Pharmacology & Pharmacy
Vol.3 No.3(2012), Article ID:20648,6 pages DOI:10.4236/pp.2012.33046
Modulation of Interval-Force Dependence of Rat Papillary Muscle at Acute and Course Exposure of Amiodarone
![]()
Federal State Budgetary Institution Research Institute for Сardiology of Siberian Branch under the Russian Academy of Medical Sciences, Tomsk, Russia.
Email: dina@cardio.tsu.ru
Received March 14th, 2012; revised April 24th, 2012; accepted May 7th, 2012
Keywords: Acute and Course Amiodarone; Sarcoplasmic Reticulum Function; Postextrasystolic and Post-Rest Contractions; Papillary Muscles of Rat
ABSTRACT
Change of interval-force dependence of rat papillary muscles at acute and course use of amiodarone was investigated. Methods: The experiments were performed on isolated papillary muscles of Wistar rats. The extrasystolic (0.2 - 1.5 s), postextrasystolic contractions and post-rest (4 - 60 s) reactions of rat left papillary muscles perfused Krebs-Henseleit solution with amiodarone (1 μM) and papillary muscles of rat treated amiodarone in dose 20 mg/kg/day for 14 days were investigated. Results: It has been found that both acute and course use of amiodarone decreases the amplitude of extrasystolic contraction. At the same time, only acute use of amiodarone leads to additional enhancement of potentialtion of contractile response at extrasystolic impulse and performance of post-rest test. Conclusion was made, that both acute and course use of amiodarone decreases excitability of cardiomyocytes but only at course use this antiarrythmic drug increases effective refractory period of myocardium. Acute exposure of amiodarone is accompanied with change of functional state of cardiomyocyte sarcoplasmatic reticulum.
1. Introduction
Amiodarone is the most effective antiarrythmic drug in therapy of life-threaten ventricular tachyarrhytmias and in comparison with other antiarrythmic preparation has low proarrhytmic activity [1,2]. Effect of amiodarone is stipulated by its impact on transmembrane ionic currents. According Vaughn-Williams classification amiodarone is a class III antiarrythmic drug, which mechanism of action is stipulated by inhibition of outgoing potassium current leading to prolongation of transmembrane action potential (AP). Prolongation of AP leads to increase of effective refractory period of cardiomyocyte ventriculars, atrium, atrioventricular node, additional conduction ways, and lengthening of the QT interval on electrocardiogram (ECG) [3,4]. At the same time drug-induced effects of amiodarone do not limited by potassium channel blockade but are characterized with integrated effects on electric parameters of caridomyocyte membranes [5]. Amiodarone is able to inhibit partially sodium and potassium channels as well as to block β-adrenoceptors. It is reputed, that this antiarrhytmic drug shows high antiarrhytmic efficiency owing to this properties. Amiodarone effect on electric properties of membrane is defined to a large extent by duration of its use. It may have important significance at clinical use of the drug. It is shown that short-term use of amiodarone inhibits fast potassium currents and long-term use inhibits slow potassium currents [4]. Short-term use of amiodarone leads to decreasing of Na+-K+-ATPase activity [6] and particulate blockade of incoming sodium and calcium currents that is accompanied with suppression of Na+-K+-dependent conductivity and excitability of membranes [7]. Chronic use of amiodarone influences on expression of ion channel genes [5]. Probably, this property of amiodarone can lead to “antiarrhytmic” remodeling of cardiomyocytes. As far as modulation of electrophysiological properties of cardiomyocytes at action of amiodarone is realized in dependence on exposure duration, influence of this preparation on inotropic ability of the heart muscle may depend on duration of use of this preparation, too. Mechanism of action of amiodarone and many other antiarrhytmic preparations is considered, traditionally, only from positions of their impact on ion channels in sarcolemma. Question of effect of antiarrhytmic drugs on function of intracellular systems, which regulate ion homeostasis in cells, to a large extent remains open. One of intracellular structure responsible for regulation of calcium homeostasis in heart cells is sarcoplasmic reticulum (SR) [8]. Contractile abilities of myocardium depend to a large extent on functional state of SR. It is shown, that analysis of single contraction-relaxation cycle of separated heart muscle preparations at change of electric stimulation mode allows to study the function of SR [9]. This approach is used to estimate ability of cardiomyocyte SR to release and to absorb Са2+. On the basis of above mentioned the purpose of our investigation was to study dependence of interval-force of rat papillary muscle at acute and course use of amiodarone.
2. Materials and Methods
2.1. Subject
The study was performed on 32 adult male Wistar rats of line with mass 200 - 250 g. All procedures with the experimental animals were performed in accordance with the National Guidance on the Operation of the experimental animals (1977). Papillary muscles were rapidly isolated from the left ventricle of rat heart. The following groups of papillary muscles have been studied: I groupintact papillary muscles perfused with Krebs-Henseleit solution; II group-intact papillary muscles, perfused with Krebs-Henseleit solution, containing amiodarone in dose of 1 μМ/l (Sanofi Pharma, France), in 15 min.; III grouppapillary muscles of rats, which obtained preliminary amiodarone in dose 20 mg/kg/day during 14 days and perfused with Krebs-Henseleit solution.
2.2. Experimental Procedure
Papillary muscles were placed into the thermostabilized flow chamber. One end of muscle was fixed to chamber wall and other end was fastened on the rod of force transducer (mechanoelectrical transducer 6МХ1С). Perfusion of muscles was performed with Krebs-Henseleit solution of following composition (in mM): NaCl: 120; KCl: 4.8; CaCl2: 2.0; MgSO4: 1.2; KH2PO4: 1.2; NaHCO3: 20.0; glucose-10.0 at 36.5˚C. The gas mixture-carbogen (О2: 95%, СО2: 5%) used to oxygenate solution. Stimulation of muscles was performed with electric pulses of rectangular shape with duration of 5 ms, applied on two massive silver electrodes located in perfusion chamber. Frequency of stimulating pulses was 0.5 Hz.
2.3. Experimental Protocol
Inotropic reaction of papillary muscles on change of electric stimulation mode was studied. The following interval-force dependence were investigated:
1) Extrasystolic test. Change of contraction amplitude after applying of single extraordinary stimulus on the background of basic stimulation in 0.2 - 1.5 sec (extrasystolic interval) after beginning of regular stimulating pulse was estimated [10].
2) Post-rest test. Change of contraction amplitude after reinitiation of basic frequency of electric stimulation after its suspension for fixed time from 4 to 60 s was estimated (post-rest test). Mechanical restitution curve was obtained as dependence between duration of rest period and amplitude of the first contraction after reinitiation of stimulation. Restitution curve was used to calculate t2(T50)-duration of rest corresponding to the first contraction after rest with amplitude increase being 50% of maximal increase. Index t2(T50) was used to estimate velocity of Са2+ capture in cardiomyocyte SR. Extrasystolic test and post-rest test are performed after adaptation of papillary muscles to perfusion mode during 60 min [11]. Registration of isometric contraction was carried out with the help of special software. The maximal voltage-amplitude developed by the muscle was calculated.
3. Statistical Analysis
Data were processed with statistical method, statistical significance of the obtained result was estimated by the t-Student’s test and Wilcoxon criterion for paired values.
4. Results
4.1. Inotropic Reaction of Papillary Muscle on Extrasystolic Exposure
Investigation carried out by us showed that stimulation of papillary muscles of intact myocardium (the group I) with extraordinary electric pulse in 0.2 s after beginning of regular cycle did not caused appearance of extrasystoles, however duration of regular contraction-relaxation cycle was increased. According to the literature data [10], that testified that extraordinary electric pulse falls into phase of absolute refractoriness and therefore additional extrasystolic contraction did not appear. However, such exposure induced entry of additional calcium ions into cardiomyocytes myoplasme that caused lengthening of contraction-relaxation cycle. Application of electric stimulus in 0.225 s induced appearance of extrasystoles with amplitudes of 27% ± 1.42% of value of regular cycle amplitude (Figure 1). As one can see on Figure 1, the amplitude of extrasystolic contraction increased at following increasing of time interval between regular and extrasystolic stimulating pulses. It is known, that electric stimulation pulse appeared in the 3rd phase of action potential leads to lengthening of action potential plateau in which time additional quantity of external calcium ions enters into myoplasm of cardiomyocytes which are accumulated in SR and take part in the first postextrasystolic contraction-relaxation cycle [10,12,13]. In our experiment such effect became apparent the most obviously in the time of the shortest extrasystolic interval (0.2 s). In this case postextrasystolic contraction exceeded amplitude of regular contraction on 23% ± 1.78% (Figure 2). We observed decreasing of postextrasystolic potentiation with appearance of independent extrasystole and increasing of its amplitude, at that evidence of post-extrasystolic potentiation became minimal on the longest extrasystolic intervals (Figures 1-2).
Treatment of papillary muscles of intact rats with amiodarone during 15 min (the group II) caused reliably decreasing of extrasystole amplitudes induced by extraordinary electric pulses in 0.25, 0.75, 1.0, 1.25, 1.5 s (р < 0.05, Figure 1). Long-term (course) use of amiodarone (the group III) led to more significant change of inotropic reaction of papillary muscles on extrasystolic exposure. So, extrasystole amplitude was less than in control group on 9% - 12% (р < 0.05, Figure 1). In addition extraordinary pulse in 0.225 s in this case did not cause appearance of extrasystoles (Figure 1) that shows on prolongation of effective refractoriness period. Obtained results allow to suppose that use of amiodarone decreases the excitability of the myocardium at that this effect be-
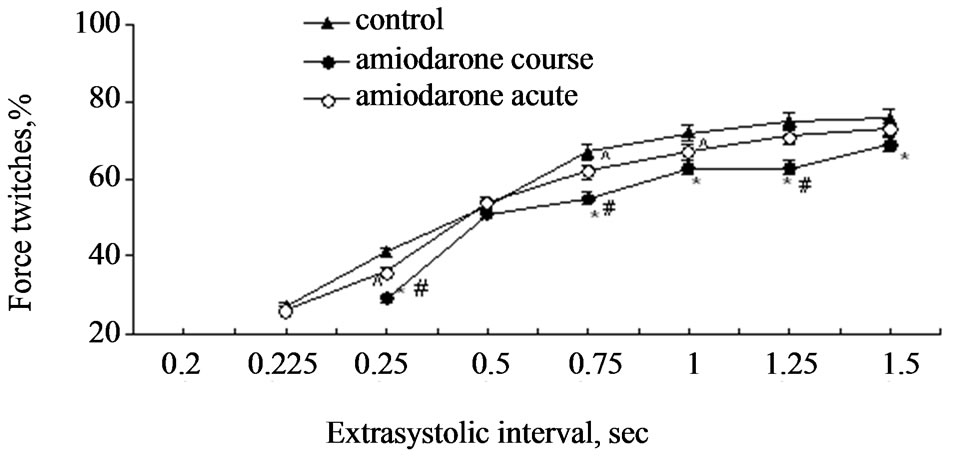
Figure 1. Extrasystolic contraction of rat papillary muscles on the background of acute and course use of amiodarone. Notes: ^p < 0.05 and *p < 0.01 are reliability of difference in comparison with the control, #p < 0.01 is reliability of difference between groups with acute and course use of amiodarone. On the ordinate axe: contraction amplitude in percents in relation to initial values. On the absciss axe: extrasystolic interval (in seconds).
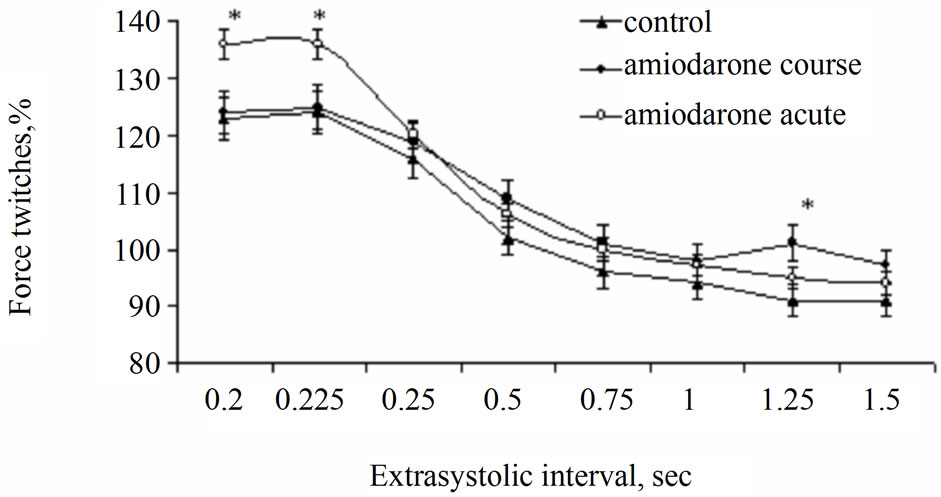
Figure 2. Effect of acute and course use of amiodarone on postextrasystolic contraction of rat papillary muscles. Note: See Figure 1. *p < 0.01 is reliability of differences in comparison with control and group with course use of amiodarone.
comes apparent in a greater degree at course use.
Amplitude of postextrasystolic contraction in the conditions of acute treatment of papillary muscles with amiodarone (the group II) exceeded control values (the group I) on 12 % (р < 0.05) after extrasystolic exposure rendered in 0.2 and 0.225 s. At the same time, at prolonged use of amiodarone (the group III) these parameters did not differ from control values (Figure 2). These results may testify about action of amiodarone on SR functions. Probably, increasing of SR ability to take up Са2+ occurs and/or leakage current of Са2+ from SR decreases. In addition possibility of more mobilization of Са2+ from intracellular depot in the time of postextrasystolic contraction is not excluded. So far as we did not reveal similar effect at course use of amiodarone (the group III), it is possible to suppose that exposure of amiodarone of SR function has short-termed character.
4.2. Inotropic Reaction of Papillary Muscle on the Post-Rest Test
It is known, that suspension of regular electrical stimulation of rat papillary muscles causes accumulation of Са+2 in SR [14,15]. This leads to increasing of amplitude of the first contraction registered at renewal of regular electric stimulation. Our experiments showed that independently on duration of the post-rest test, amplitude of the first contraction of intact papillary muscles after rest period (the group I) exceeded the base level and potentialtion of contraction amplitude increased with increasing of rest duration (Figure 3). However, increasing of contraction amplitude decreased considerably after prolonged rest period. This testifies saturation of SR with calcium ions. Maximal amplitude of muscle contraction in the group I after delay in 60 s was 187.8% ± 12.12% in comparison with amplitude of regular contraction. Perfusion of muscle strips with amiodarone (the II group) caused considerable increasing of potentiation (Figure 3). So, potentiation of inotropic response of rat papillary muscles with short-term treatment with amiodarone (the group II) was reliably more (р < 0.01) than potentialtion obtained in the group I on all rest intervals. Maximal contraction amplitude was 251.2% ± 10.61% that is almost in 2 times more than one in the group I. At the same time as one can see on Table 1, the time necessary to achieve half maximum of amplitude increase t(T50) at acute and course exposure of amiodarone (the II, III groups) and in the group I did not differ for certain. This fact can testify that velocity of take up Са2+ in SR from cardiomyocyte myoplasm after treatment of papillary muscles with amiodarone remained the same as in intact papillary muscles. At the same time, post-rest test showed increasing of contraction potentiation on the background of amiodarone (the group II), that testifies large
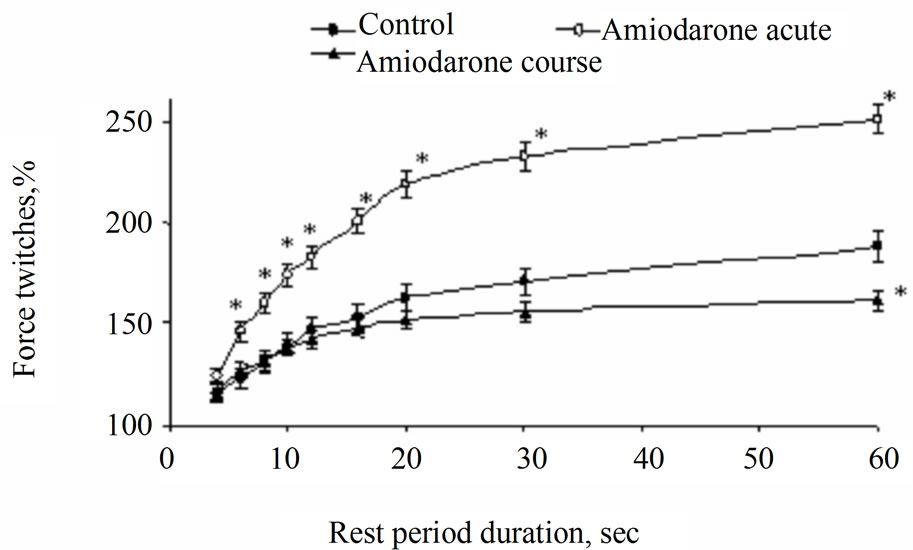
Figure 3. Change of dynamics of mechanical restitution of rat papillary muscles at acute and course use of amiodarone. Note: *p < 0.01 is reliability of differences in comparison with the group I and the group with course use of amiodarone (the group II).
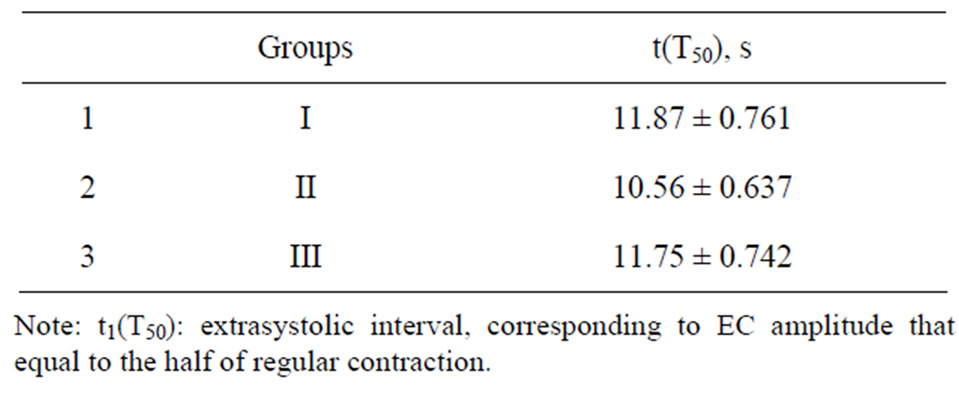
Table 1. Influence of amiodarone on t(T50) of rat papillary muscle.
quantity of Са2+, rejected from SR in the moment of the first contraction after renewal of stimulation. These facts allowed us to suppose that amiodarone can promote more effective “retention” of Са2+ in SR and, thereby, to prevent current of Са2+ leakage from SR in the rest period. Dynamics of mechanical restitution of myocardium of rat obtained amiodarone in 14 days (the group III) did not differ significantly from control value.
5. Discussion
It is known that electrophysiological effects of amiodarone are connected with inhibition of both incoming and outgoing ionic currents [5,16]. One of the effects noted at course use of amiodarone is decreasing of Na+-K+-ATPase activity in cardiomyocytes [17,18]. In addition long use of amiodarone leads to electrophysiological remodeling of cardiomyocytes connected with expression of ion channel genes and proteins [4]. Probably these changes provide more effective decreasing of the excitability of the cardiomyocytes than at acute exposure of amiodarone.
In our experiments we obtained that amiodarone acute in intact myocardium is seemed more effective on SR function compare with chronic administration. Acute amiodarone is able to modulate calcium transport in SR system, potentiating postextrasystolic and post-rest contractions of intact rat papillary muscles. There are convincing data that phenomenon of postextrasystolic and post-rest contraction is connected with releasing of calcium ions from sarcoplasmic reticulum. At the same time calculation of coefficients of Са2+ recirculation gives basis to suppose that amiodarone effects on SR function is not connected with modulation of activity of SR Са2+-ATPase and most probably are stipulated by activetion of Са2+ transport from places of capture to places of ejection inside SR or limitation of Са2+ leakage current of the same intracellular structure. It is known amiodarone decrease the adenylate cyclase activity and noncompetitive inhibit β-adrenoceptors [19-23]. β-adrenergic receptor activation enhances of SR calcium leak in cardiac myocytes [24,25], so amiodarone can limitation of Са2+ leakage current of SR by this way.
Analyzing our results it is possible to suppose that long-term use of amiodarone leads to balancing of intracellular structures of cardiomyocytes to changed homeostasis of Са2+ and as result inotropic reaction of myocardium on short term suspension of electrical stimulation and manifestation of phenomenon of increasing of postextrasystole does not differ form values of papillary muscles which was not exposed to amiodarone exposure. At that effects of acute and course use of this preparation on intracellular homeostasis of calcium ions are different. Course use of amiodarone does not influence practically postextrasystolic and post-rest potentiation. These data allow us to suppose that action of amiodarone on functional activity of SR has short term character in intact myocardium.
6. Conclusion
Course and acute use of amiodarone leads to decreasing of the exitability of the myocardial cells at that its longterm use increases effective refractory period of myocardium. Acute effect of amiodarone influence on processes of intracellular homeostasis of calcium ions promoting more effective “retention” of calcium ions in SR and/or preventing of Са2+ leakage from SR.
REFERENCES
- S. J. Connolly, “Evidence-Based Analysis of Amiodarone Efficacy and Safety,” Circulation, Vol. 100, 1999, pp. 2025-2034. doi:10.1161/01.CIR.100.19.2025
- L. M. Letelier, K. Udol, J. Ena, B. Weaver and G. H. Guyatt, “Effectiveness of Amiodarone for Conversion of Atrial Fibrillation to Sinus Rhythm: A Meta-Analysis,” Archives of Internal Medicine, Vol. 163, No. 7, 2003, pp. 777-785. doi:10.1001/archinte.163.7.777
- M. D. Freedman and J. C. Somberg, “Pharmacology and Pharmacokinetics of Amiodarone,” Journal of Clinical Pharmacology, Vol. 31, 1991, pp. 1061-1069.
- I. Kodama, K. Kamiya and J. Toyama, “Cellular Electropharmacology of Amiodarone,” Cardiovascular Research, Vol. 35, No. 1, 1997, pp. 13-29. doi:10.1016/S0008-6363(97)00114-4
- I. Kodama, K. Kamiya, H. Honjo and J. Toyama, “Acute and Chronic Effects of Amiodarone on Mammalian Ventricular Cells,” Japanese Heart Journal, Vol. 37, No. 5, 1996, pp. 719-730. doi:10.1536/ihj.37.719
- F. Forini, G. Nicolini, S. Balzan, G. M. Ratto, B. Murzi, V. Vanini and G. Iervasi, “Amiodarone Inhibits the 3,5,3’- Triiodothyronine-Dependent Increase of Sodium/Potassium Adenosine Triphosphatase Activity and Concentration in Human Atrial Myocardial Tissue,” Thyroid, Vol. 14, No. 7, 2004, pp. 493-499. doi:10.1089/1050725041517084
- H. Yoshida, A. Sugiyama, Y. Satoh, Y. Ishida, M. Yoneyama, K. Kugiyama and K. Hashimoto, “Comparison of the in Vivo Electrophysiological and Proarrhythmic Effects of Amiodarone with Those of a Selective Class III Drug, Sematilide, Using a Canine Chronic Atrioventricular Block Model,” Circulation, Vol. 66, No. 8, 2002, pp. 758-762. doi:10.1253/circj.66.758
- M. E. Diaz, H. K. Grahama, S. C. O’Neill, A. W. Trafford and D. A. Eisner, “The Control of Sarcoplasmic Reticulum Ca Content in Cardiac Muscle,” Cell Calcium, Vol. 38, No. 3-4, 2005, pp. 391-396. doi:10.1016/j.ceca.2005.06.017
- M. Endoh, “Force-Frequency Relationship in Intact Mammalian Ventricular Myocardium: Physiological and Pathophysiological Relevance,” European Journal of Pharmacology, Vol. 500, No. 1-3, 2004, pp. 73-86. doi:10.1016/j.ejphar.2004.07.013
- D. V. Vassallo, E. Q. Lima, P. Campagnaro, A. N. Faria and J. G. Mill, “Mechanisms Underlying the Genesis of Post-Extrasystolic Potentiation in Rat Cardiac Muscle,” Brazilian Journal of Medical & Biological Research, Vol. 28, No. 3, 1995, pp. 377-383.
- S. N. Wu, A. Y. Shen and T. L. Hwang, “Analysis of Mechanical Restitution and Post-Rest Potentiation in Isolated Rat Atrium,” Chinese Journal of Physiology, Vol. 39, 1996, pp. 23-29.
- D. M. Bers, “Calcium Cycling and Signaling in Cardiac Myocytes,” Annual Review of Physiology, Vol. 70, 2008, pp. 23-49. doi:10.1146/annurev.physiol.70.113006.100455
- J. Mizuno, J. Araki, S. Mohri, H. Minami, Y. Doi, W. Fujinaka, K. Miyaji, T. Kiyooka, Y. Oshima, G. Iribe, M. Hirakawa and H. Suga, “Frank-Starling Mechanism Retains Recirculation Fraction of Myocardial Ca(2+) in the Beating Heart,” Japanese Journal of Physiology, Vol. 51, No. 6, 2001, pp. 733-743.
- W. F. Bluhm, M. Meyer, E. A. Swanson and W. H. Dillmann, “Postrest Potentiation of Active Force in Mouse Papillary Muscles Is Greatly Accelerated by Increased Stimulus Frequency,” Annals of New York Academy of Sciences, Vol. 853, 1998, pp. 304-307. doi:10.1111/j.1749-6632.1998.tb08285.x
- J. Layland and J. C. Kentish, “Positive Forceand [Ca2+]i-Frequency Relationships in Rat Ventricular Trabeculae at Physiological Frequencies,” American Journal of Physiology, Vol. 276, 1999, pp. H9-H18.
- D. P. Zankov, W. G. Ding, H. Matsuura and M. Horie, “Open-State Unblock Characterizes Acute Inhibition of I Potassium Current by Amiodarone in Guinea Pig Ventricular Myocytes,” Journal of Cardiovascular Electrophysiology, Vol. 16, No. 3, 2005, pp. 314-322. doi:10.1046/j.1540-8167.2005.40561.x
- D. F. Gray, A. S. Mihailidou, P. S. Hansen, K. A. Buhagiar, N. L. Bewick, H. H. Rasmussen and D. W. Whalley, “Amiodarone Inhibits the Na(+)-K+ Pump in Rabbit Cardiac Myocytes after Acute and Chronic Treatment,” The Journal of Pharmacology and Experimental Therapeutics, Vol. 284, No. 1, 1998, pp. 75-82.
- A. D. Pitt, C. Fernandes, N. L. Bewick, P. D. Hemsworth, K. A. Buhagiar, P. S. Hansen, H. H. Rasmussen, L. Delbridge and D. W. Whalley, “Chronic AmiodaroneInduced Inhibition of the Na+-K+ Pump in Rabbit Cardiac Myocytes Is Thyroid-Dependent: Comparison with Dronedarone,” Cardiovascular Research, Vol. 57, No. 1, 2003, pp. 101-108. doi:10.1016/S0008-6363(02)00650-8
- P. Chatelain, L. Meysmans, J. R. Matteazzi, Ph. Beaufort and M. Clinet, “Interaction of the Antiarrhythmic Agents SR 33589 and Amiodarone with the β-Adrenoceptor and Adenylate Cyclase in Rat Heart,” British Journal of Phamocology, Vol. 116, No. 3, 1995, pp. 1949-1956.
- V. Drvota, J. Häggblad, I. Blange, Y. Magnusson and S. Sylvén, “The Effect of Amiodarone on the Beta-Adrenergic Receptor Is Due to a Downregulation of Receptor Protein and Not to a Receptor-Ligand Interaction,” Biochemical and Biophysical Research Communications, Vol. 255, No. 2, 1999, pp. 515-520. doi:10.1006/bbrc.1998.0138
- P. Schnabel, F. Mies, C. Maack, S. Rosenkranz, O. Zolk and M. Böhm, “Beneficial Effects of Amiodarone in Heart Failure: Interaction with Beta-Adrenoceptors Rather Than g Proteins,” European Journal of Pharmacology, Vol. 369, No. 3, 1999, pp. 391-394. doi:10.1016/S0014-2999(99)00101-6
- H. Tachikawa, M. Kodama, K. Watanabe, T. Takahashi, M. Ma, T. Kashimura, M. Ito, S. Hirono, Y. Okura, K. Kato, H. Hanawa and Y. Aizawa, “Amiodarone Improves Cardiac Sympathetic Nerve Function to Hold Norepinephrine in the Heart, Prevents Left Ventricular Remodeling, and Improves Cardiac Function in Rat Dilated Cardiomyopathy,” Circulation, Vol. 111, No. 7, 2005, pp. 894-899. doi:10.1161/01.CIR.0000155610.49706.D2
- X.J. Du, M. D. Esler and A. M. Dart, “Sympatholytic Action of Intravenous Amiodarone in the Rat Heart,” Circulation, Vol. 91, 1995, pp. 462-470. doi:10.1161/01.CIR.91.2.462
- J. Curran, M. J. Hinton, E. Rios, D. M. Bers and T. R. Shannon, “β-Adrenergic Enhancement of Sarcoplasmic Reticulum Calcium Leak in Cardiac Myocytes Is Mediated by Calcium/Calmodulin-Dependent Protein Kinase,” Circulation Research, Vol. 100, 2007, pp. 391-398. doi:10.1161/01.RES.0000258172.74570.e6
- P. Ferrero, M. Said, G. Sánchez, L. Vittone, C. Valverde, P. Donoso, A. Mattiazzi and C. Mundiña-Weilenmann. “Ca2+/Calmodulin Kinase II Increases Ryanodine Binding and Ca2+-Induced Sarcoplasmic Reticulum Ca2+ Release Kinetics during β-Adrenergic Stimulation,” Journal of Molecular and Cellular Cardiology, Vol. 43, No. 3, 2007, pp. 281-291. doi:10.1016/j.yjmcc.2007.05.022

