American Journal of Plant Sciences
Vol.4 No.5A(2013), Article ID:32264,8 pages DOI:10.4236/ajps.2013.45A013
Polyamine Metabolism of the Cotton Flower and Its Subtending Leaf under Water-Deficit Stress in the Field
![]()
1Department of Energy,The Politecnico di Milano, Milano, Italy; 2Department of Tecnologie e Progettazione, Università di Bergamo, Bergamo, Italy.
Email: *dloka@uark.edu
Copyright © 2013 D. A. Loka et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 6th, 2013; revised April 8th, 2013; accepted May 1st, 2013
Keywords: Cotton; Polyamines; Reproductive Stage; Water-Deficit Stress
ABSTRACT
Polyamines, putrescine, spermidine and spermine are low molecular weight polycations implicated in flowering and seed set and plants’ responses under adverse environmental conditions. Research in other crops has shown that polyamine metabolism is greatly affected by water-deficit stress, however, no information exists on cotton (Gossypium hirsutum L.). A field study was conducted in 2011 in two contrasting locations (Fayetteville, AR, and Lubbock, TX) in order to investigate the effect of water-deficit stress during flowering on polyamine metabolism of the cotton flower and its subtending leaf. Treatments consisted of control (well watered) and water-stress (irrigation withheld for two weeks at the onset of flowering) in a split plot design. First day white flowers and their subtending leaves were collected at the end of each week of the stress period for determination of polyamine concentrations. Water-deficit stress resulted in significant increases in PUT and SPD levels of pistils and leaves compared to the control. However, pistil and leaf SPM content significantly increased under drought conditions in one location and remained unaltered in the other one. Leaf and pistil polyamine metabolism of cotton appeared to be greatly affected by limited water supply, however further research is needed to elucidate the ways polyamines can be used to increase cotton drought tolerance.
1. Introduction
Cotton (Gossypium hirsutum L.) is a perennial with an indeterminate complex growth habit and is very sensitive to adverse environmental conditions [1]. Since it originates from hot and arid areas [2], cotton is well equipped with stress mitigation mechanisms such as leaf and root osmotic adjustment [3,4], production and accumulation of compatible osmolytes and heat shock proteins [5,6]. However, as a result of its domestication and cultivation as an annual crop, modern cultivars are characterized by sensitivity to drought since the effectiveness of stress alleviation mechanisms is closely related to its indeterminate growth habit [7]. Even though debate still exists on which stage of development cotton is most susceptible to water stress, limited supply of water during flowering and boll development has been reported to be very detrimental for yield [8-11]. Extensive research conducted to elucidate water-deficit stress effects on cotton, has focused mainly on leaf physiology and metabolism, with limited attention to cotton’s reproductive units despite their great importance as yield determinants [12].
Polyamine metabolism has been reported to be affected by conditions of water-deficit stress [13]. Polyamines are low molecular weight cationic polycations, with the main forms occurring in plants being the diamine, putrescine (PUT) and its derivatives, the triamine spermidine (SPD) and the tetramine spermine (SPM). They have been observed to participate in a variety of physiological and metabolic functions [14] while their presence is indispensable during flowering [15,16]. Furthermore, changes in polyamine concentrations have been reported to be a common plant response to a variety of abiotic stresses such as salinity and high or low temperatures, as well as drought [13,17].
Regarding polyamine metabolism in cotton under abiotic stresses, Bibi et al. (2010) [18] reported that application of PUT on cotton flowers resulted in increases in PUT levels of the ovary and seed set efficiency under conditions of heat stress, while SPD and SPM concentrations were significantly decreased. Considering that water-deficit stress is the most important abiotic factor affecting crop production in almost 40% of cultivated areas around the world [19] and that drought events are projected to intensify in the future [20], it is imperative that research efforts should be focused on finding ways to ameliorate the consequences of water-deficit stress. Therefore, the objectives of our study were to monitor and evaluate the alterations caused by water-deficit stress on the polyamine metabolism of the cotton pistil and its subtending leaf.
2. Materials and Methods
2.1. Plant Material
Cotton cultivar ST5288B2F seeds were sown at a density of ten plant per meter in a Captina silt loam (Typic Fragidult) soil on June 6th, 2011, at the University of Arkansas Agricultural Research and Extension Center Crop, Soil and Environmental Sciences Farm in Fayetteville, AR and in a sandy loam (Typic Amarillo) soil on May 30th, 2011 at Quaker Avenue Research Farm of Texas Tech University in Lubbock, TX. Plots were 4 m × 7 m with 1m borders between each plot. To maintain wellwatered conditions until stress was imposed, plants in Fayetteville, AR were irrigated to soil saturation every six days in the absence of saturating rainfall, while in Lubbock, TX subsurface drip irrigation was provided daily. Fertilizer application, weed control, and insecticide applications were performed according to extension center recommendations and practices.
Irrigation was withheld when plants reached the flowering stage (approximately 50% of the plants in the field had white flowers) which was July 20th in Fayetteville, AR and July 13th in Lubbock, TX. First sympodial branch fruiting position white flowers and their subtending leaves were sampled at 1200 h at the end of the firstand second week after irrigation was withheld. Tissues were immediately placed in an ice chest filled with dry ice and transferred to the laboratory, and stored at −80˚C in Fayetteville, while tissues from Lubbock were shipped overnight to Fayetteville for further determination of polyamine concentrations.
2.2. Soil Moisture Content
Six soil samples were collected from each treatment plot in both locations at the end of the first and the second week after irrigation was withheld using a soil auger. Fresh weight of the samples was determined after sampling, after which the samples were placed in a dryer (50˚C) until they were completely dry. Samples were weighed and their dry weight was determined. Soil moisture content was expressed as the ratio of dry weight/ fresh weight.
2.3. Stomatal Conductance Measurements
Stomatal conductance rates were taken at the end of the first and second week only at Fayetteville. Measurements were taken from the subtending leaf (n = 20) from 1100 h until 1300 h using a Decagon SC-1 Porometer (Decagon Inc., Pullman, WA). Due to the small surface area of the cuvette, three measurements on various areas of the leaf were taken and then averaged. The results were expressed as mmol/m²s.
2.4. Polyamine Extraction and Analysis
Polyamines were extracted according to Smith and Davies (1985) [21] with modifications. Cotton ovary and leaf tissue, 0.1 and 0.2 g, respectively, were excised and homogenized in mortars with pestles in 0.2 N HClO4. To monitor the extraction and quantification procedure unfortified and fortified samples were prepared. For unfortified samples 100 µl of 1mM hexamethylenediamine in 0.2 N HClO4 was added to the tissue prior to homogenization as an internal standard. The final volume of 2 ml was obtained by adding 1900 µl 0.2 N HCLO4. For the fortified samples that contained a certain amount of the three polyamines, 100 µl hexamethylenediamine 1 mM in 0.2 N HClO4 was added plus the desired volume of fortification solution in 0.2 N HClO4 which was 120 µl 1 mM putrescine in 0.2 N HCLO4, 120 µl 1 mM spermidine in 0.2 N HClO4, and 120 µl 1 mM spermine of 0.2 N HCLO4. The final volume of 2 ml was obtained by adding 1540 µl of 0.2 N HClO4. An aliquot of 1.5 ml of the homogenate was transferred to 2 ml micro centrifuge tubes and the samples were centrifuged at 4˚C for 20 min at 14,000 rpm. The supernatant was collected and used for dansylation of polyamines.
The polyamines were derivatized by adding 100 µl aliquots of the supernatant to 1000 µl 21.2 mM of aqueous Na2CO3, 400 µl of 99.9% acetone and 50 µl of 12.5 mM and 100 µl of 87.5 mM of dansyl chloride in acetone. The mixture was incubated in a thermal reaction block at 60˚C for 1 h in the dark. After 1 h in the thermal block, the samples were removed and cooled to near room temperature, and 100 µl 1 N HClO4 were added to the mixture and mixed. The samples were then centrifuges at 4˚C for 20 min at 14,000 rpm, after which 500 µl of centrifugate were transferred into 2 ml sample vial and 500 µl of 0.02 N HClO4 were added. The samples were capped and mixed before injection into the High Performance Liquid Chromatography (HPLC). Derivatization needs to be in a basic solution, whereas the final solution for HPLC needs to be acidic.
A total of 5 standards were used for the preparation of the standard curves. The standards included putrescine, spermidine, spermine and the internal standard hexamethylenediamine. The concentrations of putrescine and spermidine in the five standards ranged from 5 to 30 nmoles/ml, whereas the concentrations of spermine ranged from 10 to 60 nmoles/ml. A 500 µl aliquot of hexanemethylenediamine was added to the standards. All standards were brought to a final volume of 10 ml with 0.2 N HClO4.
HPLC analysis was performed using a Hitachi HPLC (Hitachi High Technologies America, Inc., Canada) system that included a model L-7100 pump, and L-7200 autosampler, a D-7000 interface, and an ERC-3415a degasser and an L-7480 fluorescence detector. The column used in this analysis was a 25 cm × 2 mm, i.d. 0.5 micron Phenomenex Gemini C18. Injection volume was 50 µl. Polyamines were eluted from the column at 0.3 ml/min with methanol:water (v/v) gradient from 70% methanol to 95% methanol over 6 min and then remaining at 95% methanol for 16.4 min. The system was re-equilibrated with 70% methanol for 15 min before the next injection. For dansyl polyamines, an excitation wavelength of 510 nm. Data collection and processing were done with Hitachi System Manager (HSM) software on the internal standard concentration.
2.5. Statistical Analysis
The experimental design used was a split plot and treatments consisted of control, receiving optimum quantity of water throughout the season, and water stress, where irrigation was withheld for two weeks during flowering. Fifty first position white flowers and their subtending leaves were randomly selected from each treatment plot at the end of each week from both locations and each sample was considered as an experimental unit. Analysis of polyamine results was done utilizing a three factor factorial analysis with factors being water-regime, time (week) and location, with fifty replications. JMP 8 software (SAS institute, Cary, NC) was used to evaluate the results while interactions and main effects were tested with Analysis of Variance (ANOVA) at α ≤ 0.05.
3. Results
3.1. Soil Moisture Content and Environmental Data
Significant differences were observed between the water-stressed and control plots in Fayetteville, AR at the end of the first and second week (Figure 1(a)), however the opposite was observed in Lubbock, TX, where moisture content of both water-stressed and control plots were not significantly different at the end of the first and second week of the stress (Figure 1(b)). Additionally, similar mean temperatures were recorded in both locations (Figure 2) all through the stress period, while the contrary was recorded for relative humidity (Figure 2). Values of relative humidity were consistently higher in Fayetteville, AR compared to Lubbock, TX resulting in substantial differences in vapor pressure deficit (VPD) with average VPD during the stress period in Fayetteville, AR being 28 KPa and in Lubbock, TX being 39.5 KPa.
3.2. Stomatal Conductance
Water-deficit stress resulted in significant decreases in leaf stomatal conductance rates. Water-stressed plants had significantly lower stomatal conductance rates compared
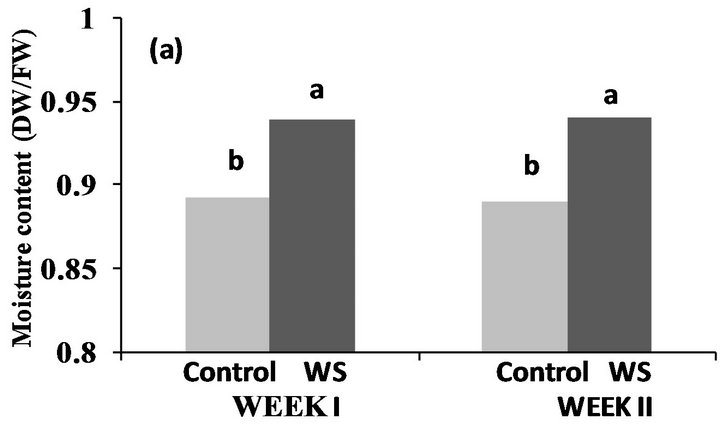
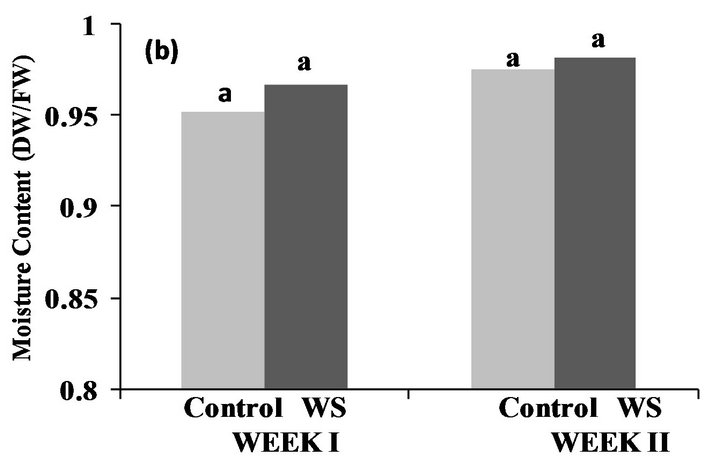
Figure 1. Effect of water-deficit stress on soil moisture content in Fayetteville, AR (a) and Lubbock, TX (b) at the end of the first and second week. Pairs of columns with the same letter are not significantly different (P ≤ 0.05) (Week 1: LSDFAY = 0.04632, LSDLUB = 0.01493; Week 2: LSDFAY = 0.04835, LSDLUB = 0.00562).
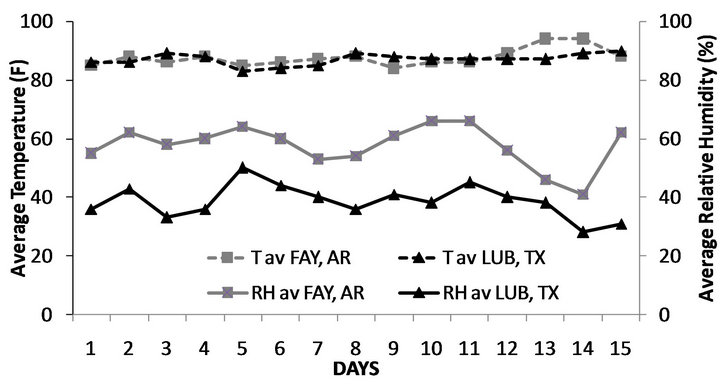
Figure 2. Mean day temperatures and relative humidity during the two weeks of the water-deficit stress period for Fayetteville, AR and Lubboc, TX. Samples were taken on days 7 and 14 (T av FAY, AR: average temperature in Fayetteville, AR; T av LUB, TX: average temperature in Lubbock, TX; RH av FAY, AR: average relative humidity in Fayetteville, AR; RH av LUB, TX: average relative humidity in Lubbock, TX).
to the control for the first week (P ≤ 0.0001) as well as the second week (P ≤ 0.0001) (Figure 3). Stomatal conductance rates were 38% and 42% lower compared to the control at the end of the first and second week of stress, respectively.
3.3. Polyamine Concentrations
Polyamine content of fifty white flowers (ovaries) and their subtending leaves were sampled randomly from each treatment plot at the end of each week from both locations. No interaction was observed in ovary PUT or SPD concentrations between water-regime, time (weeks) and location (P PUT = 0.9112, P SPD = 0.3674), while a significant interaction was observed in levels of SPM (P SPM = 0.0198). However, results were analyzed separately for each location for all three polyamines using a two factor factorial with the factors being water-regime and time (weeks). No interaction was observed between water-regime and time (weeks) in Lubbock, for ovary PUT, SPD and SPM (P PUT = 0.4297, P SPD = 0.3873, P SPM = 0.0926), while a significant interaction was observed for ovary PUT and SPD but not for SPM in Fayetteville (P PUT = 0.0079, P SPD = 0.0011, P SPM = 0.0896), and the effects of water-deficit stress were analyzed separately for each week using Student’s t-test at α ≤ 0.05.
A significant interaction was observed in leaf PUT concentrations between water-regime, time (weeks) and location (P PUT = 0.00029) whereas no interaction was observed in leaf SPD and SPM concentrations (P SPD = 0.3584, P SPM = 0.3127), however again all results were analyzed separately for each location using a two factor factorial with the factors being water-regime and time (weeks). In Lubbock, a significant interaction was found between the two factors in leaf PUT levels (P PUT = 0.0001) while not interaction was observed in leaf SPD and SPM levels (P SPD = 0.2559, P SPM = 0.3387) and
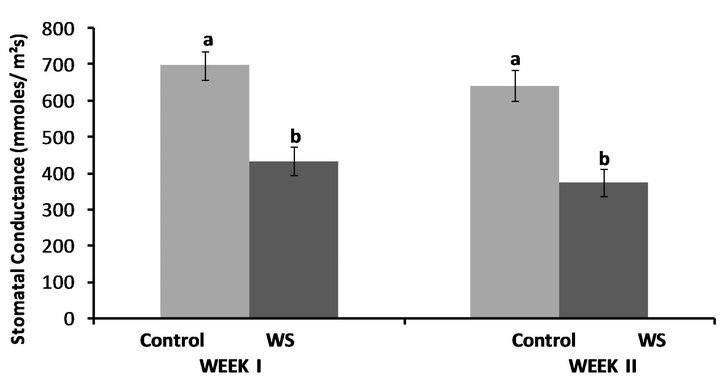
Figure 3. Effect of water-deficit stress on leaf stomatal conductance rates at the end of the first and the second week in Fayetteville. Pairs of columns in the same time interval (week) connected with different letters are significantly different (P = 0.05). Error bars indicate ±1 standard error.
a similar pattern was notices in Fayetteville where a significant interaction between water-regime and time (weeks) was found only in leaf PUT levels (P PUT = 0.0092) while the opposite was observed in leaf SPD and SPM (P SPD = 0.8589, P SPM = 0.6117), and the effects of water-deficit stress were analyzed separately for each week using Student’s t-test at α ≤ 0.05.
Water-deficit stress in Fayetteville had no effect on ovary PUT levels at the end of the first week, however leaf PUT levels were nearly 3-fold higher compared to the control (Figure 4). Leaf PUT concentrations were again increased at the end of the second week by 256% compared to the control while ovary PUT concentrations significantly increased as well by 54% compared to the control (Figure 4). Conversely, both ovary SPD (Figure 5) and SPM (Figure 6) concentrations significantly decreased at the end of the first week, by 30% and 31%, respectively whereas leaf SPD significantly increased by 27% (Figure 5), while leaf SPM levels remained similar to the control (Figure 6). Nevertheless, ovary and leaf
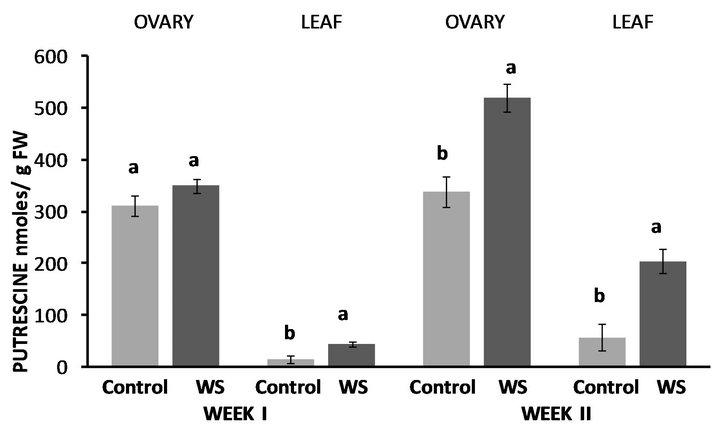
Figure 4. Effect of water deficit stress on ovary and leaf putrescine (PUT) concentrations in Fayetteville at the end of the first and second week. Pairs of columns within each type of tissue connected with the same letter are not significantly different (P = 0.05). Error bars represent ±1 standard error.
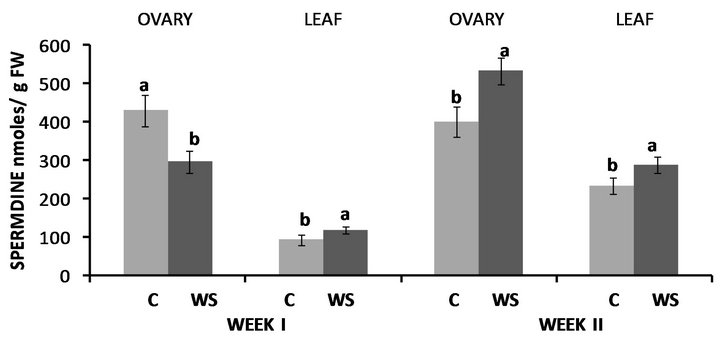
Figure 5. Effect of water deficit stress on ovary and leaf spermidine (SPD) concentrations in Fayetteville at the end of the first and second week. Pairs of columns within each type of tissue connected with the same letter are not significantly different (P = 0.05). Error bars represent ±1 standard error.
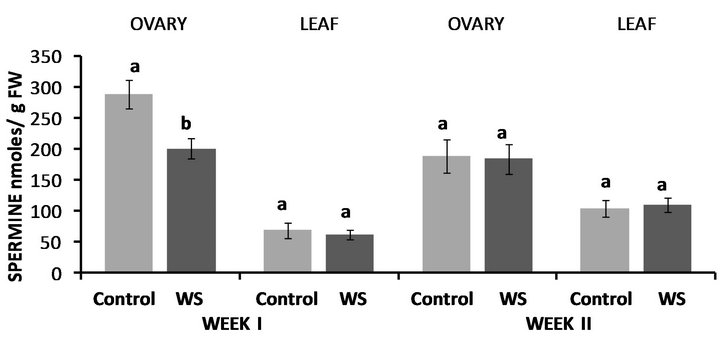
Figure 6. Effect of water deficit stress on ovary and leaf spermine (SPM) concentrations in Fayetteville at the end of the first and second week. Pairs of columns within each type of tissue connected with the same letter are not significantly different (P = 0.05). Error bars represent ±1 standard error.
SPD levels were significantly higher compared to the control at the end of the second week (Figure 5), while both ovary and leaf SPM levels remained unaffected by water stress (Figure 6).
In Lubbock, PUT concentrations of water-stressed ovaries were almost 2.5-fold higher compared to the control (Figure 7) and the same was observed in PUT levels of water-stressed leaves with them being though almost 9- fold higher compared to the control (Figure 7). Similarly, at the end of the second week, both ovary and leaf PUT concentrations were significantly higher compared to the control with the increases in the leaf being more pronounced (Figure 7). In accordance to PUT levels, both SPD (Figure 8) and SPM (Figure 9) concentrations of water-stressed ovaries were higher compared to the control by 46% and 47%, respectively, while leaf SPD (Figure 8) and SPM (Figure 9) levels were also significantly enhanced compared to the control at the end of the first week by 90% and 40%, respectively. Unlike ovary PUT levels, however, water-deficit stress had no effect on either ovary SPD (Figure 8) or ovary SPM (Figure 9) levels at the end of the second week, while the opposite was observed in leaf SPD and SPM concentrations where water-stressed leaves contained 67% and 30% more SPD (Figure 8) and SPM (Figure 9) respectively, compared to the control.
4. Discussion
Water supply is a key factor regulating cellular growth and development [22] and ultimately crop yield since it determines the function of several physiological and metabolic processes. Stomatal conductance has been reported to greatly decrease under limited water supply conditions [23,24]. Experiments with potted plants have reported that water-deficit stress decreased cotton leaf stomatal conductance [25,26]. In our study, in accordance with the majority of the previous research, significant decreases in leaf stomatal conductance rates of waterstressed plants were observed compared to the control.
Nevertheless, contrary to the insignificant responses in glutathione reductase activity in both tissues analyzed [27] pistil and leaf polyamine metabolism was significantly affected by water-deficit stress in both locations.
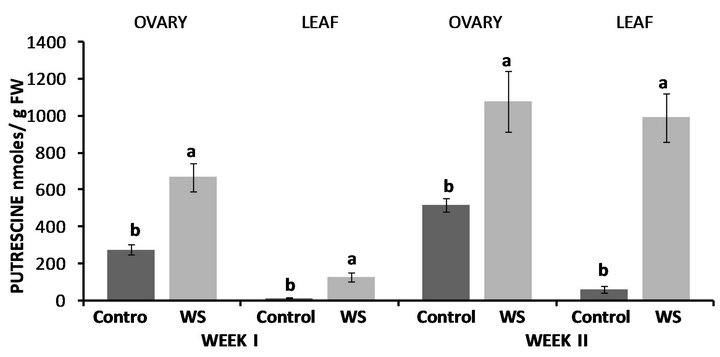
Figure 7. Effect of water deficit stress on ovary and leaf putrescine (PUT) concentrations in Lubbock at the end of the first and second week. Pairs of columns within each type of tissue connected with the same letter are not significantly different (P = 0.05). Error bars represent ±1 standard error.
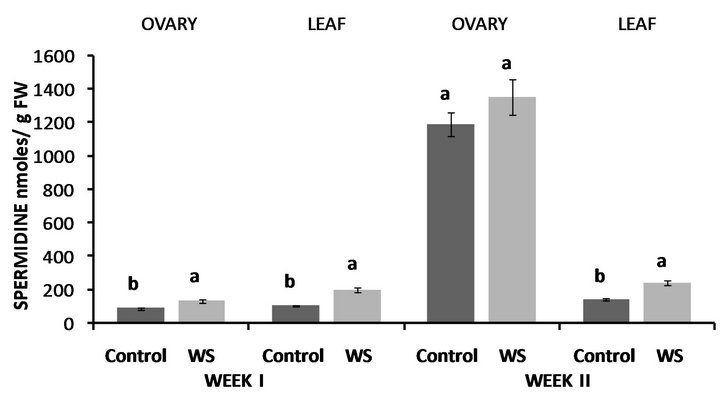
Figure 8. Effect of water deficit stress on ovary and leaf spermidine (SPD) concentrations in Lubbock at the end of the first and second week. Pairs of columns within each type of tissue connected with the same letter are not significantly different (P = 0.05). Error bars represent ±1 standard error.
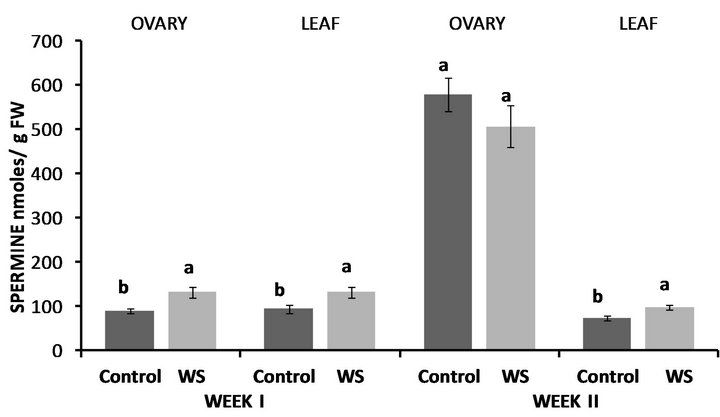
Figure 9. Effect of water deficit stress on ovary and leaf spermine (SPM) concentrations in Lubbock at the end of the first and second week. Pairs of columns within each type of tissue connected with the same letter are not significantly different (P = 0.05). Error bars represent ±1 standard error.
PUT concentrations of water-stressed leaves were significantly increased in both locations, compared to the control, at the end of the first and the second week and a similar pattern was observed in leaf SPD levels. However, water stress resulted in a significant increase in SPM concentrations only in Lubbock, whereas in Fayetteville its levels remained similar to the control. Increased polyamine concentrations have been reported as a response to water-deficit stress in a variety of other crops [28-31]. Furthermore, Alcazar et al. (2010) [32] reported that transgenic Arabidopsis plants transformed to overproduce PUT were more drought-tolerant compared to the wild types due to their ability to control and induce stomatal closure more effectively, while Liu et al. (2000) [33] made similar observations for SPD and SPM. Stomatal conductance rates of water-stressed leaves in Fayetteville, were significantly decreased at the end of the first and the second week, similar to the increases observed in PUT and SPD concentrations, while SPM remained unaffected leading us to assume that in cotton, PUT and SPD are more efficient in inducing stomatal closure and protecting plants from water loss compared to SPM. Additionally, we speculate that the lack of response in leaf SPM concentrations in Fayetteville compared to Lubbock was due to the environmental differences with the stress being more severe in Lubbock.
Regarding ovary polyamine concentrations, a differential response was observed between the two locations at the end of first week as well as at the end of the second week. Water-stressed PUT concentrations were significantly higher compared to the control in Lubbock at the end of the first and second week, while ovary SPD and SPM levels were significantly increased only at the end of the first week, but they remained unaffected at the end of the second. Conversely, in Fayetteville ovary PUT concentrations remained unaffected at the end of the first week, whereas SPD and SPM levels significantly decreased, while at the end of the second week ovary PUT, SPD and SPM concentrations were significantly higher in water-stressed ovaries compared to the control. Similar to leaves, increases in polyamine concentrations in reproductive units of chickpea (Cicer arietinum L.) have been observed [28]. In addition, Capell et al. (2004) [34] observed that PUT concentrations in rice have to surpass a certain threshold in order for SPD and SPM biosynthesis to be triggered. Similar results were reported also by Alcazar et al., (2010) [32] in experiments with Arabidopsis. We speculate that the decreases in SPD and SPM concentrations in Fayetteville in the first week were due to the insignificant increases in PUT levels that failed to initiate SPD and SPM biosynthesis, while the stress resulted in activation of SPD and SPM catabolic pathway, which has also been observed by Capell et al. (1993) [35].
Conversely, in Lubbock at the end of first week, the stress was enough to enhance PUT levels past the threshold, resulting in significant increases in both SPD and SPM concentrations. A similar pattern was observed in Fayetteville at the end of the second week, with both SPD and SPM levels of water-stressed being significantly higher compared to control after PUT concentrations were apparently past the critical level to induce their biosynthesis. However, the over-production of PUT observed at the end of the second week in Lubbock resulted in SPD and SPM levels of water-stressed ovaries remaining similar to the control, leading us to speculate either that nitrogen pools of the plants were depleted or plants were unable to utilize them, as has also been reported by Lazcano-Ferrat and Lovett (1999) [36]. Hence, polyamine metabolism in cotton appears to be greatly affected by water stress, with leaf polyamine metabolism being more sensitive compared to ovary polyamine metabolism, which is in accordance with the observed enhanced drought tolerance of cotton reproductive units compared to leaves.
The results of our study indicated that leaf and ovary polyamine metabolism were significantly affected by limited water supply, suggesting that polyamines have a critical role in cotton protection under adverse environmental conditions, especially PUT and SPD. However, more research needs to be conducted in order to elucidate the exact function of each polyamine and the ways polyamines can be used to enhance drought-tolerance in cotton.
REFERENCES
- D. M. Oosterhuis, “A Post Mortem of the Disappointing Yields in the 1993 Arkansas Cotton Crop,” In: D. M. Oostehuis, Ed., Summaries of Arkansas Cotton Research, Fayetteville, 1994, pp. 22-26.
- J. A. Lee “Cotton as a World Crop,” In: R. J. Kohel and C. F. Lewis, Eds., Cotton Agronomy Monogram 24, ASA, CSSA, SSSA, Madison, 1984, pp. 1-25.
- D. M. Oosterhuis and S. D. Wullschleger, “Osmotic Adjustment in Cotton (Gossypium Hisrsutum L.) Leaves and Roots in Response to Water Stress,” Plant Physiology, Vol. 84, No. 4, 1987, pp. 1154-1157. doi:10.1104/pp.84.4.1154
- A. L. Nepomuceno, D. M. Oosterhuis and J. M. Stewart, “Physiological Responses of Cotton Leaves and Roots to Water Deficit Induced by Polythelene Glycol,” Environmental and Experimental Botany, Vol. 40, No. 1, 1998, pp. 29-41. doi:10.1016/S0098-8472(98)00018-5
- J. J. Burke, J. L. Hatfield, R. R. Klein and J. E. Mullet, “Accumulation of Heat Shock Proteins in Field Grown Soybean,” Plant Physiology, Vol. 78, No. 2, 1985, pp. 394-398. doi:10.1104/pp.78.2.394
- V. V. Kuznetsov, V. Rakitin and V. N. Zholkevich, “Effects of Preliminary Heat-Shock Treatment on Accumulation of Osmolytes and Drought Resistance in Cotton Plants during Water Deficiency,” Physiologia Plantarum, Vol. 107, No. 4, 1999, pp. 399-406. doi:10.1034/j.1399-3054.1999.100405.x
- J. E. Quisenberry and B. Roark, “Influence of Indeterminate Growth Habit on Yield and Irrigation Water-Use Efficiency in Upland Cotton,” Crop Science, Vol. 16, 1976, pp. 762-765. doi:10.2135/cropsci1976.0011183X001600060005x
- G. A. Constable and A. B. Hearn, “Irrigation of Crops in a Subhumid Climate, 6: Effects of Irrigation and Nitrogen Fertilizer on Growth, Yield and Quality of Cotton,” Irrigation Science, Vol. 2, 1981, pp. 17-28.
- P. O. Cull, A. B. Hearn and R. C. G. Smith, “Irrigation Scheduling of Cotton in a Climate with Uncertain Rainfall. I. Crop Water Requirements and Response to Irrigation,” Irrigation Science, Vol. 2, 1981, pp. 127-140. doi:10.1007/BF00257975
- P. O. Cull, R. G. C. Smith and K. McCaffery, “Irrigation Scheduling of Cotton in a Climate with Uncertain Rainfall. II. Development and Application of A Model for Irrigation Scheduling,” Irrigation Science, Vol. 2, 1981, pp. 141-154. doi:10.1007/BF00257976
- N. C. Turner, A. B. Hearn, J. E. Begg and G. A. Constable, “Cotton (Gossypium hirsutum L.) Physiological and Morphological Responses to Water Deficits and Their Relationship to Yield,” Field Crops Research, Vol. 14, 1986, pp. 153-170. doi:10.1016/0378-4290(86)90054-7
- D. W. Grimes, W. L. Dickens and W. D. Anderson, “Functions for Cotton (Gossypium hirsutum L.) Production from Irrigation and Nitrogen Fertilization Variables. Ii. Yield Components and Quality Characteristics,” Agronomy Journal, Vol. 61, No. 5, 1969, pp. 773-776. doi:10.2134/agronj1969.00021962006100050036x
- A. Bouchereau, A. Aziz, F. Lahrer and J. Martin-Tanguy, “Polyamines and Environmental Challenges: Recent Developments,” Plant Science, Vol. 140, No. 2, 1999, pp. 103-125. doi:10.1016/S0168-9452(98)00218-0
- R. Kaur-Sawhney, A. F. Tiburcio, T. Altabella and A. W. Galston, “Polyamines in Plants: An Overview,” Journal of Cell Molecular Biology, Vol. 2, No. 1, 2003, pp. 1-12.
- P. T. Evans and R. L. Malmberg, “Do Polyamines Have Roles in Plant Development?” Annual Reviews of Plant Physiology and Plant Molecular Biology, Vol. 40, 1989, pp. 235-269. doi:10.1146/annurev.pp.40.060189.001315
- R. K. Kakkar and V. K. Rai, “Plant Polyamines in Flowering and Fruit Ripening,” Phytochemistry, Vol. 33, No. 6, 1993, pp. 1281-1288. doi:10.1016/0031-9422(93)85076-4
- M. D. Groppa and M. P. Benavides, “Polyamines and Abiotic Stresses: Recent Advances,” Amino Acids, Vol. 34, No. 1, 2008, pp. 35-45. doi:10.1007/s00726-007-0501-8
- A. C. Bibi, D. M. Oosterhuis and E. D. Gonias, “Exogenous Application of Putrescine Ameliorates the Effect of High Temperature in Gossypium hirsutum L. Flowers and Fruit Development,” Journal of Agronomy and Crop Science, Vol. 196, No. 3, 2010, pp. 205-211. doi:10.1111/j.1439-037X.2009.00414.x
- A. Massaci, S. M. Nabiev, L. Petrosanti, S. K. Nematov, T. N. Chernikova, K. Thor and J. Leipner, “Response of the Photosynthetic Apparatus of Cotton (Gossypium hirsutum L.) to the Onset of Drought Stress under Field Conditions Studied by Gas-Exchange Analysis and Chlorophyll Fluorescence Imaging,” Plant Physiology and Biochemistry, Vol. 46, No. 2, 2008, pp.189-195. doi:10.1016/j.plaphy.2007.10.006
- IPCC (Intergovernmental Panel on Climate Change), “Climate Change 2007: Impacts, Adaptation and Vulnerability,” In: M. L. Parry, O. F. Cnaziani, J. P. Palutikof, P. J. van der Linden and C. E. Hanson, Eds., Contribution of Working Group II to Fourth Assesment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, 2007, pp. 1-49.
- M. A. Smith and P. J. Davies, “Separation and Quantification of Polyamines in Plant Tissue by High Performance Liquid Chromatography of Their Dansyl Derivatives,” Journal of Plant Physiology, Vol. 78, 1985, pp. 89-91. doi:10.1104/pp.78.1.89
- T. C. Hsiao, “Plant Responses to Water Stress,” Annual Reviews of Plant Physiology, Vol. 24, No. 1, 1973, pp. 519-570. doi:10.1146/annurev.pp.24.060173.002511
- F. X. Socias, M. J. Correia, M. M. Chaves and H. Medrano, “The Role of Abscisic Acid and Water Relations in Drought Responses of Subterranean Clover,” Journal of Experimental Botany, Vol. 48, 1997, pp. 1281- 1288. doi:10.1093/jxb/48.6.1281
- M. M. Chaves, J. S. Pereira, J. Maroco, M. L. Rodriguez, C. P. P. Ricardo, M. L. Osorio, I. Carvalho, T. Faria and C. Pinheiro, “How Plants Cope with Water Stress in the Field. Photosynthesis and Growth,” Annals of Botany, Vol. 89, No. 7, 2002, pp. 907-916. doi:10.1093/aob/mcf105
- S. D. Wullschleger and D. M. Oosterhuis, “Photosynthetic and Respiratory Activity of Fruiting Forms within the Cotton Canopy,” Plant Physiology, Vol. 94, No. 2, 1990, pp. 463-469. doi:10.1104/pp.94.2.463
- V. A. Da Costa and J. T. Cothren, “Drought Effects on Gas Exchange, Chlorophyll, and Plant Growth of 1-Methylcyclopropene Treated Cotton,” Agronomy Journal, Vol. 103, No. 4, 2011, pp. 1230-1241. doi:10.2134/agronj2010.0479
- D. A. Loka, D. M. Oosterhuis, B. L. McMichael and C. J. Fernandez, “Water-Deficit Stress and Reproductive Development in Cotton,” Procedures of the Beltwide Cotton Production and Research Conference of National Cotton Council of America, Memphis, 4-8 January 2010.
- H. S. Nayyar, S. Kumar, K. J. Singh and K. K. Dhir, “Involvement of Polyamines in the Contrasting Sensitivity of Chickpea (Cicer arietinum L.) and Soybean (Glycine max L. Merrill) to Water-Deficit Stress,” Botanical Bulletin of Academia Sinica, Vol. 46, No. 4, 2005, pp. 333-338.
- H. P. Liu, Z. X. Zhu, T. X. Liu and Z. H. Li, “Effects of Osmotic Stress on the Kinds, Forms and Levels of Polyamines in Wheat Coleoptiles,” Journal of Plant Physiology and Molecular Biology, Vol. 32, 2006, pp. 293-299.
- J. Yang, J. Zhang, K. Liu, Z. Wang and L. Liu, “Involvement of Polyamines in Drought Resistance of Rice,” Journal of Experimental Botany, Vol. 58, No. 6, 2007, pp. 1545-1555. doi:10.1093/jxb/erm032
- K. Yamaguchi, Y. Takahashi, T. Berberich, A. Imai, T. Takahashi, A. J. Michael and T. Kusano, “A Protective Role for the Polyamine Spermine against Drought Stress in Arabidopsis,” Biochemistry and Biophysics Research Community, Vol. 352, 2007, pp. 486-490. doi:10.1016/j.bbrc.2006.11.041
- R. Alcazar, J. Planas, T. Saxena, X. Zarza, C. Bortolotti, J. Cuevas, M. Bitrian, A. F. Tiburcio and T. Altabella, “Putrescine Accumulation Confers Drought Tolerance in Transgenic Arabidopsis Plants Over-Expressing the Homologous Arginine decarboxylase 2 Gene,” Plant Physiology and Biochemistry, Vol. 48, 2010, pp. 547-552. doi:10.1016/j.plaphy.2010.02.002
- K. Liu, H. Fu, Q. Bei and S. Luan, “Inward Potassium Channel in Guard Cells as a Target of Polyamine Regulation of Stomatal Movements,” Plant Physiology, Vol. 124, No. 3, 2000, pp. 1315-1325. doi:10.1104/pp.124.3.1315
- T. Capell, L. Bassle and P. Christou, “Modulation of the Polyamine Biosynthetic Pathway in Trasngenic Rice Confers Tolerance to Drought Stress,” Proceedings of National Academy of Science, Vol. 101, No. 26, 2004, pp. 9909-9914. doi:10.1073/pnas.0306974101
- T. Capell, J. L. Campos and A. F. Tiburcio, “Antisenescence Properties of Guazatine in Osmotically Stressed Oat Leaves,” Phytochemistry, Vol. 32, No. 4, 1993, pp. 785-788. doi:10.1016/0031-9422(93)85205-6
- I. Lazcano-Ferrat and C. J. Lovatt, “Relationship between Relative Water Content, Nitrogen Pools and Growth of Phaseolus vulgaris L. and P. acutifolius, A. Gray During Water Deficit,” Crop Science, Vol. 39, No. 2, 1999, pp. 467-475. doi:10.2135/cropsci1999.0011183X0039000200028x
NOTES
*Corresponding author.

