International Journal of Clinical Medicine
Vol.3 No.3(2012), Article ID:19410,4 pages DOI:10.4236/ijcm.2012.33045
Milk Intoxication—A Case Report
![]()
1Department of Internal Medicine, Maasstad Hospital, Rotterdam, The Netherlands; 2Department of Clinical Chemistry, Maasstad Hospital, Rotterdam, The Netherlands.
Email: *BootsJ@maasstadziekenhuis.nl
Received January 20th, 2012; revised February 29th, 2012; accepted March 18th, 2012
Keywords: Triglycerides; Milk
ABSTRACT
Drinking milk is generally considered to fit in a healthy diet. In this case report however, it is demonstrated that drinking an excessive amount of milk in a relatively short period of time can form a serious health risk, mainly due to the extremely elevated level of triglycerides. To our knowledge, the levels of triglycerides that were measured in this patient are one of the highest ever reported. Moreover in this case report, etiology, diagnostic challenges and therapeutic options for such increased levels of triglycerides are discussed.
1. Introduction
Drinking milk has been considered to fit in a healthy diet. However, in certain circumstances one’s opinion may be changed as we will demonstrate in the following extraordinary case.
2. Case Report
A 54 year old man was admitted to our emergency room with an eight day history of polyuria, polydipsia, excessive perspiration and progressive dyspnoea. He had visited his general practitioner two days before, who had diagnosed “diabetes de novo” based on a measured blood-glucose level of approximately 16 mmol/l (288 mg/dl, reference values: 4.4 - 5.5 mmol/l; 79 - 99 mg/dl). Metformin 500 mg was started twice daily, but the patient’s complaints persisted. Medical history revealed that to quench his thirst, the patient had drunk approximately twenty-two liters (i.e. five and a half gallons) of full fat milk per day for the past two days. According to the nutritional value of milk (Table 1), this implies 1540 grams of protein, 1980 grams of sugar and 1496 grams of fat in just two days.
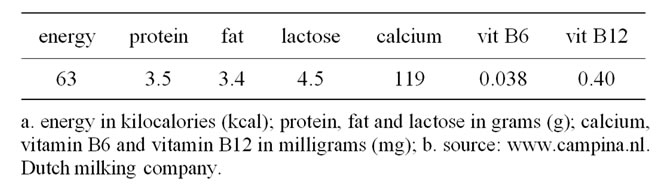
Table 1. Nutritional value of full fat milk, per 100 ml.
The patient took no other medication. His past medical history stated a single event of syncope, however no further follow-up or drugs were initiated. The patient smoked approximately 10 cigarettes per day and drank alcohol occasionally.
On arrival in our emergency room, the patient’s consciousness level was normal. His blood pressure was 165/108 mmHg, he had a regular pulse rate of 138 beats per minute, respiration rate of 40 breaths per minute, O2- saturation 96% with three liters of oxygen per minute and temperature 37.5˚C (ear). Physical examination showed an adipose, sweating and dyspnoeic Caucasian male. Auscultation of the lungs was normal, besides slight basal crackles bilaterally. There were normal heart sounds and no murmurs. The abdomen was swollen and distended, but peristalsis was normal and neither resistance nor intra-abdominal pain could be appreciated. Blood samples were drawn for initial laboratory examination. We noticed that the blood seemed to be milky or lactescent and unfortunately, the chemistry analyzer in the laboratory could not examine this particular blood sample (Figures 1 and 2). An arterial blood gas measurement, which is performed with a different type of analyzer, showed a glucose level of 75 mmol/l (1350 mg/dl), sodium 129 mmol/l (297 mg/dl), potassium 6.1 (24 mg/dl) mmol/l, with pH 7.37, pCO2 38 mmHg, Base Excess -3,bicarbonate 22 mmol/l (134 mg/dl), pO2 107 mmHg and O2- saturation of 100% with three liters of oxygen per minute. Chest X-ray showed neither signs of heart failure nor infiltrates.
Due to the extremely high glucose level, the patient was admitted to an intensive care unit to be closely monitored whilst administering normal saline solution intensively, along with the perfusion of short-acting insulin.
After several hours further laboratory results were retrieved (Table 2). Most remarkable was the extremely high level of the serum triglycerides: 191 mmol/l (16713 mg/dl, normal values: 0 - 2.0 mmol/l; 0 - 175 mg/dl, Table 3). It was estimated that such an increased level of triglycerides implied a substantial risk for developing acute pancreatitis. Therefore, plasma exchange was instituted. Heparin was used as anti-coagulant. After two sessions in two subsequent days, the serum triglyceride level was reduced to an acceptable level (Table 3). The plasma retrieved from the patient had a milky appearance (Figure 3). Further analysis was performed by determining apolipoprotein (Apo) A and Apo B levels: both were not increased (Table 3).
In addition to plasma exchange, the patient was treated with insulin (four-daily regimen), atorvastatin, metformin and lifestyle interventions. During admittance, no complications occurred. On follow-up six weeks after discharge the patient was still in good health, with a slightly elevated triglyceride level of 2.8 mmol/l (245 mg/dl, see Table 3).

Figure 1. The patient’s blood appeared rather pale. The left blood sample was taken at admission; the right blood sample was taken at discharge and is shown for comparison.

Figure 2. The same blood samples from Figure 1, now shown after centrifugation. The milky plasma is remarkable.
3. Discussion
Drinking milk is generally considered to be healthy. In this case report however, it is demonstrated that drinking a lot of milk can have serious implications. To our knowledge, such an increased level of triglycerides is one of the highest ever reported. Several interesting questions derive from this case. Firstly, what caused the extreme increase of triglyceride levels? Secondly, how can the patient’s clinical presentation be explained? Thirdly, how can the interference with regards to the estimation of the laboratory results be resolved? Finally, what is the true risk of developing acute pancreatitis and how can this risk most optimally be managed?
To further analyze the patient’s lipid disturbances, Apo A and Apo B levels were determined. Both were not increased. This implies that almost all triglycerides were stored in chylomicrons. In contrast, diabetic dyslipidemia is characterized by an increase in very low density lipoprotein (VLDL) particles and thus by an increase in Apo B levels. Lipoprotein lipase (LPL) activity is regulated by insulin and insulin resistance, as occurs in type 2 diabetes, results in less activity of LPL. Consequently triglyceride clearance is decreased [1]. Although it can never be excluded that this mechanism contributed to the patient’s hypertriglyceridemia, the main cause of increase in triglyceride levels was the ingestion of the exceptionally high dose of full fat milk. Familial hyperchylomicronemia seems unlikely because of the nearly normal value of triglycerides six weeks later.
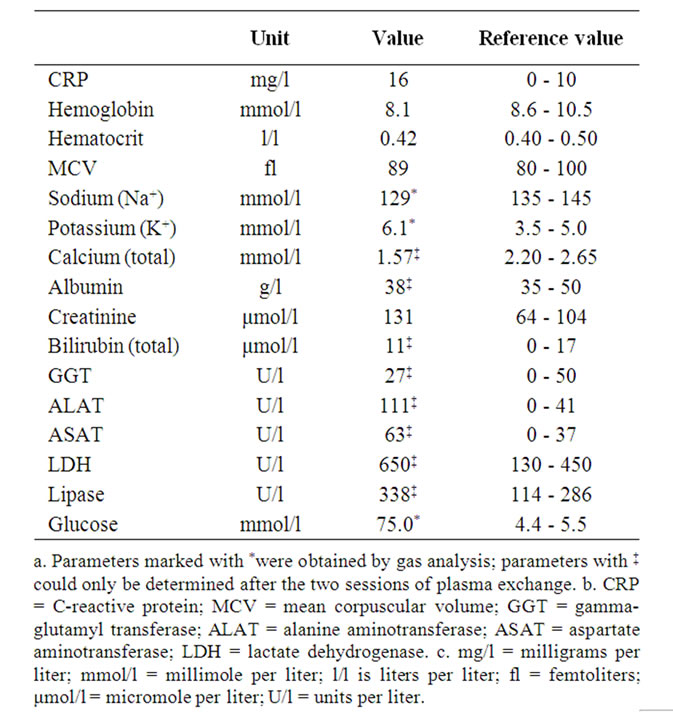
Table 2. Laboratory results retrieved after several hours.
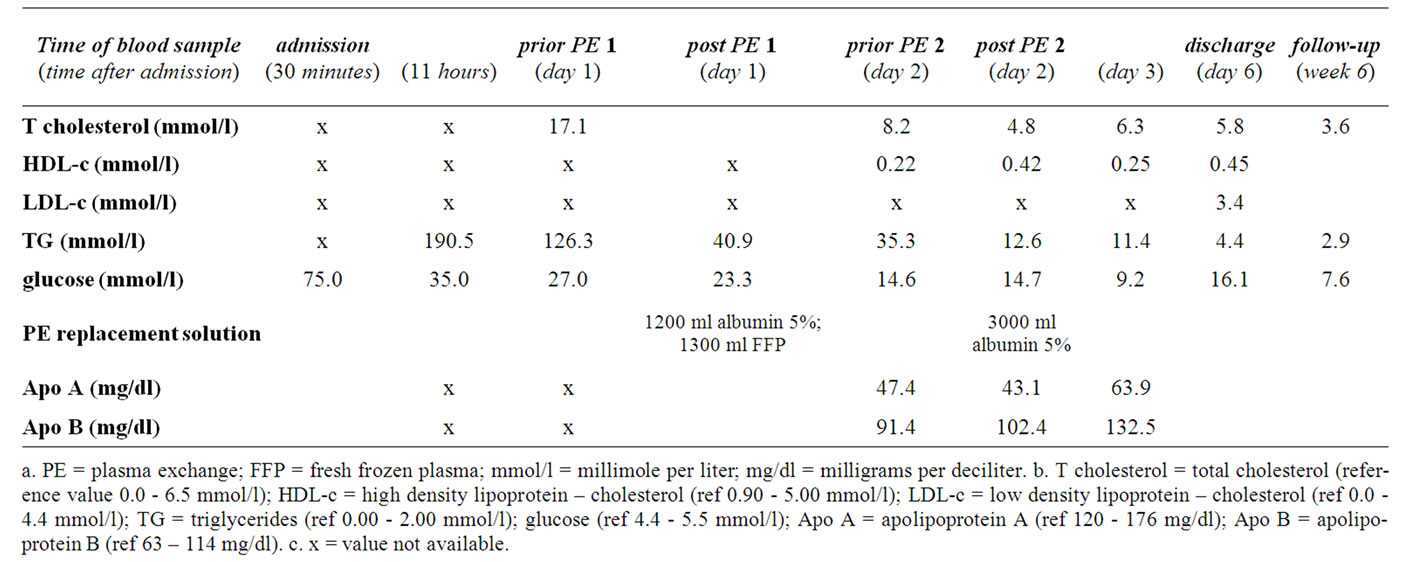
Table 3. Lipid values throughout admission (reference values are given in the footnote).
Figure 3. The patient’s plasma during plasma exchange.
The patient presented with dyspnoea and abdominal complaints. Patients with extremely high triglyceride levels (>11.3 mmol/l; 1000 mg/dl) may develop the socalled chylomicronemia syndrome, characterized by memory loss, abdominal pain and/or pancreatitis, dyspnoea, eruptive xanthoma, flushing due to alcohol consumption and lipemia retinalis [2]. Some of the symptoms of this syndrome resemble the symptoms of the hyperviscosity syndrome [3]. Perhaps, hyperviscosity is the mechanism by which the symptoms were provoked. However it is only speculation, because the real mechanism is unknown. Our patient had high triglycerides in the chylomicron fraction and neither acidosis nor pulmonary congestion was apparent as an explanation for his dyspnoea. Furthermore, the patient rapidly recovered after removal of the excessive triglycerides. Thus, it was concluded that the chylomicronemia syndrome might explain the dyspnoea and abdominal complaints in the patient.
The initial electrolyte measurements were obtained by blood gas analysis (ABL 725 from Radiometer). This instrument provides direct potentiometric measurement, which is free from interference by triglycerides. In contrast, the routine chemistry analyzer (Dimension RxL, Dade Behring) relies on colorimetric and turbidimetric measurement and therefore can not produce correct results with these levels of gross lipidemia. The indirect electrode measurement in this analyzer showed an initial sodium concentration of 104 mmol/l (not reported). This pseudohyponatremia results from the high triglyceride level, in this case producing a 19% lower sodium concentration, which is in line with the 17% triglyceride level (16.7 g/dl).
The removal of high levels of triglycerides from a blood sample can be achieved in several ways: firstly, by ultracentrifugation (15 minutes at 90000 rpm); secondly, by repeated high speed centrifugation (15 minutes at 13000 rpm); or thirdly, by treatment with a commercial lipid-clearing polymer (Lipoclear). None of these techniques were available in our laboratory. Ultracentrifugation seems to be superior to high speed centrifugation for triglycerides exceeding 50 mmol/l (4375 mg/dl) [4]. Lipoclear has been reported to be less efficient when triglycerides exceed 28 mmol/l (2450 mg/dl) [5].
Hypertriglyceridemia accounts for 1.3% - 3.8% of causes for acute pancreatitis, following alcohol and gallstone induced disease [6]. There are a number of theories on the pathogenesis of developing acute pancreatitis due to elevated triglyceride levels, however the exact mechanism remains unknown [7]. The risk of developing acute pancreatitis depends on the level of triglycerides in the serum and is considered to be triggered when triglyceride levels exceed 11.4 mmol/l (1000 mg/dl). Patients with a triglyceride level above 40.7 mmol/l (3600 mg/dl) have a fourfold higher risk compared to patients with a triglyceride level between 10.7 and 20.3 mmol/l (950 - 1800 mg/dl) [8]. The average level of triglycerides in a series of patients with acute pancreatitis due to hypertriglyceridemia was just above 50 mmol/l (4500 mg/dl) [6,8]. Our patient presented with a triglyceride level of 191 mmol/l (16713 mg/dl) and therefore he must be classified in the highest risk category.
Treatment of this type of hyperlipidemia consists of decreasing the ingestion of fat and the correction of the diabetes and lipid levels. However, it was estimated that by this therapy alone the time to reach safe levels of triglycerides might be too long and that the risk of developing acute pancreatitis was severe. Therefore, other therapies were considered.
Insulin activates lipoprotein lipase, the responsible enzyme for degradation of chylomicron triglycerides. It can be used even in the absence of diabetes. Heparin stimulates the release of lipoprotein lipase from the endothelium into the circulation and is also used for the treatment of hyperlipidemia [7]. Insulin was already used in our patient to control his diabetes. The use of insulin might have accounted for the initial lowering of the triglycerides besides dilution by sodium-chloride infusion. Heparin was initially not used.
Triglycerides are wrapped in lipoproteins and form large particles in the blood. The pores of a hemodialysis filter are too small to extract these particles. However, filters used for plasma exchange consist of larger pores: large enough to extract large proteins like γ-globulins. Experience with plasma exchange is limited in hyperlipidemic pancreatitis patients, but recent studies have shown that plasma exchange dramatically reduces triglyceride levels and, consequently reduces the risk of (acute) pancreatitis [9-11]. In this case, it was demonstrated that by two sessions of plasma exchange, triglycerides were reduced remarkably and acute pancreatitis was successfully prevented. The use of heparin as the anticoagulant during plasma exchange might have stimulated lipoprotein lipase release from the endothelium and could have contributed to the results.
In conclusion, severe hypertriglyceridemia and the chylomicronemia-syndrome can be provoked by the consumption of large amounts of full fat milk. The lipidemia interferes with common laboratory determinations. Immediate laboratory results can be obtained by use of the blood-gas analysis potentiometric assay. Severe hyperlipidemia is a serious risk factor for the development of acute pancreatitis. Plasma exchange can extract triglycerides and can be helpful in preventing acute pancreatitis.
REFERENCES
- I. J. Goldberg, “Diabetic Dyslipidemia: Causes and Consequences,” Journal of Clinical Endocrinology and Metabolism, Vol. 86, No. 3, 2001, pp. 965-71. doi:10.1210/jc.86.3.965
- S. Santamarina-Fojo, “The Familial Chylomicronemia Syndrome,” Endocrinology and Metabolism in Clinics of North America, Vol. 27, No. 3, 1998, pp. 551-67. doi:10.1016/S0889-8529(05)70025-6
- H. C. Kwaan, “Role of Plasma Proteins in Whole Blood Viscosity: A Brief Clinical Review,” Clinical Hemorheology Microcirculation, Vol. 44, No. 3, 2010, pp. 167-176.
- G. Dimeski, “Interference Management,” Clinical Biochemists Reviews, Vol. 29, Suppl. 1, 2008, pp. S43-S48.
- C. M. Mak, K. F. Ng, R. W. C. Pang and S. Tam, “Severe Acquired Chylomicronaemia Syndrome—A Challenge to the Routine Laboratory,” British Journal of Biomedical Science, Vol. 61, No. 2, 2004, pp. 94-95.
- M. R. Fortson, S. N. Freedman and P. D. Webster III, “Clinical Assessment of Hyperlipidemic Pancreatitis,” American Journal of Gastroenterology, Vol. 90, No. 12, 1995, pp. 2134-2139.
- W. Tsuang, U. Navaneethan, J. B. Palascak and A. Gelrud, “Hypertriglyceridemic Pancreatitis: Presentation and Management,” American Journal of Gastroenterology, Vol. 104, No. 4, 2009, pp. 984-991. doi:10.1038/ajg.2009.27
- C. Lloret Linares, A. L. Pelletier, S. Czernichow, A. C. Vergnaud, D. Bonnefont-Rousselot, P. Levy, et al., “Acute Pancreatitis in a Cohort of 129 Patients Referred for Severe Hypertriglyceridemia,” Pancreas, Vol. 37, No. 1, 2008, pp 13-18. doi:10.1097/MPA.0b013e31816074a1
- A. Piolot, F. Nadler, E. Cavallero, J. L. Coquard and B. Jacotot, “Prevention of Recurrent Acute Pancreatitis in Patients with Severe Hypertriglyceridemia: Value of Regular Plasmapheresis,” Pancreas, Vol. 13, No. 1, 1996, pp. 96-99. doi:10.1097/00006676-199607000-00013
- A.V. Kyriakidis, P. Karydakis, N. Neofytou, M. Pyrgioti, D. Vasilakakis, P. Digenis, et al., “Plasmapheresis in the Management of Acute Severe Hyperlipidemic Pancreatitis: Report of 5 Cases,” Pancreatology, Vol. 5, No. 2-3, 2005, p.p 201-204. doi:10.1159/000085272
- C. Stefanutti, S. Di Giacomo, A. Vivenzio, G. Labbadia, F. Mazza, G. D’Alessandri, et al., “Therapeutic Plasma Exchange in Patients with Severe Hypertriglyceridemia: A Multicenter Study,” Artificial Organs, Vol. 33, No. 12, 2009, pp. 1096-1102. doi:10.1111/j.1525-1594.2009.00810.x
NOTES
*Corresponding author.

