American Journal of Plant Sciences
Vol.3 No.7(2012), Article ID:20694,4 pages DOI:10.4236/ajps.2012.37112
Improving Effect of Salicylic Acid on the Multipurpose Tree Ziziphus spina-christi (L.) Willd Tissue Culture
![]()
Botany Department, Faculty of Science, Sohag University, Sohag, Egypt.
Email: nasergalal@gmail.com
Received April 18th, 2012; revised May 16th, 2012; accepted May 25th, 2012
Keywords: Callus; Growth Regulator; Multiplication; Shoot-Tips; Somatic Embryogenesis; Ziziphus spina-christi
ABSTRACT
Salicylic acid (SA) is one of numerous phenolic compounds found in plants with numerous important physiological events. Exogenously application of Salicylic acid (SA) is useful for the growth and development of plants. This investigation aimed to study the improving effect of salicylic acid on the tissue culture of Ziziphus spina-christi. To study its improving effect were used shoot-tip explants cultured on Murashige and Skoog (MS) solid medium supplemented with different concentrations and various combinations of auxins, cytokinins and Salicylic acid. Media without growth regulators enhanced the growth and elongation of shoot-tip explants not its proliferation. Lower concentrations of cytokinin were better for shoot-tip proliferation than higher ones. 6-Benzylaminopurine (BAP) was superior to kinetin (KN) in shoot-tip proliferation. The optimum culture conditions for shoot-tip proliferation were achieved on MS medium supplemented with 0.25 mg/l 6-Benzylaminopurine (BAP) and 25 mg/l Salicylic acid (SA). The optimum culture conditions for callus formation were obtained on MS medium supplemented with 3 mg/l 2,4-Dichlorophenoxyacetic acid (2,4-D) and 25 mg/l Salicylic acid (SA), while the optimum culture conditions for somatic callus formation were obtained on MS medium supplemented with 2.0 mg/l 2,4-Dichlorophenoxyacetic acid (2,4-D) and 10 mg/l Salicylic acid (SA). The optimum culture conditions for rooting of shoots were obtained on MS medium supplemented with 0.25 mg/l of Indole-3-butyric acid (IBA) and 10 mg/l Salicylic acid (SA). The highest percentage of survival plants was obtained in the soil mixture supplied with 10 mg/l Salicylic acid (SA). Salicylic acid (SA) showed positive effect and good response on callusing, shooting and rooting of Z. spina-christi. Cultures received small amounts of salicylic acid were better than those lack it.
1. Introduction
Salicylic acid is a phenolic derivative, distributed in a wide range of plant species. It is a natural product of phenylpropanoid metabolism. It appears to be just like in mammals an effective therapeutic agent for plants. Beside this function during biotic and abiotic stress plays a crucial role in the regulation of physiological and biochemical processes during the entire lifespan of plant [1]. Its application might be safe and more useful for plant growth improving. Salicylic acid stimulates the growth and development of shoots and roots of the treated plants [2]. It is ubiquitous in plants generating a significant impact, transpiration, ion uptake and transport. Enhancement of the level of chlorophyll and carotenoid pigments, photosynthetic rate and modifying the activity of some of the important enzymes are other roles assigned to Salicylic acid. It induces specific changes in leaf anatomy and chloroplast structure [3]. Salicylic acid involves in endogenous signaling mediating in plant defense against pathogens. Signaling network of salicylic acid confirms its important role in both plant health and disease. It plays a role in the resistance to pathogens by inducing the production of pathogenesis-related protein [4]. Zizyphus spina-christi (L.) Willd is one of the most important trees in the dry parts of tropical Arabia. It is a multipurpose tree belonging to the botanical family Rhamnaceae [5]. All parts of the plant are used by the local Arab people to help maintain a healthy lifestyle. The plant has also been used for its soothing properties [6]. Fruit has a very high energy value and it can be eaten raw or dried. It tastes like a mixture of dates and apples [5]. The flowers are important for the production of wild bee honey [7]. Plant leaves are also used in folk medicine as which provides potential for large scale production of genetically identical superior strains for an antiseptic anti-inflamematory agent, and for healing skin diseases such as atopic dermatitis [8]. In vitro shoot multiplications were obtained successfully from shoot-tips of Ziziphus spinachristi. Lower concentrations of cytokinin were better for lateral bud proliferation than higher ones [6]. Root initiation was observed in media containing lower concentrations of IBA and the percentage of adventitious root formation was low [8]. Seed germination of Zizyphus spinachristi is poor, and the tree is in a great demand due to its economic importance. Therefore, this investigation aimed to study the stimulatory effect of salicylic acid on the tissue culture of Ziziphus spina-christi to improve its proliferation efficiency.
2. Materials and Methods
2.1. Plant Materials Preparation
Shoot-tip cuttings of Z. spina-christi were collected at the midsummer from healthy mature tree, and then were washed in soap water prior to surface sterilization, followed by Shoot-tip explants preparing by removing all the expanded leaves leaving the shoot meristem with 2 - 3 leaf primordia. The shoot-tip explants were surface sterilized by immersing in 70% ethanol, for a minute, followed by immersing in 0.1% mercuric chloride for 2 min, then rinsed with three changes of sterile distilled water.
2.2. Culture Conditions
The explants were, aseptically, placed on agar-solidified MS medium [9] containing 3% sucrose and various growth regulators combinations. All media were adjusted to pH 6.0 at 25˚C with KOH or HCL and were autoclaved for 20 min. at 121˚C (15 PSI nominal steam pressure). For shooting, the shoot-tip explants were placed on basal solid medium (MS medium) supplemented with different concentrations of BAP, KN and SA. The cultures were incubated in a 16 h light and 8 h dark cycle at 25˚C ± 2˚C and were illuminated with Fluorescent light at 2000 lux. For callus induction, the shoot-tip explants were placed on MS solid medium supplemented with different concentrations and various combinations of BAP, 2,4-D and SA. The cultures were incubated in the dark at 25˚C ± 2˚C. For rooting, the excised shoots were transferred into the same solid medium (MS medium) supplemented with different concentrations and various combinations of NAA, IBA and SA. The well developed plantlets derived from the germinated embryoids were grown in autoclaved soil mixture contained with sand, peat moss and well rotted cow-dung manure by the ratio of 1:1:1 (w/v), then placed in the green house conditions. Irrigation was carried out using ordinary tap water contained with different concentrations of SA and 100 mg/l NPK fertilizer twice a week.
2.3. Statistical Analysis
Data were statistically analyzed using SAS program, version 9.1. [10].
3. Results
Shoot multiplications were obtained successfully from the shoot-tip explants of Z. spina-christi on MS media supplemented with different concentration of BAP, KN and SA (Table 1). The developed lateral buds were healthy and grew vigorously in all growth media used. There was no lateral bud induction on MS medium lacked growth regulators. MS medium lacked growth regulators stimulated the growth and elongation of shoots not its proliferation. There was no sign of callus formation at the cut end of the explants during lateral bud induction. Prolonged culture in media showed yellowing of the older leaves and hardened stems. BAP was better than KN for shoot-tip proliferation. In the case of KN treatments, MS medium supplemented with 0.50 mg/l KN was the most effective one, associated with the highest percentage (56.7%) of responding explants, number (2.4) of lateral buds, length (2.05 cm) of shoot buds and number (2.30) of nodes. In the case of SA and KN interaction, the highest percentage of responding explants, number of lateral buds, length of shoot buds and number of nodes showed high increase. MS medium supplemented with 0.50 mg/l KN and 25 mg/l SA was the most effective one, associated with the highest percentage (67.3%) of responding explants, number (2.9) of lateral buds, length (2.73 cm) of shoot buds and number (2.83) of nodes. In the case of BAP treatments, the lower concentration was better than higher ones. MS medium supplemented with 0.25 mg/l BAP was the most effective medium, associated with the highest percentage (70.4%) of responding explants, number (3.1) of lateral buds, length (2.67 cm) of shoot buds and number (2.80) of nodes. In the case of SA and BAP interaction, the highest percentage of responding explants, number of lateral buds, length of shoot buds and number of nodes showed high increase compare with media lack it. MS medium supplemented with 0.25 mg/l BAP and 10 mg/l SA was the best overall, associated with the highest percentage (78.7%) of responding explants, number (3.8) of lateral buds, length (3.87 cm) of shoot buds and number (3.67) of nodes (Table 1). Callus was initiated from the shoot tip explants on MS basal medium supplemented with different concentrations and various combinations of BAP, 2,4-D and SA (Table 2). Within 10 - 12 days the shoot-tip explants enlarged and developed calluses at the surface of shoot-tip explants in most of media tested, subsequently covered the whole surface of explants within 4 weeks. The response of explants varied with the different concentrations and the various combinations of BAP, 2,4-D and SA. The response of callus growth varied in the different treatments. The results of callus growth
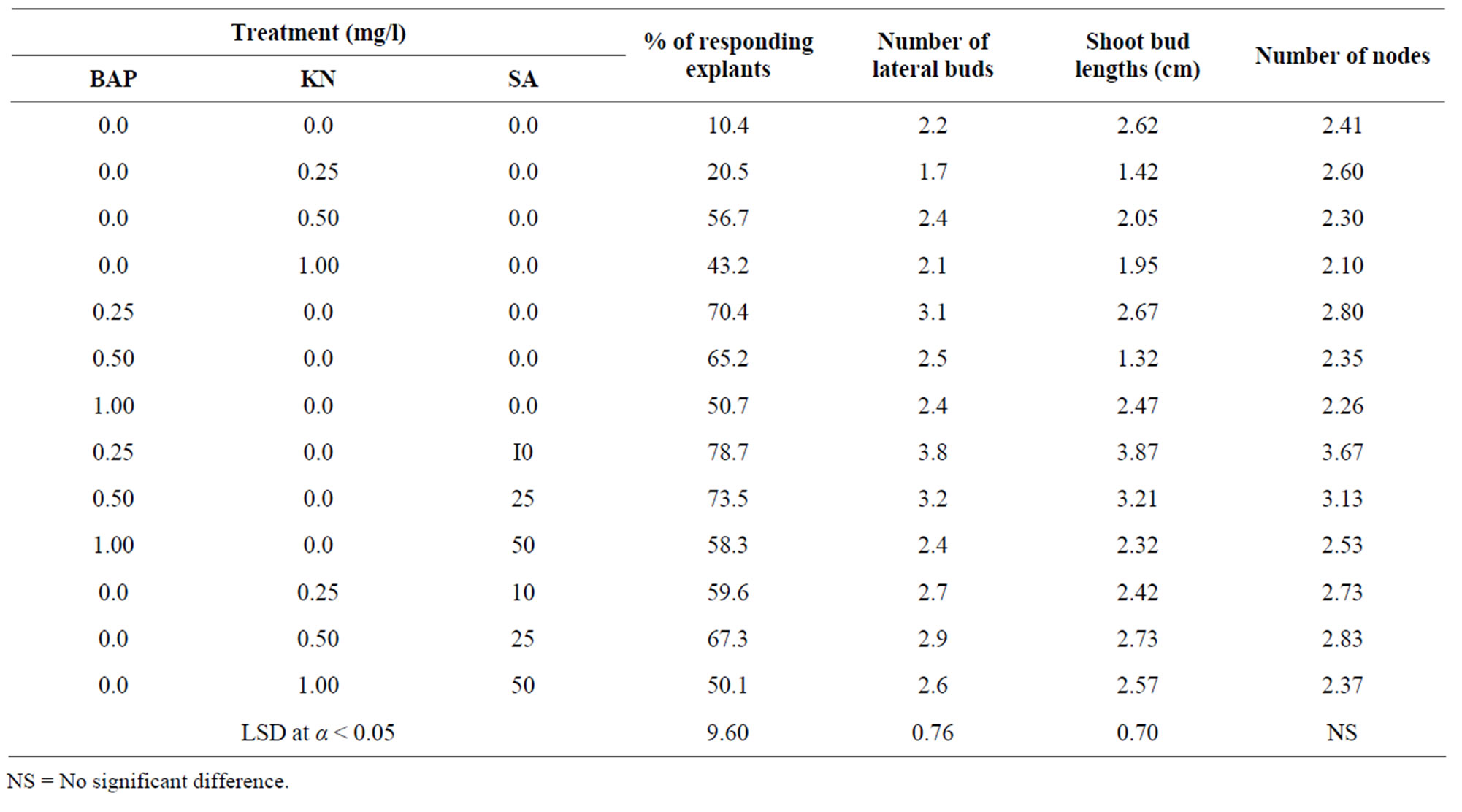
Table 1. Effect of different concentrations and various combinations of BAP, KN and SA on proliferation of Zizyphus spinachristi shoot-tips cultured on MS-solid medium for four weeks.
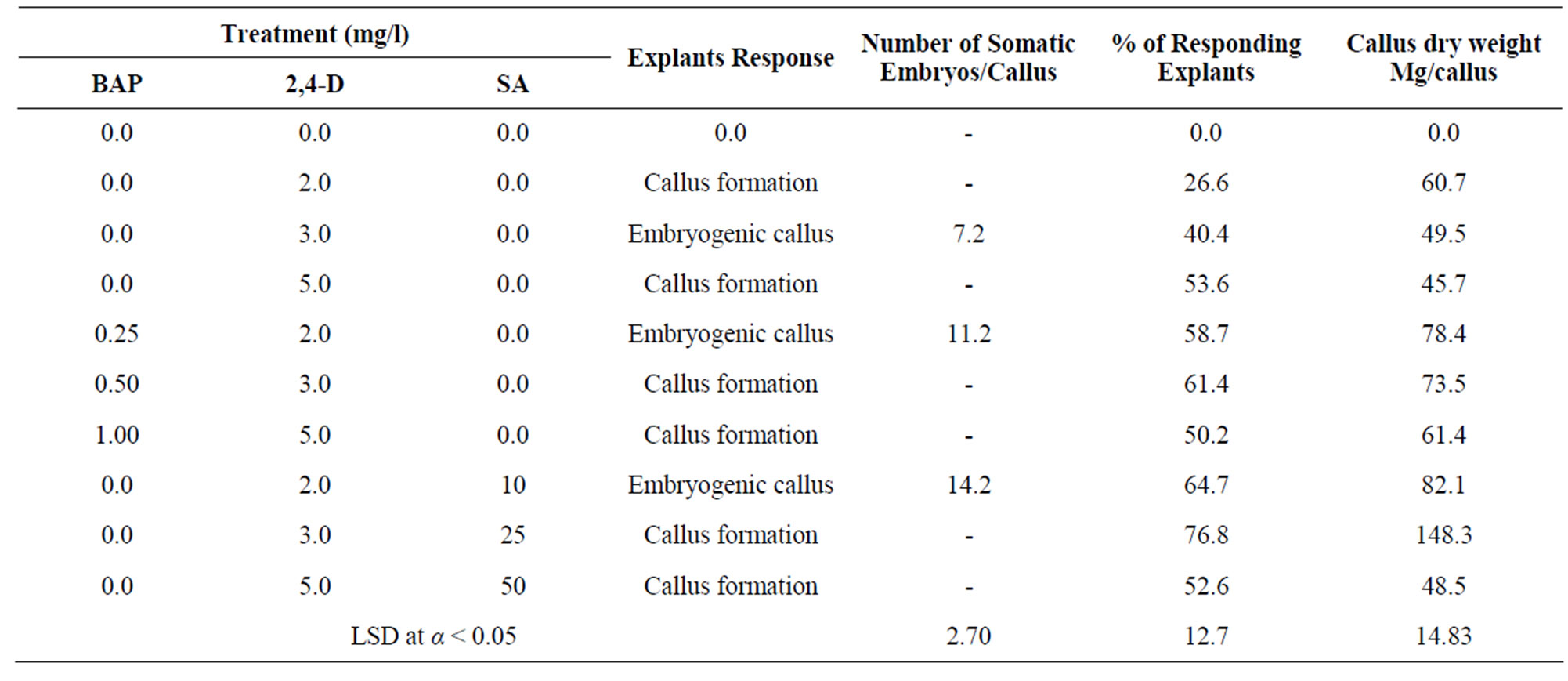
Table 2. Effect of different concentrations and various combination of BAP, 2,4-D and SA on the callus formation form shoot-tip explants cultured on MS-solid medium for four weeks. Two independent experiments were conducted with 25 replicates per treatment.
capacity after 4 weeks showed highly significant differences in the most of callus dry weights of treatments. SA significantly increased the callus dry weight compare with those lack it. MS medium supplemented with 3 mg/l 2,4-D and 25 mg/l SA was the most effective one, associated with the highest percentage (76.8%) of responding explants and the highest callus dry weight (148.3 mg/callus). Within 3 weeks some calluses induced embryos, these calluses were pale-yellow and friable in its appearance. SA significantly increased the number of somatic embryos compare with media lacked it (Table 2). MS medium supplied with 2 mg/l 2,4-D and 10 mg/l SA was the most effective one associated with the highest number (14.2) of somatic embryos. There were no callus formation and somatic embryos induction in media lacked growth regulators. The addition of SA exhibited positive effect and good response on the callus formation and somatic embryos induction in all media tested. The production and growth of callus decreased with the increase of SA concentration. Roots emerged after 2 weeks of culture initiation (Table 3). MS medium lacked growth regulators produced few number of roots. These roots were rather short and differ in its phenotype. In the case of NAA treatments, the rooting capacity was very low in most of media tested. MS medium supplied with NAA produced calluses and tufted roots at the bases of shoots. Interaction of IBA and NAA exhibited lower effect in the root formation. In the case of IBA treatments, the lower concentration of IBA for root formation was better than higher ones. MS medium supplemented with 0.25 mg/l IBA was the most effective one, associated with the highest percentage (63%) of shoots developed roots, the highest number (6.80) of roots per shoot and the tallest (2.53) roots. The effect of various concentrations of SA and IBA on the excised shoots rooting (Table 3) showed that the percentage of excised shoots induced roots and the length of growing roots increased compare with media lacked it. MS solid medium supplemented with 0.25 mg/l IBA and 25 mg/l SA was the best overall, associated with the highest percentage (84%) of shoots developed roots, the highest number (12.20) of roots per shoot and the tallest (3.74 cm) root. There was no increase on the root capacity with the increase of SA concentration. The results showed that the addition of different concentration of SA to the soil mixture of acclimated plantlets twice a week increased the numbers of survival plants (Table 4). The soil mixture supplied with 10 mg/l SA was the most effective one, associated with the highest percentage (92%) of survival plants.
4. Discussion
Salicylic acid (SA) has significant impacts on the various aspects of plant life. In vitro shoot multiplications were obtained successfully from shoot-tips of Ziziphus spinachristi. Lower concentrations of cytokinin were better for lateral bud proliferation than higher ones [6]. In this study the exogenous application of relatively low levels of SA in the culture media improved the proliferation efficiency of Z. spina-christi tissue culture. These results are in agreement with some earlier studies [11] in which the exogenous application of SA promoted the cellular growth and somatic embryogenesis in Coffea arabien tissue culture. The positive effect of SA on the proliferation of plant tissue cultures could be a reflection of an increase in the number of meristematic cells [12]. It was reported that SA could act as a morphoregulator signal and this supports the hyposthesis that SA is a growth regulator [12]. SA stimulates the organogenesis and embryogenesis by regulation the cell division, enlargement and/or activating DNA replication without concomitant nuclear division [13]. SA stimulates the growth and development of shoots and roots of the treated plants [2]. Aqueous solutions of SA applied as a spray to the shoots of soybean (Glycine max (L.) Merr. cv. Cajeme), significantly increased the growth of shoots and roots measured
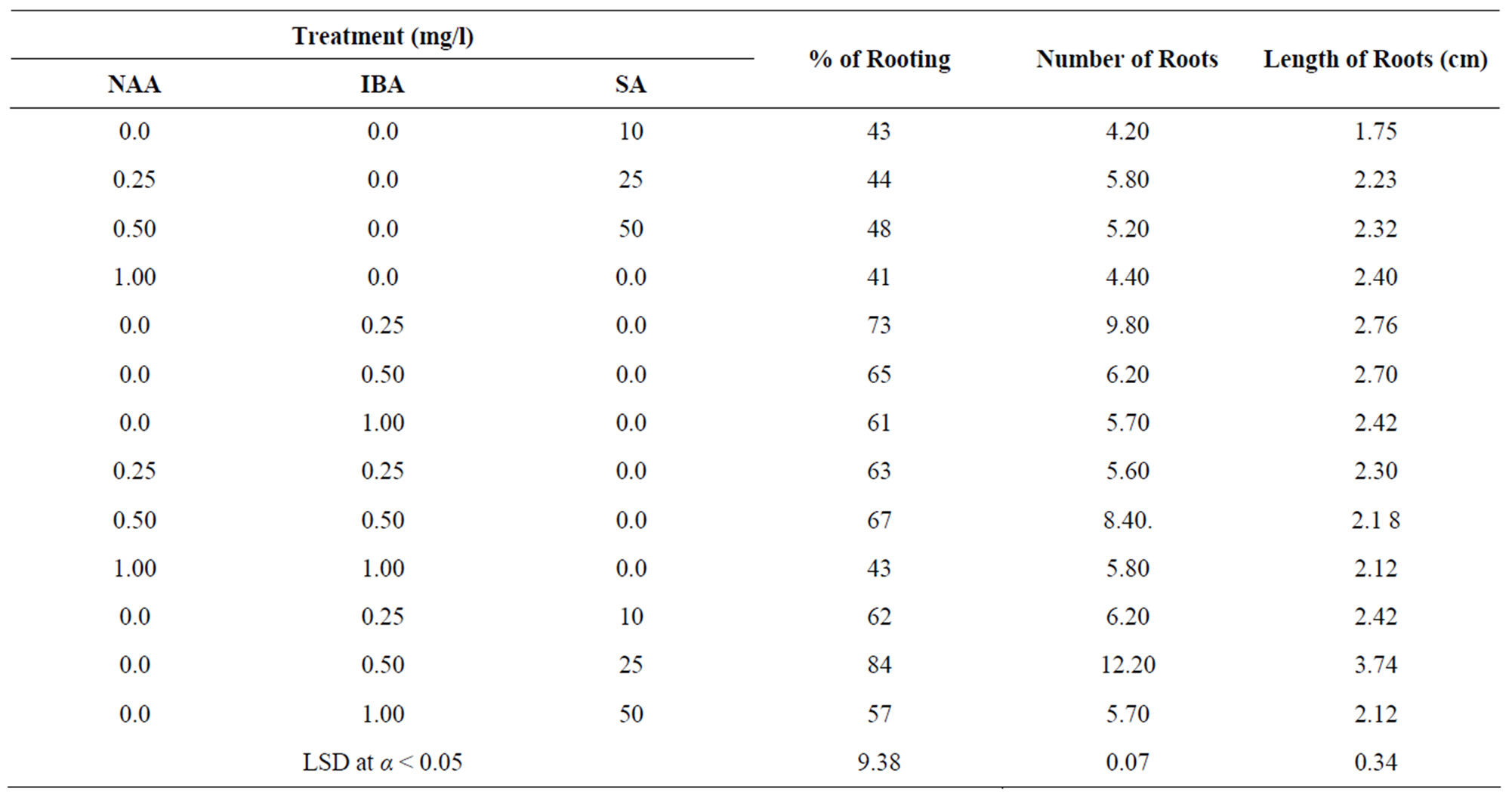
Table 3. Effect of different concentrations and various combinations of NAA, IBA and SA on the rooting of shoots cultured on MS-solid medium for four weeks. Two independent experiments were conducted with 25 replicates per treatment.
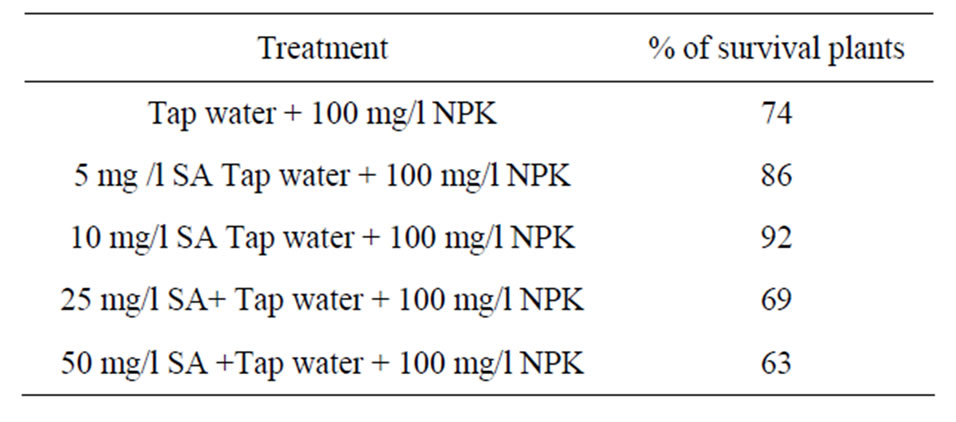
Table 4. Effect of different concentration of Salicylic acid on the viability of acclimatized plants in the green house conditions. Control = Tap water + 100 mg/l NPK.
after seven days of treatment [14]. The results of this study are in agreement with some earlier studies [15] in which SA increased the dry weights of calluses and this increase was attributed to the acceleration processes connected to the cell division and its elongation. It was pointed out that the positive effect of SA on callus growth on dry basis resulting in the increase in ethylene production in the atmosphere of callus cultures which may be responsible for the enhancement of growth and maturation of callus cultures [15]. The results of this study showed high decrease in the proliferation efficiency of Z. spina-christi tissue culture with the increase of SA concentration. There is a relationship between plant growth and the amount of SA fed to cultures. The number and length of roots were varied in all excised shoots tested in the various treatments of SA, since relatively low concentration of SA had a stimulatory effect and a good response on the rooting of shoots [12]. It was reported that the number and the length of growing roots varied with the different concentrations of SA used in the culture media [11]. It was found that increasing the concentration of auxin promoted rooting without callusing [8]. From the recoded data of this study it was appeared that the addition of low concentration of SA (antitranspirant) increased the number of survival plants during the acclimatization stage. It is well known that the regenerated plants exert a great quantity of water during the acclimatization stage of the tissue culture. SA and its related compounds reduced the water loss and improved the water use efficiency through closing of stomata [1]. SA inhibit catalase activity and increase the concentration of H2O2 in guard cell cytoplasm. H2O2 oxidizes the plasma membrane and increase the membrane permeability of K+. The mass efflux of K+ induces the loss of pressure and lead to stomata closing [16]. The inhibition of photosynthetic activity by SA suggests that stomata closing by SA are also related to the decrease of photosynthetic activity [3]. In conclusion, from the recorded data of this study, it was appeared that the exogenous application of SA in the culture media had a positive effect and good response on callus, shoot and root proliferations. Cultures received relatively small amounts of SA were better than those lacked it. During the acclimatization stage, plantlets supplied with relatively low concentration of SA grew and developed well compared to those lacked it. These results of improving effect of salicylic acid on Ziziphus spina-chriti tissue culture have thrown up many questions in need of further investigations, using wide range of growth regulator components and physical change in plant growth environment.
REFERENCES
- P. Karuppaiah, S. Rameshkumar, K. Shah and R. Marimusthu, “Effect of Antitranspirants on Growth, Photosyhthetic Rate and Yield Characters Brinjal,” Indian Journal of Plant Physiology, Vol. 8, No. 2, 2003, pp. 189-192.
- Z. A. M. Sanaa, S. L. Ibrahim and E. H. A. Sharaf, “The Effect α-Naphthaline Acetic Acid (NAA), Salicylic Acid (SA) and Their Combinations on Growth, Fruit Setting. Yield and Some Correlate Components in Dry Bean (Phaseolus vulgaris L.),”Annals of Agricultural Science, Ain Shams University, Cairo, Vol. 46, No. 2, 2001, pp. 451-463.
- L. Popova, L. Maslenkova, R. Yordanova, A. Krantev, G. Szalai and T. Janda, “Salicylic Acid Protects Photosynthesis against Cadmium Toxicity in Pea Plant,” General and Applied Plant Physiology, Vol. 34, No. 3-4, 2008, pp. 133-148.
- M. Heil and R. M. Bostock, “Induced Systemic Resistance (ISR) against Pathogens in the Context of Induced Plant Defenses,” Annals of Botany, Vol. 89, No. 5, 2002, pp. 503-512. doi:10.1093/aob/mcf076
- A. M. Pawlowska and F. Camangi, A. Bader and A. Braca, “Flavonoids of Zizyphus jujuba L. and Zizyphus spina-christi (L.) Willd (Rhamnaceae) Fruits,” Food Chemistry, Vol. 112, No. 4, 2009, pp. 858-862. doi:10.1016/j.foodchem.2008.06.053
- M. A. AI-Sulaiman and M. N. Barakat, “In Vitro Shoot Multiplication of Ziziphus spina-christi by Shoot Tip Culture,” African Journal of Biotechnology, Vol. 9, No. 6, 2010, pp. 850-857.
- A. S. Saied, J. Gebauer, K. Hammer and A. Buerker, “Ziziphus spina-christi (L.) Willd.: A Multipurpose Fruit Tree,” Genetic Resources and Crop Evolution, Vol. 55, No. 7, 2008, 929-937. doi:10.1007/s10722-007-9299-1
- C. Sudhersan and J. Hussain, “In Vitro Clonal Propagation of a Multipurpose Tree, Ziziphus spina-christi (L.) Desf,” Turkish Journal of Botany, Vol. 27, 2003, pp. 167- 171.
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassay with Tobacco Tissue Cultures,” Physiologia Plantarum, Vol. 15, No. 3, 1962, pp. 473-497. doi:10.1111/j.1399-3054.1962.tb08052.x
- SAS, “PROC User’s Manual, Version 9.1, 2003,” SAS Institute, Cary.
- F. Quiroz-Figueroa and M. M. Zeel, “Picomolar Concentrations of Salicylate Induce Cellular Growth and Enhance Somatic Embryogenesis in Coffea arabien Tissue Culture,” Plant Cell Reports, Vol. 20, No. 8, 2001, pp. 679-684. doi:10.1007/s002990100386
- H. F. Sakhanokho and R. Y. Kelley, “Influence of Salicylic Acid on in Vitro Propagation and Salt Tolerance in Hibiscus acetosella and Hibiscus moscheutos (cv ‘Luna Red’),” African Journal of Biotechnology, Vol. 8, No. 8, 2009, pp. 1474-1481.
- J. P. Luo, S. T. Jiang and L. L. Pan, “Enhanced Somatic Embryogenesis by Salicylic Acid of Astragalus adsurgens; Relationship with H2O2 Production and H2O2 Metabolizing Enzyme Activities,” Plant Science, Vol. 161, No. 1, 2001, pp. 125-132. doi:10.1016/S0168-9452(01)00401-0
- M. A. Gutiérrez-Coronado, C. Trejo-López and A. Larqué-Saavedra, “Effects of Salicylic Acid on the Growth of Roots and Shoots in Soybean,” Plant Physiology and Biochemistry, Vol. 36, No. 8,1998, pp. 563-565. doi:10.1016/S0981-9428(98)80003-X
- L. Hao, L. Zhou, X. Xu, J. Cao and T. Xi, “The Role of Salicylic Acid and Carrot Embryogenic Callus Extracts in Somatic Embryogenesis of Naked Oat (Avena nuda),” Plant Cell, Tissue and Organ Culture, Vol. 85, No. 1, 2006, pp. 109-113. doi:10.1007/s11240-005-9052-4
- L. Joon-Sang, “The Mechanism of Stomatal Closing by Salicylic Acid in Commelina communis L,” Journal of Plant Biology, Vol. 41, No. 2, 1998, pp. 97-102. doi:10.1007/BF03030395

