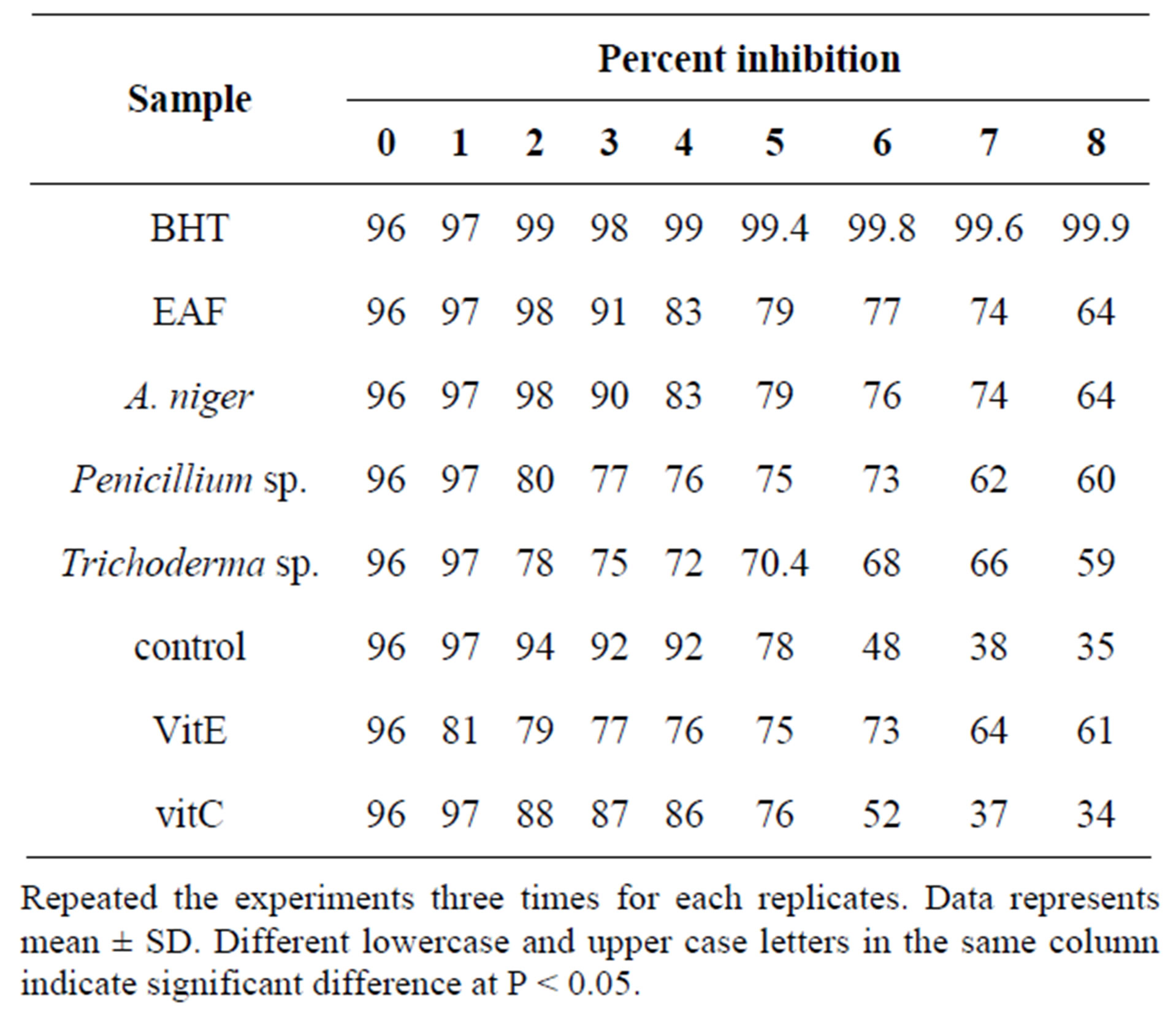
Table 9. Percentage of inhibition of linoleic acid and peroxidation by endophytic extracts in FTC method.
Repeated the experiments three times for each replicates. Data represents mean ± SD. Different lowercase and upper case letters in the same column indicate significant difference at P < 0.05.
of vitamin C (37) at 7 and 8 with vitamin E (Table 9).
In the present experiment, lipid peroxidation was elevated through 8 days (optical density of the control was maximal at 7 day). The control had increasing absorbance values from 0 day until the absorbance reduced the maximum level at 7 day and the absorbance value dropped at 8 day. Table 9 shows the absorbance and percentage of inhibition of all samples. The absorbance value of endophytic methanol extract were significantly (p < 0.05) higher than the absorbance value of BHT and vitamin E. Among endophytes, Trichoderma sp. the absorbance value was significantly (P < 0.05) lower than the BHT and vitamin E. The TBA results were in agreement with FTC results.
The antioxidant activity of the methanol extract was measured by the ability to scavenge DPPH free radicals, was compared with the standards Butylated Hydroxy Toluene (BHT). It was observed that methanol extract of the Aspergillus niger, Penicillium sp. had higher activity than that of Trichoderma sp. At a concentration of 0.1 mg/ml, the scavenging activity of ethanol extract of the A. niger and Penicillium sp. reached 88.61% and 86.72% respectively while at the concentration, that of Trichoderma sp. was 51.66%. Though the DPPH radical scavenging abilities of the extract were less than those of (97.8%) at 0.1 mg/ml, the study showed that the extracts have the proton donating ability and could serve as free radical inhibitors or scavenging, acting possibly as primary antioxidants (Figure 1). The performance of methanol extract of endophytic fungi, Phyllosticta sp. was higher than that the standard μ-tocopherol [28] and from different endophytes [30]. ABTS a stable free radical with the characteristic absorption at 734 nm was used to study the radical scavenging effect of endophytic extract reacted with ABTS at different concentration ranging from 100, 200, 400, 800 and 1600 μg/ml respectively and readings were observed by measuring the reduction of radical cation generated by ABTS+ at 734 nm (Figure 2).
The methanol extract of A. niger and Penicillium sp. showed maximum decolouration of 1600 μg/ml with the EC50 587.06 ± 0.74 and 566.71 ± 45 and the other endophyte showed less decolouration (Table 10). ABTS assay is an excellent tool for determining the antioxidant activity of phytochemicals [31]. The edible basidiomycetes and endophytes assayed against ABTS radical and reported to have scavenging ability against these radicals [28]. The reducing ability of the endophytic extracts was in the range of 448.26 - 1266.14 μm Fe (II)/mg (Table 11). The antioxidant potentials of the ethanol extracts of A. niger and F. oxysporum were estimated from their ability to reduce TPRZ-Fe (III) complex to TPTZ-Fe (II). The FRAP values for the ethanol endophytic extracts significantly lower that of ascorbic acid but higher that of BHT. Antioxidant activity increased proportionally to the polyphenol content. According to recent reports, a highly positive relationship between total phenol and antioxidant activity appears to be the trend in many plant species [32]. Phenolic and flavonoid compound seems to have an important role in stabilizing lipid oxidation, associated with antioxidant activity [33]. Total phenol found to be in Aspergillus niger and Penicillium sp. of 19.20 and 18.23 mg/GAE/g dry weight and flavonoid content of Aspergillus niger and Penicillium sp. of 7.25 and 6.41 μg/mg equivalent respectively (Table 12). The present investigation results reveal that ethanol extract of Aspergillus niger and Penicillium sp. contains significant amount of phenols and flavonoids. Results of our findings confirmed the use Aspergillus niger and Penicillium sp. extract can be as traditional medicine. We found strong antioxidants activities specifically in the ethanol
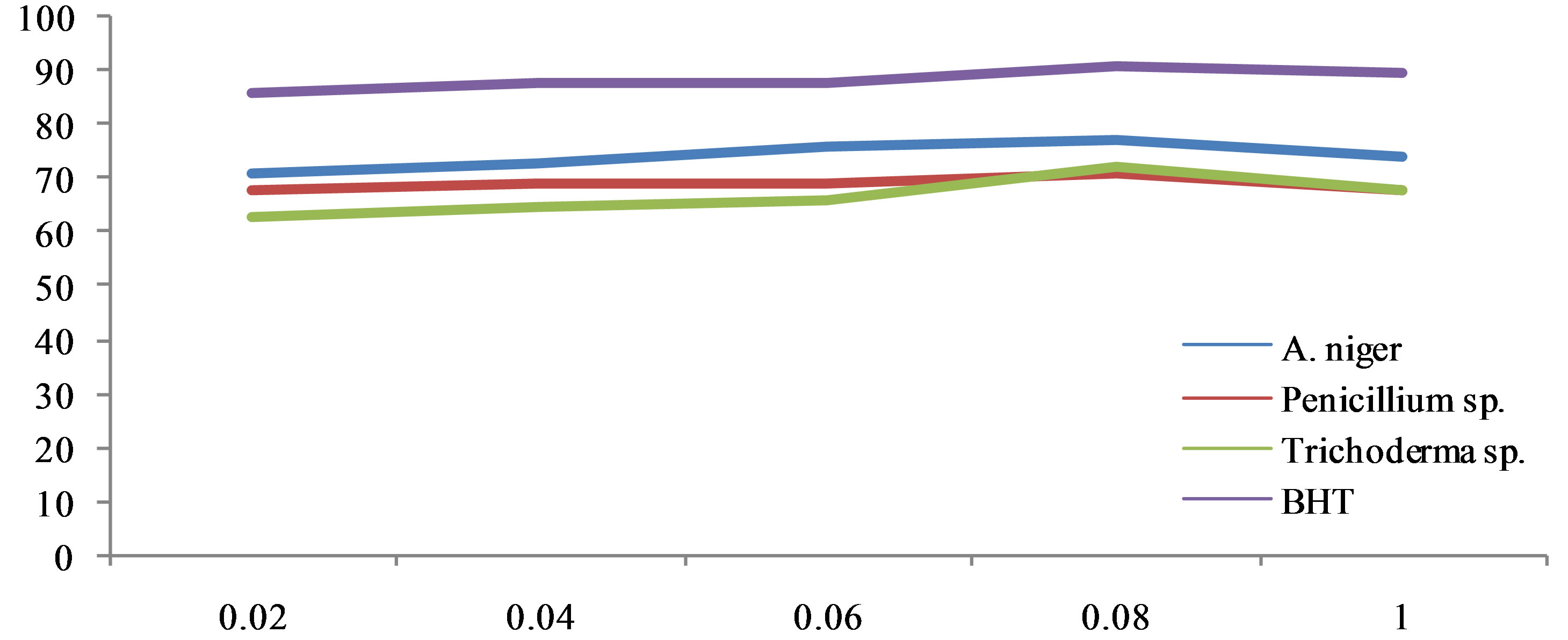
Figure 1. DPPH scavenging activities of methanol endophytes extract.
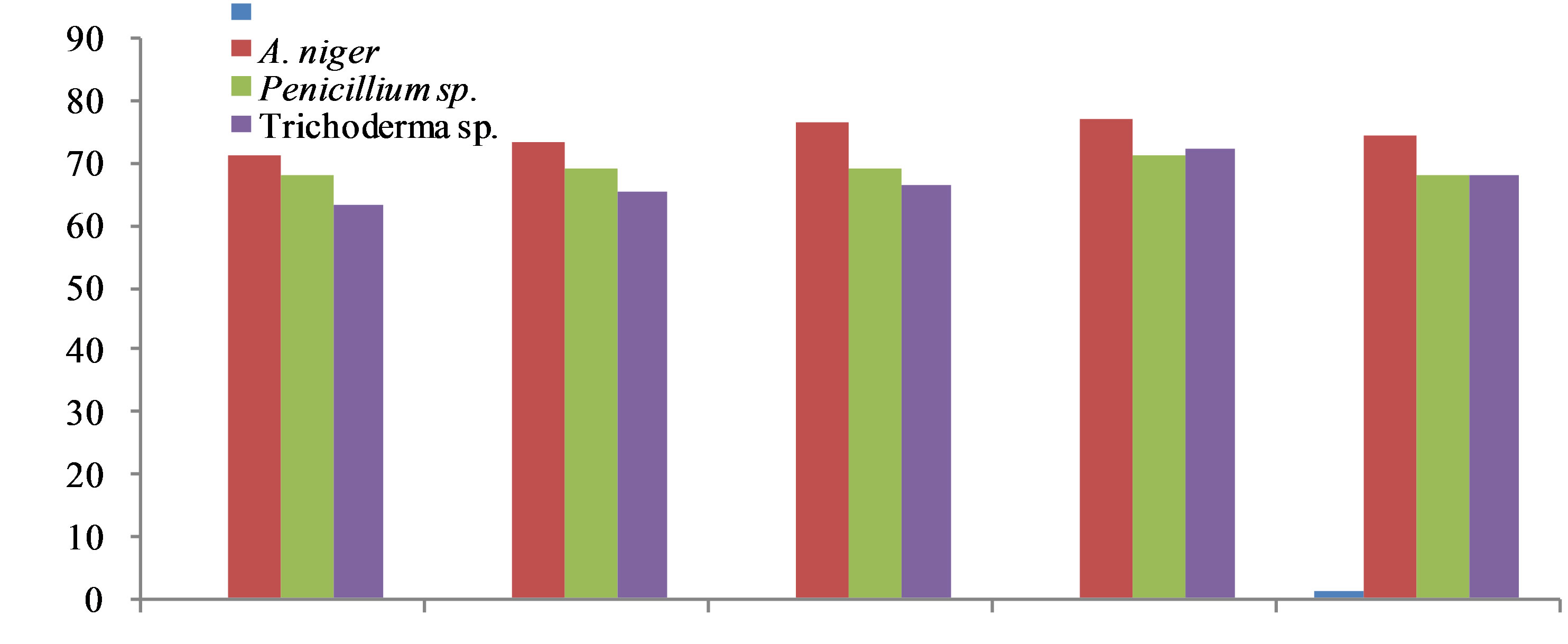
Figure 2. Free radical scavenging effect of different endophytes extracts against ABTS.
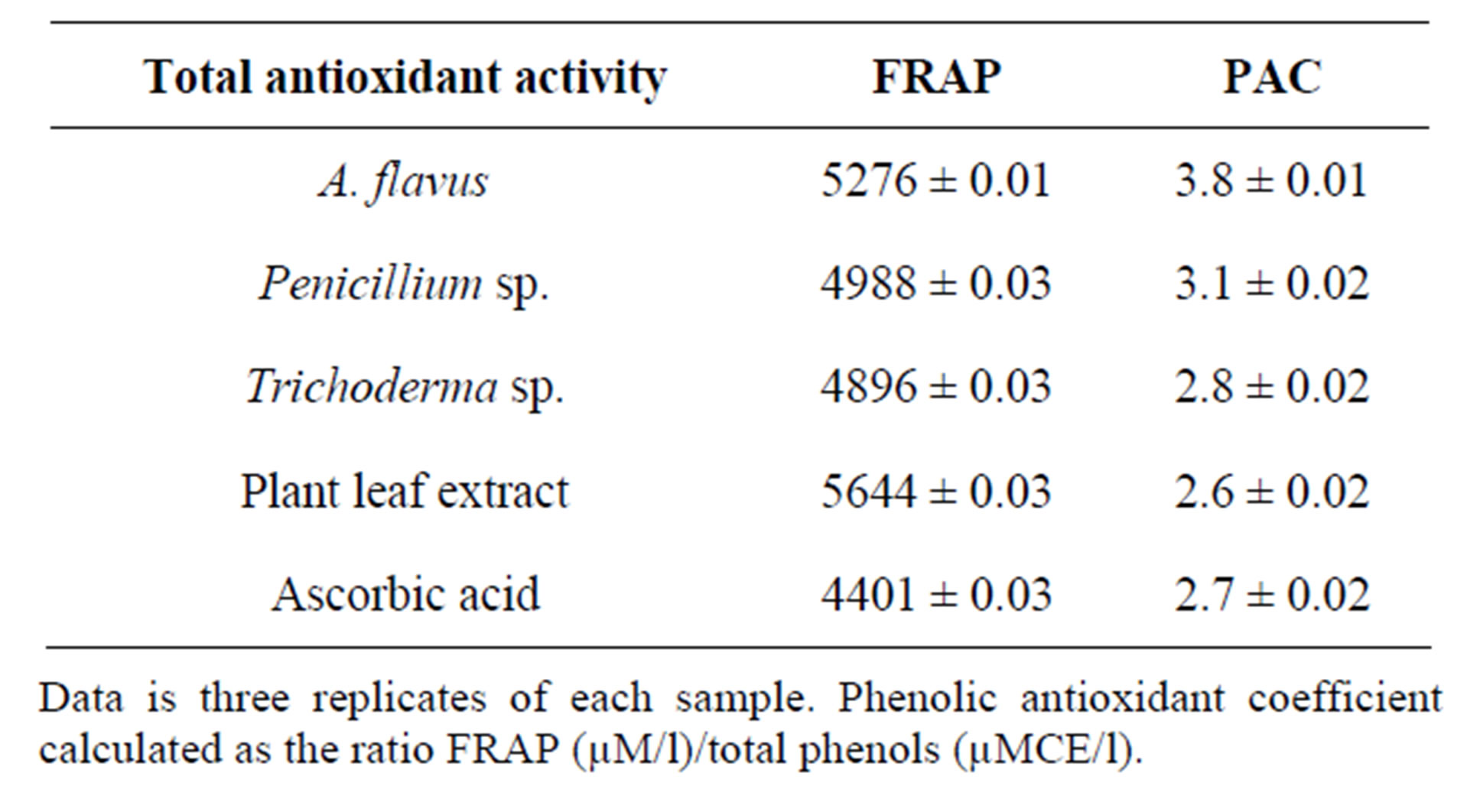
Table 10. Total antioxidant activity of the different endophytes and plant extract of T. argetea.
Data is three replicates of each sample. Phenolic antioxidant coefficient calculated as the ratio FRAP (µM/l)/total phenols (µMCE/l).
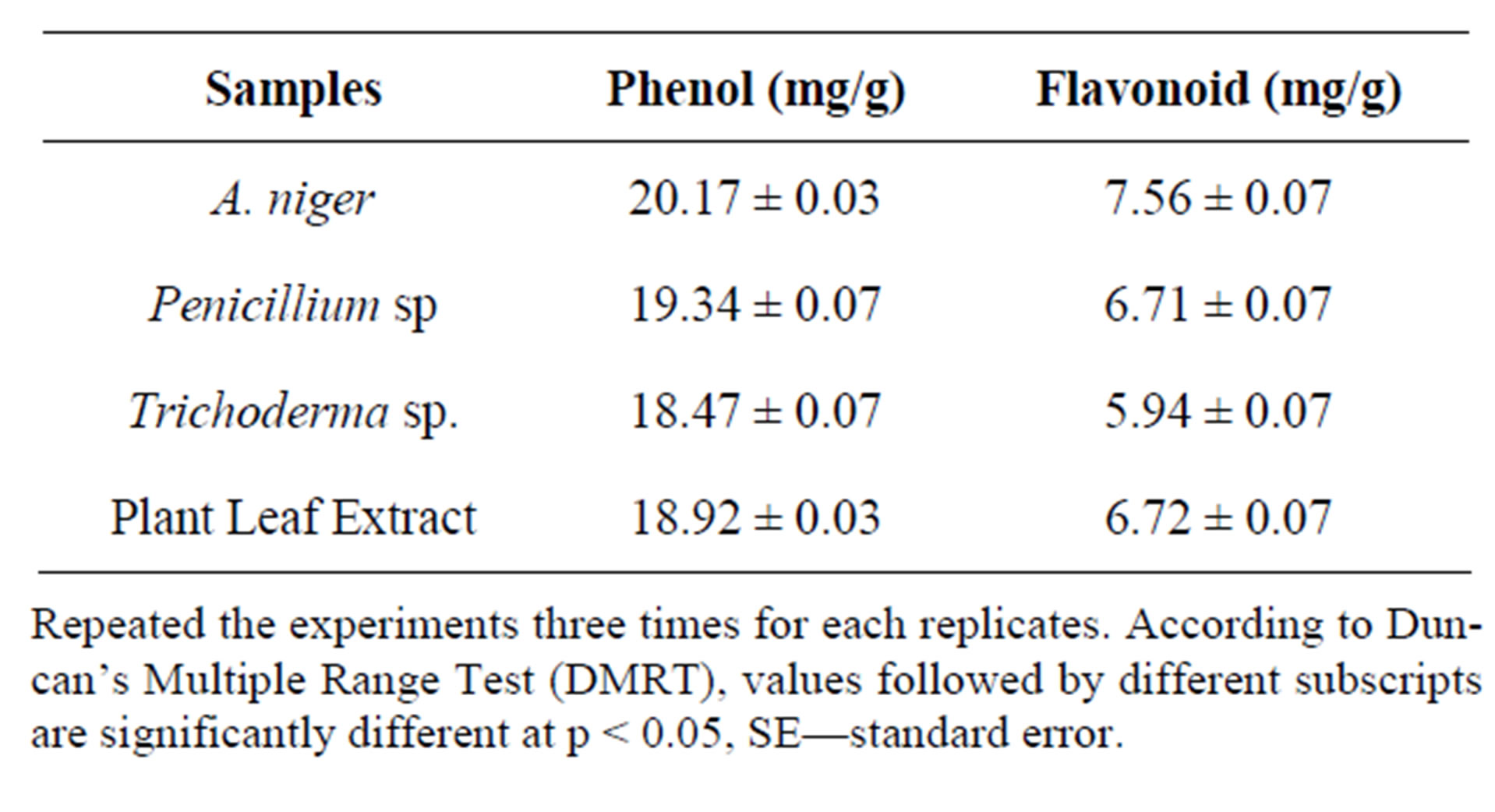
Table 11. Determination of phenols and flavonoid from endophytes extract of Tabebuia argentea.
Repeated the experiments three times for each replicates. According to Duncan’s Multiple Range Test (DMRT), values followed by different subscripts are significantly different at p < 0.05, SE—standard error.
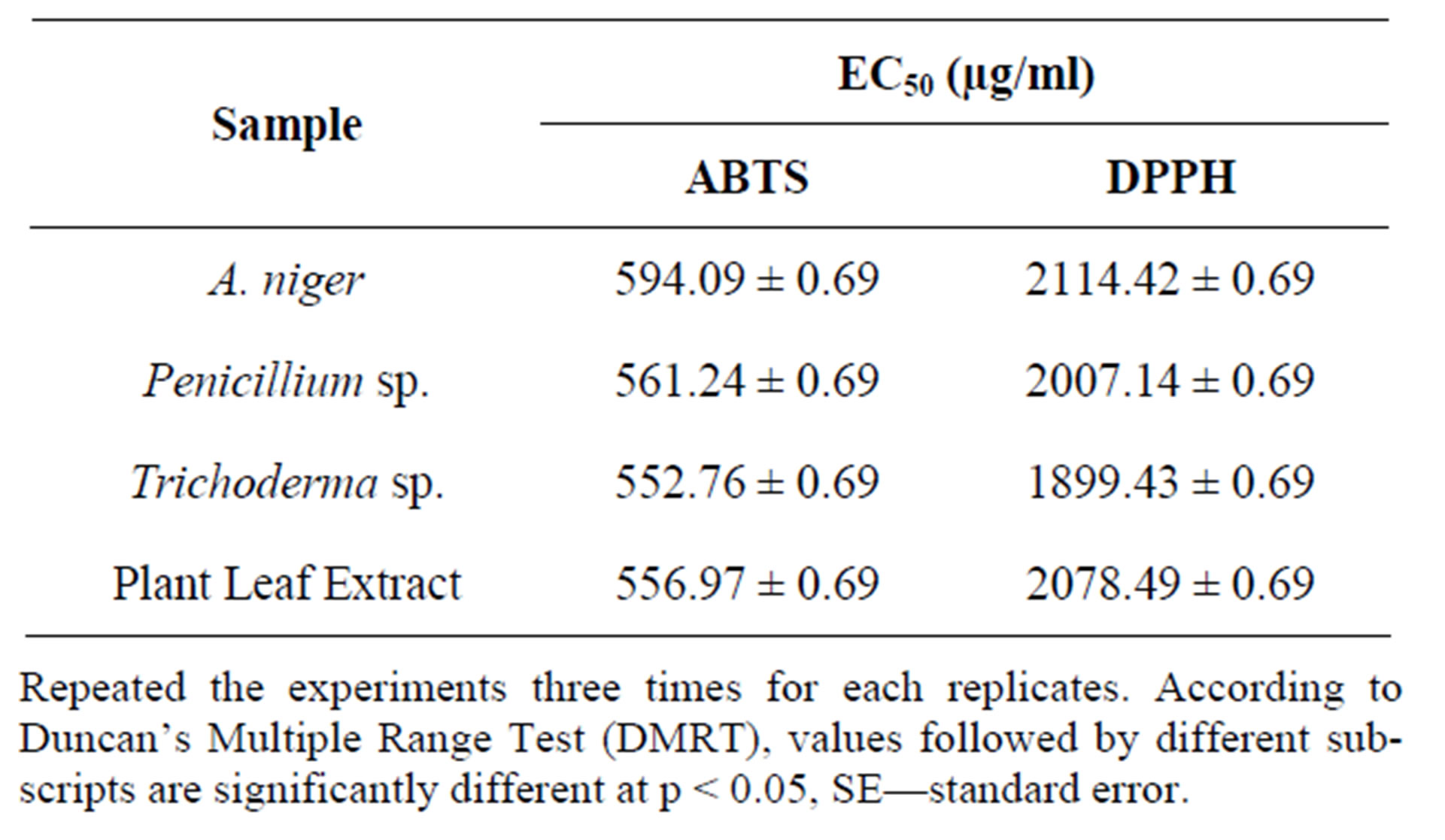
Table 12. Table of EC50 values of endophytic extracts.
Repeated the experiments three times for each replicates. According to Duncan’s Multiple Range Test (DMRT), values followed by different subscripts are significantly different at p < 0.05, SE—standard error.
Aspergillus niger and Penicillium sp. endophytic stem extracts. High phenol and flavonoid values found in ethanol Aspergillus niger and Penicillium sp. extracts imply the role of phenol compounds in contributing these activities. Plant phenol compounds have been found to possess potent antioxidants [34]. The flavonoids from endophytic extracts have been found to possess antioxidants properties in various studies. The terpenoids act as antioxidant [35]. Strong presence of tannins and saponinsin all extracts may explain its potent bioactivities are known to possess potent antioxidants [36]. The present investigation has shown that the endophytic extracts have active phytochemicals and exhibited strong antioxidant properties. These activities may be due to strong occurrence of polyphenolic compounds such as flavonoids, tannins, terpenoids phenols and saponins. The antioxidant activity was comparable with standard ascorbic acid and BHT. These findings provide scientific evidence to support endophytic fungal medicinal uses and indicate a promising potential for the development of an antioxidant agent plant. These endophytic fungi by in vitro results appear as interesting and promising and may be effective as potential sources of novel antioxidant drugs.
4. Discussion
Endophytic fungiare reported ubiquitously from each mad every higher plant, which has been investigated for their antimicrobial complement [37]. The endophytes have made greater interest in the use reservoir of natural bioactive compounds that they (host) produced [38]. Endophytic fungal species are now considered as exciting novel sources for obtaining new bioactive compounds and have been reported from several hosts [39]. Investigations on the phytochemical screening of the Tabebuia argentea revealed the presence of saponins, glycosides, alkaloids, flavonoids etc. The presence of phytochemical within endophytes can be potential source for medicinal and industrial use. The presence of phytochemicals in the endophytes can be potential source of precursors in the development of synthetic drugs [14].
Based on the above results, endophytes, Aspergillus niger, Penicillium sp. and Trichoderma sp. of Tabebuia argentea yielded medically important phytochemical compounds, may be due to these endophytes possess potent antioxidant potentials of methanol extracts in all in vitro study. In addition, allendophytic extracts was found to possess significant amount of total phenolic content. Different ingredients of endophytes, Aspergillus niger and Penicillium sp. of Tabebuia sp. have been claimed in different studies to possess biological properties related to antioxidant mechanisms. Hence, the significant antioxidant activity of methanol extract in the present study may be attributed to these aforementioned potent antioxidant ingredients of endophytes, Aspergillus niger and Penicillium sp. The ferric thiocyanate (FTC) results showed that methanolic extract had greater antioxidant activity. Previous studies have claimed that, the aqueous extract of herbal plants has high antioxidant activity against lipid peroxidation. The reaction in the FTC method is due to the malondialdehyde (MDA) compounds from the linoleic acid oxidation in which, peroxide reacts with ferrous chloride to form a reddish ferric chloride pigment [40]. In this study, however, the high antioxidant activity of Aspergillus niger and Penicillium sp. methanol extract may be due to the majority of the active compounds being dissolved in the methanol solvent (strong polar). In this study, the results of the thiobarbituric acid (TBA) test were confirmed with the FTC data. Based on these results, endophytic methanol extract had higher antioxidant activity. Therefore, the higher antioxidant activity found from the ferric thiocyanate method indicated that, the amount of peroxide in the initial stage of lipid peroxidation was greater than the amount of peroxide in the secondary stage. Thus, these data suggest that methanol extract has a better beneficial effect against lipid peroxidation when compared with chloroform extract. The DPPH assay is one of the most common and relatively quick methods used for testing radical scavenging activity of various plant extracts [25]. The results of this study indicated that, the IC50 in methanol extract was significantly lower than the IC50 in chloroform extract suggesting that the methanol extract had better scavenging activity than the choloroform extract. In this study, we found a dose dependent relationship in the DPPH assay. The activity increased as the concentration increased for both extracts. The increased formation of free radicals was associated with the increase in lipid peroxidation. One of the important roles of antioxidants is to inhibit the chain reaction of lipid peroxidation. We conclude that, the methanol extract (polar extract) with maximal inhibition of free radicals is a more potent extract when compared with the chloroform extract (non-polar).
The present study estimated the phenol and flavonoid contents of methanol extract and chloroform extract of Tabebuia argentea. Our results showed that methanol extract contained significantly higher phenol (approximately 15 fold) and flavonoid (approximately 19 fold) contents than chloroform extract. Phenol and flavonoid contents in methanol extracts (polar solvent) of endophytic Xylaria sp. are higher than the contents in hexane extracts (nonpolar solvent) [41]. Because of the hydroxyl groups in the phenol compounds, they may directly contribute to the antioxidant activity and have a critical role in scavenging free radicals [25]. Recent studies have shown that, fruit and vegetable phenols and polyphenols such as flavonoids are one of the major groups that indicate a large spectrum of biological activities that are principally ascribed to their antioxidant property. They prevent free radical damage and lipid peroxidation. The high content of total phenol components in the methanol extract may have led to the better results found in the total antioxidant activity and free radical scavenging ability when compared with the chloroform extract. In addition, the differential activities may have been due to the higher solubility of phenol components in the methanol solvent than in the chloroform solvent. In this study, the radical scavenging ability was significantly increased in methanol extract when compared with chloroform extract. The reason for the increase in radical scavenging ability may have been due to the increase in total phenol compounds. Thus, it was important to calculate the correlation between the total phenol contents and total antioxidant activity. A good correlation was found between the total phenol contents and DPPH scavenging ability for methanol extract and chloroform extract. Our results are in accordance with previously published results that indicated a high correlation between antioxidant activity and total phenols. Thus, this indicates that the antioxidant activity of many plant extracts is related to their phenol components. Furthermore, the methanol extract containing the higher concentration of phenols had significant free radical scavenging activity in this study. The results suggest that, phenols are important components of endophytes and that some of its pharmacological effects may be attributed to the presence of these valuable constituents.
Superoxide is a highly reactive molecule that reacts with various substances produced through metabolic processes. Superoxide dismutase enzymes present in aerobic and anaerobic organisms catalyses the breakdown of superoxide radical. Superoxide scavenging ability of plant extract might primarily be due to the presence of flavanoids.
Iron is essential for life because it is required for oxygen transport, respiration and activity of many enzymes. However, iron is an extremely reactive metal and catalyzes oxidative changes in lipids, proteins and other cellular components. It causes lipid peroxidation through the lipid hydroxide into peroxyl and Alkoxyl radicals that can perpetuate the chain reactions.
Based on the above results indicated, the methanol extract of Aspergillus niger and Penicillium sp. of Tabebuiaargentea was found to most effective in exhibiting in vitro antioxidant activity in various method. The results indicted the endophytes, Aspergillus niger and Penicillium sp. possess iron biding capacity which might be due to the presence of polyphenols that averts the cell from free radical damage by reducing of transition metal ions. Various plant extracts were proved to be good chelators and correlation exists between phenols, flavonoids and chelating activity. Further in vivo experiment is needed to recommend the endophytic extracts can be used as antioxidant drug.
5. Conclusion
In this study, all antioxidant methods (ferric thiocyanate, thiobarbituric acid, and DPPH) showed that the methanol endophytic extracts of Tabebuia argentea contain more antioxidant activities than the chloroform extract. Moreover, this study demonstrated that, endophytes Aspergillusniger and Penicillium sp. are an important source of phenol compounds, which are a good source of antioxidant activity. The phenol component has a high inhibitory effect that prevents lipid peroxidation. However, the solvent type has an important role in detecting phenol compounds and antioxidant factors. Thus, we concluded that endophytes Aspergillusniger and Penicillium sp. react via its free radical scavenging to prevent lipidperoxidation. Therefore, natural antioxidants and phenol compounds in endophytes Aspergillus niger and Penicillium sp. have the capability to be used medically and in food systems to preserve food quality.
6. Acknowledgements
The authors are grateful to Visvesvaraya Technological University (VTU), Belgaum, Karnataka, India for providing financial support for this investigation under Research Grants. We thank Dr MR Hulinaykar, Managing Trustee, Sri Shridevi Charitable Trust (R.) and Dr Sukumaran K, Principal, SIET, Tumkur, India for encouragement and Dr S Lokesh, DOS in Applied Botany and Biotechnology, University of Mysore, Mysore, India for assistance in identifying endophytic fungi.
REFERENCES
- Y. Shen, C. Chen and Y. Kou, “New Sewquiterpenehydroquinones from a Taiwanese Marine Songe, Hippospongiametachromia,” Journal of Natural Products, Vol. 64, No. 6, 2002, pp. 801-803. doi:10.1021/np000610c
- W. Hills, “Heartwood and Tree Exudates,” Springer Series in Wood Science, Syracuse, New York, 1987, p. 267.
- S. M. Wuerzberger, J. J. S. M. Pink, Planch, K. L. Byers, W. G. Bornmann and D. A. Boothman, “Induction of Apoptosis in MCF-7: WS8 Breast Cancer Cells by 3-Lapachone1,” Cancer Research, Vol. 58, 1998, pp. 1876- 1885.
- M. T. Murray and J. E. Pizzorno, “Encyclopedia of Natural Medicine,” 2nd Edition, PA4 Rocklin, Prima Pub, 1998, pp. 967-972.
- I. I. Balassiano, S. A. Paulo, N. H. Silva, M. C. Cabral and M. C. Carvalho, “Demonstration of Lapachol as a Potential Drug for Fighting Cancer Metastases,” Oncology Reports, Vol. 13, 2005, p. 329.
- M. N. Da Silva, M. C. B. V. Da Souza, V. F. Ferreira, A. V. Pinto, M. C. R. F. Pinto, M. S. V. Solange, S. M. S. V. Wardell and J. L. Wardell, “Synthesis of New Aldehyde Derivatives from β-Lapachone and Nor-β-Lapachone,” Arkivoc, 2003, pp. 156-168. doi:10.3998/ark.5550190.0004.a16
- J. Breger, B. B. Fuchs, G. Aperis, T. I. Moy, F. M. Ausubel and E. Mylonakis, “Antifungal Chemical Compounds Identified Using a C. elegans Pathogenicity Assay,” PLoS Pathogens, Vol. 3, No. 2, 2007, pp. 168-178. doi:10.1371/journal.ppat.0030018
- E. R. Almeida, “Anti-Inflammatory Action of Lapachol,” Journal of Ethnopharmacology, Vol. 29, 1990, pp. 239- 241. doi:10.1016/0378-8741(90)90061-W
- M. J. Teixeira, Y. M. Almeida, J. R. Viana, J. G. HolandaFilha, T. P. Rodrigues, J. R. C. Prata, I. C. B. Coelho, V. S. Rao and M. M. L. Pompeu, “In Vitro and in Vivo Leishmanicidal Activity of 2-Hydroxy-3-(3-Methyl-2-Butenyl)-1,4-Naphthoquinone (Lapachol),” Phytotherapy Research, Vol. 15, 2001, pp. 44-48. doi:10.1002/1099-1573(200102)15:1<44::AID-PTR685>3.0.CO;2-1
- T. M. S. Silva, C. A. Camara, T. P. Barbosa, A. Z. Soares, L. C. Cunha, A. C. Pinto and M. D. Vargas, “Design, Synthesis and Antifungal Activity of a Novel Water Soluble Prodrug of Antifungal Triazole,” Bioorganic & Medicinal Chemistry Letters, Vol. 13, 2005, pp. 193-196. doi:10.1016/j.bmc.2004.09.043
- T. Finkel and N. J. Holbrook, “Oxidants, Oxidative Stress and the Biology of Ageing,” Nature, Vol. 408, No. 6809, 2000, pp. 239-247. doi:10.1038/35041687
- L. S. Cozma, “The Role of Antioxidant Therapy in Cardiovascular Disease,” Current Opinion in Lipidology, Vol. 15, No. 3, 2004, pp. 369-371. doi:10.1097/00041433-200406000-00020
- R. W. Owen, A. Giacosa, W. E. Hull, R. Haubner, B. Spiegelhalder and H. Bartsch, “The Antioxidant/Anticancer Potential of Phenolic Compounds Isolated from Olive Oil,” European Journal of Cancer, Vol. 36, No. 10, 2000, pp. 1235-1247. doi:10.1016/S0959-8049(00)00103-9
- T. S. Sadananda, R. Nirupama, K. Chaithra, M. Govindappa, C. P. Chandrappa and B. VinayRaghavendra, “Antimicrobial and Antioxidant Activities of Endophytes from Tabebuia argentea and Identification of Anticancer Agent (Lapachol),” Journal of Medicinal Plants Research, Vol. 5, No. 16, 2011, pp. 3643-3652.
- T. Theantana, K. D. Hyde and S. Lumyong, “Asparginase Production by Endophytic Fungi from Thai Medicinal Plants: Cytotoxic Properties,” International Journal of Integrative Biology, Vol. 7, No. 1, 2009, pp. 1-8.
- M. B. Ellis, “Dematiaceoushypomycetes,” Common Wealth Mycological Institute, Kew Surrey, 1971.
- H. L. Barnett and B. B. Hunter, “Illustrated Genera of Imperfect Fungi,” 2nd Edition, Burgess Publishing Company, Minnesota, 1972.
- A. L. Bandoni, M. E. Mendiondo, R. V. D. Rondina and J. D. Coussio, “Survey of Argentine Medicinal Plants, Folklore and Phytochemical Screening,” Economic Botany, Vol. 30, No. 2, 1976, pp. 161-185. doi:10.1007/BF02862962
- R. H. Thomson, “Naturally Occurring Quinones,” Recent Advances, Chapman and Hall, London, Vol. 142, 1987, pp. 144-147.
- A. Tomic, S. Petrovic, M. Pavlovic, B. Trajkovski, M. Milenkovic, D. Vucicevic and M. Niketic, “Antimicrobial and Antioxidant Properties of Methanol Extracts of Two Athamantaturbish Subspecies,” Pharmaceutical Biology, Vol. 47, No. 4, 2009, pp. 314-319. doi:10.1080/13880200902748452
- M. Gulluce, H. Ozer, O. Baris, D. Daferera, F. Sahin and M. Polissiou, Turkish Journal of Biology, Vol. 30, 2006, pp. 231-233.
- C. C. Winterbourne, R. E. Hawkins, M. Brain and R. W. Carrel, “The Estimation of Red Cell Superoxide Dismutase Activity,” Journal of Laboratory and Clinical Medicine, Vol. 85, No. 2, 1975, pp. 337-341.
- I. F. F. Benzie and J. J. Strain, “Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay,” Analytical Biochemistry, Vol. 239, No. 1, 1996, pp. 70-76. doi:10.1006/abio.1996.0292
- Đ. Gülçin, R. Elias, A. Gepdiremen, K. Taoubi and E. Köksal, “Antioxidant Secoiridoids from Fringe Tree (Chionanthusvirginicus L.),” Wood Science and Technology, Vol. 43, No. 3-4, 2009, pp. 195-212. doi:10.1007/s00226-008-0234-1
- M. Elmastas, O. Isildak, I. Turkekul and N. Temur, “Determination of Antioxidant Activity and Antioxidant Compounds in Wild Edible Mushrooms,” Journal of Food Composition and Analysis, Vol. 20, No. 3, 2007, pp. 337- 345. doi:10.1016/j.jfca.2006.07.003
- K. Saha, N. H. Lajis, D. A. Israf, A. S. Hamzah, S. Khozirah, S. Khamis and A. Syahida, “Evaluation of Antioxidant and Nitric Oxide Inhibitory Activities of Selected Malaysian Medicinal Plants,” Journal of Ethnopharmacology, Vol. 92, No. 2-3, 2004, pp. 263-267. doi:10.1016/j.jep.2004.03.007
- A. Jain, M. Soni, L. Deb, A. Jain, S. Rout, V. Gupta and K. Krishna, “Antioxidant and Hepatoprotective Activity of Ethanolic and Aqueous Extracts of Momordicadioica Roxb. Leaves,” Journal of Ethnopharmacology, Vol. 115, No. 1, 2008, pp. 61-66. doi:10.1016/j.jep.2007.09.009
- K. Srinivasan, L. K. Jagadish, R. Shenbhagaraman and J. Muthumary, “Antioxidant Activity of Endophytic Fungus Phyllosticta sp. Isolated from Guazumatomentosa,” Journal of Phytology, Vol. 2, No. 6, 2010, pp. 37-41.
- L. Barros, S. Oliveira, A. M. Carvalho and I. C. F. R. Ferreira, “In Vitro Antioxidant Properties and Characterization in Nutrients and Phytochemicals of Six Medicinal Plants from the Portuguese Folk Medicine,” Industrial Crops and Products, Vol. 32, No. 3, 2010, pp. 572-579. doi:10.1016/j.indcrop.2010.07.012
- S. Phongpaichit, N. Rungjindamai, N. Rukachaisirikul and J. Sakayaroj, “Antimicrobial Activity in Cultures of Endophytic Fungi Isolated from Garcinia Species,” FEMS Immunology & Medical Microbiology, Vol. 48, No. 3, 2006, pp. 367-372. doi:10.1111/j.1574-695X.2006.00155.x
- L. K. Jagadish, R. Shenbhagaraman, V. V. Krishnan and V. Kaviyarasan, “Studies on the Phytochemical, Antioxidant and Antimicrobial Properties of Three Pleurotus Species Collected Indigenously,” Journal of Molecular Biology and Biotechnology, Vol. 1, 2008, pp. 20-29.
- A. A. Adedapo, F. O. Jimoh, S. Koduru, A. J. Afolayan and P. J. Masika, “Antibacterial and Antioxidant Properties of the Methanol Extracts of the Leaves and Stems of Calpurnia aurea,” BMC Complementary and Alternative Medicine, Vol. 8, No. 1, 2008, pp. 53-58.
- Y. N. V. Maslarova, “Inhibiting Oxidation,” In: J. Pokorny, N. Yanishlieva and M. H. Gordon, Eds., Antioxidants in Food: Practical Applications, CRC Press, Woodhead Publishing Limited, Cambridge, 2001, pp. 22-70. doi:10.1533/9781855736160.1.22
- H. Y. Lai, Y. Y. Yau and K. H. Kim, “Blechnumorientale Linn—A Fern with Potential as Antioxidant, Anticancer and Antibacterial Agent,” BMC Complementary and Alternative Medicine, Vol. 10, No. 1, 2010, pp. 15-18. doi:10.1186/1472-6882-10-15
- J. Grassmann, “Terpenoids as Plant Antioxidants,” Vitamins & Hormones, Vol. 72, 2005, pp. 505-535. doi:10.1016/S0083-6729(05)72015-X
- I. Gulcin, V. Mshvildadze, A. Gepdiremen and R. Elias, “Antioxidant Activity of Saponins Isolated from Ivy: Alpha-Hederin, Hederasaponin-C, Hederacolchiside-E and Hederacolchiside-F,” Planta Medica, Vol. 70, No. 6, 2004, pp. 561-563. doi:10.1055/s-2004-827158
- S. K. Gond, V. C. Verma, A. Kumar, V. Kumar and R. N. Kharwar, “Study of Endophytic Fungal Community from Different Parts of Aegle marmelos Correae (Rutaceae) from Varanasi (India),” World Journal of Microbiology and Biotechnology, Vol. 23, No. 10, 2007, pp. 1371- 1375. doi:10.1007/s11274-007-9375-x
- S. H. Faeth and K. E. Hammon, “Fungal Endophytes in Oak Tree; Long Term Pattern of Abundance and Association with Leaf Miners,” Ecology, Vol. 78, No. 3, 1997, pp. 810-819. doi:10.1890/0012-9658(1997)078[0810:FEIOTL]2.0.CO;2
- V. C. Verma, S. K. Gond, A. Kumar, A. Mishra, R. N. Kharwar and A. C. Gange, “Endophyticactinomycetes from Azadirachtaindica A. Juss.: Isolation, Diversity and Anti-Microbial Activity,” Microbial Ecology, Vol. 57, No. 4, 2009, pp. 749-756. doi:10.1007/s00248-008-9450-3
- G. Al-Naqeeb, M. S. Ismail and A. Al-Zuba, “Fatty Acid Profile, α-Tocopherol Content and Total Atioxidant Activity of Oil Extracted from Nigella sativaseeds,” International Journal of Pharmacology, Vol. 5, No. 4, 2009, pp. 244-250. doi:10.3923/ijp.2009.244.250
- X. Liu, M. Dong, X. Chen, M. Jiang, X. Lv and G. Yan, “Antioxidant Activity and Phenolics of an Endophytic xylaria sp. from Gingko biloba,” Food Chemistry, Vol. 105, No. 2, 2007, pp. 548-554. doi:10.1016/j.foodchem.2007.04.008
NOTES
*Corresponding author.

