World Journal of Cardiovascular Surgery
Vol.3 No.2(2013), Article ID:33411,7 pages DOI:10.4236/wjcs.2013.32016
Midterm Results of Leaflet Augmentation in Mitral Valve Repairin Rheumatic Valves Experience in One Center
1Department of Cardiothoracic Surgery, Zagazig University Hospital, Zagazig, Egypt
2Department of Anesthesia, Zagazig University Hospital, Zagazig, Egypt
3Department of Anesthesia, Azhar University Hospital, Asuet, Egypt
Email: *alaabrik@yahoo.com
Copyright © 2013 Abd Allah Badr et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 15, 2013; revised March 15, 2013; accepted March 23, 2013
Keywords: Mitral Repair; Valve Surgery
ABSTRACT
Patients with severe mitral regurgitation (MR) should undergo surgery when they present symptoms or if asymptomatic when there is objective evidence of left ventricular dysfunction. In this work, we analyze the midterm results of leaflet augmentation in mitral valve repair of rheumatic valves with gluteraldehyde preserved autologous pericardium. Patients and Methods: In our department 48 patients were exposed to mitral valve repair by leaflet augmentation either anterior or posterior beside other technique and all patients supported by flexible annuloplasty ring and followed for five years clinically and by echocardiography. Results: Age of the patients ranging from 12 to 47 years, mean age 25.9 ± 8.9 and there were 12 males (25%) and 36 females (75%) with male to female ratio of 1:3. All patients presented with shortness of breath (100%); with 14 patients were in NYHA class III (29.17%) and 34 patients were in NYHA class IV (70.83%). During follow-up period 5 patients needed reoperation by valve replacement, causes of reoperation were restrictive valve motion in one patient, left atrial thrombus in 1 patient and sever mitral regurgitation in 3 patients. Freedom from reoperation was 87.5%. At 5 years, (92.9%) were in New York Heart Association functional class I, three patients (7.1%) were in class II. Echocardiography at follow-up showed satisfactory mitral valve function. Conclusion: leaflet augmentation is a simple and reproducible method of valve repair for rheumatic MR with good midterm result.
1. Introduction
Mitral valve repair is considered the procedure of choice for correcting mitral regurgitation.Patients undergoing repair may be younger and less likely to undergo reoperation or to have atrial fibrillation than those undergoing replacement. So rheumatic mitral valves should be repaired when technically feasible accepting a risk of reoperation to maximize survival and reduce morbidity [1]. The effect of rheumatic disease on mitral valve is usually multifactorial. The most frequent mechanism is leaflet retraction. The main cause of leaflet retraction is progressive fibrosis of leaflet and subvalvular apparatus [2].
Leaflet augmentation with autologous pericardial patch briefly fixed in 0.6% gluteraldehyde solution gets rid of fibrosis and retraction of leaflets in rheumatic mitral valve disease. The use of autologous pericardial patch briefly fixed with gluteraldehyde in mitral valve repair offers durable results with no calcification or retraction. It has been applied successfully for anterior and posterior leaflet augmentation in cases of rheumatic mitral valve disease [3]. Posterior leaflet augmentation with autologous pericardial patch allows the posterior leaflet to bulge and moves anteriorly during systole towards the anterior leaflet to coapet with it [4]. The use of artificial chordae to replace elongated or ruptured chordae responsible for mitral valve prolapse and severe mitral regurgitation has been the subject of extensive experimental work to define feasibility, reproducibility, and effectiveness of this procedure. Artificial chordae is made of expanded polytetrafluoroethylene (PTFE), a material with the unique property of becoming covered by host fibrous and endothelium [5]. Aim of this study: In this work, we analyze the midterm results of leaflet augmentation in mitral valve repair of rheumatic valves with gluteraldehyde preserved autologous pericardium.
2. Patients and Methods
From 2006 to 2011, 48 patients underwent mitral valve repair with leaflet augmentation in patients presented with sever mitral regurgitation for rheumatic causes, in cardiothoracic department, Zagazig University Hospitals, diagnosis and judgment was made on the bases of clinical, Echocardiographic and intra operative findings. The study includes those patients with severe mitral valve insufficiency included the following criteria:
1) Pure or predominant mitral regurgitation and valve suitable for repair by leaflet augmentation;
2) Concomitant tricuspid valve disease, whether organic or functional were included;
3) Age of patient ranges from 10 years to 50 years;
4) Pulmonary artery pressure is mild (<30 mmHg).
Exclusion criteria:
1) Patients with mixed mitral valve disease with mild mitral regurgitation or severly distorted valve not suitable for repair;
2) Patients with mitral insufficiency for other causes e.g. degenerative, ischemic, traumatic congenital, endocarditis;
3) Mitral repair with other procedure not included leaflet augmentation;
4) Patients with significant aortic valve disease necessitating aortic valve repair or replacement;
5) Patients with coronary artery disease requiring coronary artery bypass graft (CABG).
We had 34 patients in NYHA class 4 and 14 patients in NYHA class 3.
3. Anesthetic Management
Routine monitoring including five lead ECG, invasive blood pressure using redial artery canula, central venous pressure using internal jagularvenoun access, pluseoximetry, capnography and esophageal temperature monitoring. Arterial blood gases analysis, electrolytes and urine output were also monitored. Activated clotting time (Act) was done preoperatively, 10 minutes after heparin administration, every hour and 10 mintues after heparin reversal. General anesthesia was provided by fentanyl 3 ug/kg Propofol (1.5 - 2.5 mg/kg), 2 mg of midazolam and tracheal intubation was facilitated by rocuronium 0.6 mg /kg. Intraoperative anesthesia was maintained by isoflurane 0.5 - 1.5 MAC, propofolinfuison (50 - 150 mg/kg/ min), fentanyl (2 - 3 mg/kg/h), and rocuronim 0.1 mg/kg every 30 mintues, as judged by the abethiologist to maintain cardio-vascular stability.
After induction of general anesthesia, a transoesophageal echo probe was placed intaheoesophagus. The main goal of pre-bypass TEE examination was generally to give the surgeon important information regarding morphology of the mitral valve, condition of leaflet and subvulvular apparatus, annular dilatation or deformation, prolapsed of either the anterior or posterior mitral leaflet or both, fused marginal chordae or thickened secondary chordae tendinae, and ruptured chordae tendinae Autologous piece of pericardium was freed from any pleural or mediastinal adhesion and harvested carefully and unfolded on sponge and dipped into a bath of 0.06% gluteraldehyde solution for 7 - 10 minute period. It was then rinsed in saline bath in separate bowel for additional 20 minutes.
Operation was performed on cardiopulmonary bypass using aortic and bicaval cannulation with hypothermia (30˚C - 32˚C).
Standard blood cardioplegic arrest was initiated with antegrade flow in all patients. A standard right-sided left atriotomy was made parallel to the interatrial groove and extended and wrapping inferiorly and superiorly in the direction of the left superior and inferior pulmonary veins, to facilitate exposure After proper exposure of the mitral valve, the whole valve apparatus was systematically assessed and evaluated to decide which operative procedure will be suitable.
First, the atriumis examined to determine whether a jet lesion is present, which would indicate a prolapse of the opposing leaflet.
In segmental valve analysis, the valvular apparatus is separated into eight segments; the three scallops of the posterior leaflet are identified as P1 (anterior scallop), P2 (middle scallop), and P3 (posterior scallop). The three corresponding segments of the anterior leaflet are termed A1 (anterior part), A2 (middle part), and A3 (posterior part). The remaining two segments are the Anterior Commissure (AC) and the Posterior Commissure (PC). These eight segments are analyzed comparatively using P2 as the reference point, leaflet pliability is also explored at each segment. This segmental valve analysis provides precise information, which serves as guideline to valve reconstruction.
Annular dilatation or deformation was corrected by flexible ring annuloplasty (Cosgrofe-Edward flexible physioring), which was an important part of all of our repaired patients (100%).
In cases of fibrotic retracted posterior leaflet, augmentation with autologous pericardium which previously prepared was done. The posterior leaflet is detached from the annulus starting at middle and then extended towards the two commissures leaving about 2 ml of leaflet tissue beside the annulus. Autologous pericardial patch taked half circle shape; its length should correspond to inter commissural distance. The width was calculated from the width of the anterior leaflet with a 1:3 ratio to avoid systolic anterior motion. Once appropriately sized, the patch was sewn into the defect of the posterior leaflet. The smooth surface of the pericardium is turned toward the atrium for valve repair (Figure 1). By Prolene 5/0, take 3 stitches; one at each periphery of patch which will later taken at site commissure and 3rd stitch taken at curved part of patch which will taken at center of anterior part of detached posterior leaflet. Then, suture the patch to leaflet by two rows. We should keep height of at least 3 cm when tailoring the patch to remain enough tissue in place to allow pericardium to bulge more anteriorly during systole toward anterior leaflet.
In cases of fibrotic and short retracted anterior leaflet that does not provide adequate coaptation and obligate us to insert small annuloplasty ring (risk of stenosis), anterior patch augmentation was done with previously prepared autologous pericardial patch a curvilinear incision was made in the anterior leaflet parallel to the anterior annulus, from trigone to trigone, leaving 1 to 2 mm of leaflet tissue. This caused the anterior leaflet to “fall” forward and into the ventricle. The autologous patch was then generously fashioned to the size of the ring that was selected to restore the zone of coaptation between the anterior and posterior leaflets. Specifically, the width of the patch was cut to be at least as wide as the trigone to trigone distance. Once appropriately sized, the patch was sewn into the deficit of the anterior leaflet with two running 5 - 0 Prolene sutures. This allows good size ring and better coaptation.
By 2/0 ethibond suture, take transverse Mattress suture parallel to annulus for annuloplasty ring insertion before augmentation.
Artificial chordae by using Gortex sutures (4/0) was done in cases of rupture or elongated chordae (leaflet prolapsed) after leaflet augmentation and before ring insertion. Commissural fusion was repaired by performing open commissurotomy along line of fusion of commissures till within 2 mm from the annulus starting from orifice; it was done prior to leaflet augmentation.
Areas of localized calcification, was repaired by decalcification through removal or excision to a maximum

Figure 1. augmentation of posterior leaflet with pericardial patch and insertion of suture at mitral annulus.
10% - 15% of the anterior leaflet surface and reapproximation of the edges with interrupted 5 - 0 prolene sutures.
Thickened secondary chordae tendinae which limited the mobility of the posterior leaflet were excised.
Concomitant surgical procedure: Patients with tricuspid valve repair were included. Adevage annuloplasty was carried out When functional tricuspid regurgitation was evident, and needed surgical repair.
Evaluation of the repair techniques, this is a critical steps using:
1) Saline insufflations of the flaccid left ventricle: Through the mitral valve orifice, cold saline was injected under hand pressure via a bulb syringe to fill the left ventricle. We used this method in all patients of valve repair.
2) Though cardioplegia line in the aortic root, cardioplegia was injected while inducing aortic regurge by finger pressure on the aortic annulus to fill the left ventricle. The repair was considered satisfactory when there was good coaptation of the anterior and posterior leaflet without leakage or minimal leakage. If the repair was not satisfactory additional reparative procedures were done, followed by reassessment of the repair.
3) Intra operative trans-esophageal echocardiography (TEE): This method was mandatory for evaluation of the repair Intra operatively. Assessment of the repair by TEE was performed shortly after discontinuation of cardiopulmonary bypass and when the cardiac output and after load pressures has been optimized. No more than mild (grade 1+) regurge was considered acceptable.
All patients received warfarin for 6 weeks postoperatively. Patients with atrial fibrillation received long-term warfarin.
Follow-up is complete in all patients who survived the operation. Transthoracic echocardiography was performed at 3 monthes, 1 year, 3 years and 5 years after the operation in all patients except those who had reoperations and mitral valve replacement were performed.
All operative, early and late postoperative data of all patients were collected and analyzed.
Statistical analysis:
All data are expressed as mean 9 S.D. Survival, reoperation-free and cardiac event-free rates were calculated by means of the life-table method. Inter individual comparisons were made by the paired Student’s t-test whereas comparison between different groups were conducted by unpaired t-test.
4. Results
Age of the patients ranging from 12 to 47 years, mean age 25.9 ± 8.9 and there were 12 males (25%) and 36 females (75%) with male to female ratio of 1:3. All patients presented with shortness of breath (100%); with 14 patients were in NYHA class III (29.17%) and 34 patients were in NYHA class IV (70.83%) (Table 1).
We had 38 (79.17%) patients with sinus rhythm and 10 (20.83%) patients with atrial fibrillation (AF).
As regard preoperative pathology; all patients had severe mitral regurgitation (100%), leaflet prolapsed due to elongated or ruptue chordae 15 patients, posterior leaflet retraction in 42 patients, anterior leaflet retraction in 6 patients, Commissural fusion in 16 patients, Thickening and fusion of the secondary chordae tendinae were found in 6 cases, Annular calcification 5 patients and Combined moderate to severe tricuspid regurge in 14 patients (Tables 2 and 3).
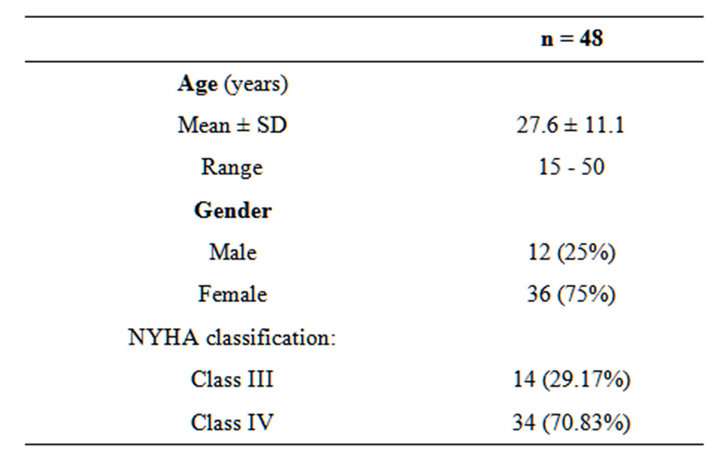
Table 1. Age, gender distribution and NYHA classification.
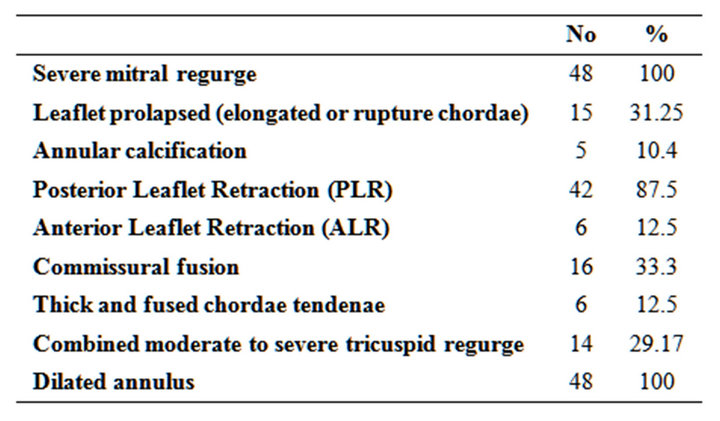
Table 2. Preoperative pathology of valve lesions.
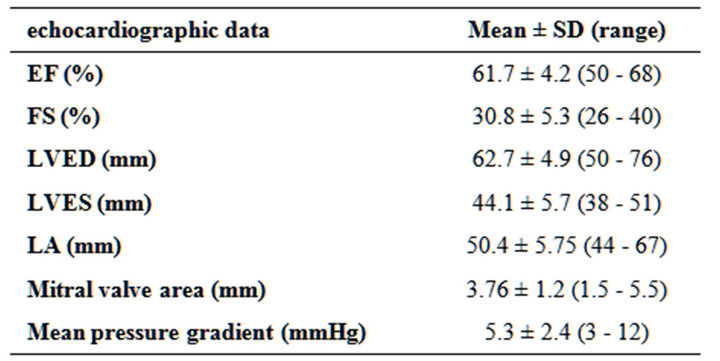
Table 3. Preoperative echocardiographic data.
All patients received flexible rings (annuloplasty 100%) while Posterior leaflet augmentation were performed In 42 patients, Anterior leaflet augmentation in 6 patients, Annular deformation with fused commissure who needed commissurotomy of one or both commissures were performed in 16 cases, Thickening and fusion of the seconddary chordae tendinae were found in 6 patients who needed resection and Artificial chordae using PTFE suture in 12 patients, chordal transfer done for 3 patients, annular decalcification done for 5 patients (Tables 4 and 5).
There was 1 death (2.08%) due to refractory heart failure, this patients had heart failure preoperative and after operation she transferred to ICU in high inotropic support and mechanical ventilation, she arrested in the 3rd day postoperatively and method of resuscitation fail to restore cardiac function.
Postoperative complications included: Re exploration in 2 patients due to post operative bleeding, cause of bleeding was oozing from surgical field, Superficial wound infection occurred in 2 cases who need only medical treatment and regular dressing and one patient developed acute renal impairement who required dialysis with marked recovery of renal function (Tables 6 and 7).
Follow-up done using clinical and echocardiographic data 3 months, one year ,three years and five years postoperatively (Tables 8-10).
Progression of leaflet retraction after initial repair, complicated sometimes by valvular and subvalvular calcification over time, is the most important cause of late
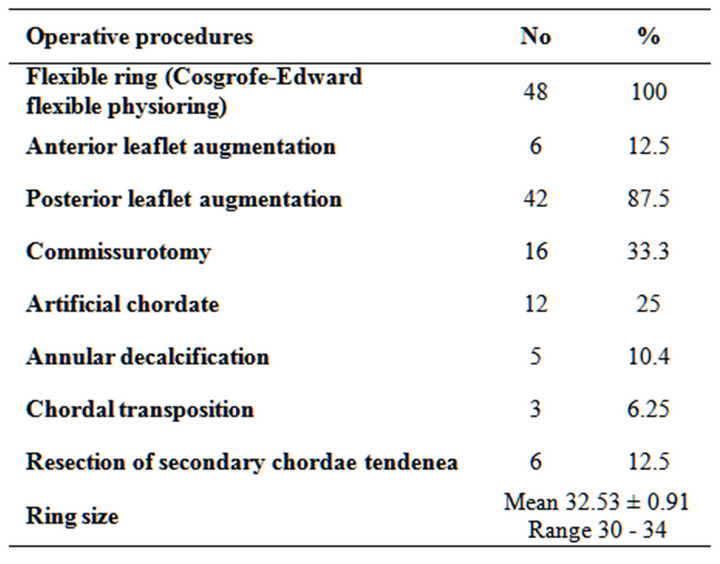
Table 4. Operative procedures.
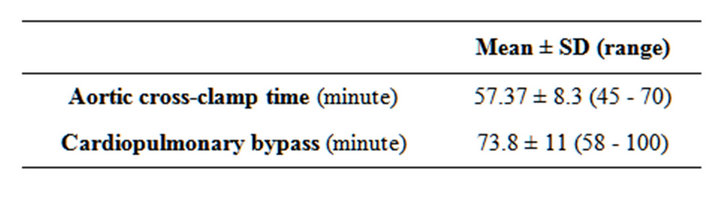
Table 5. Aortic cross-clamp time and cardiopulmonary bypass time.

Table 6. Post-bypass trans oesophageal echo (TEE).
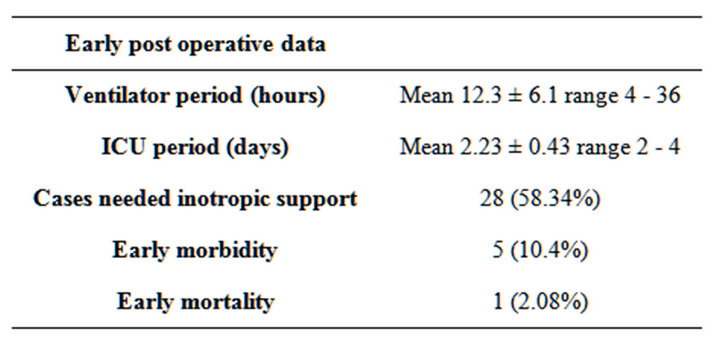
Table 7. Early post-operative data.
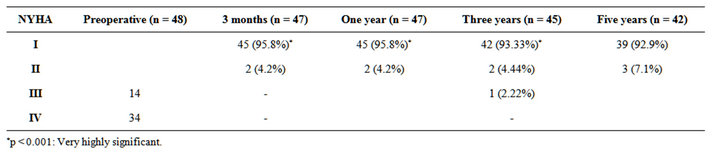
Table 8. NYHA functional class preoperative, after 3 monthes and after 1, 3, 5 years postoperatively.
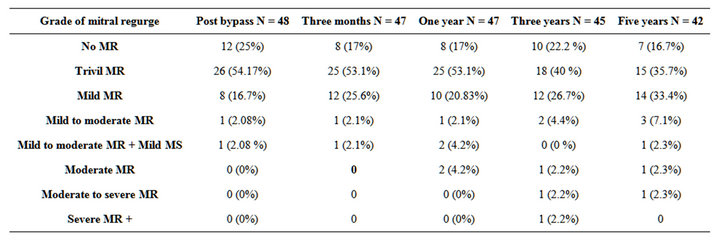
Table 9. Degree of MR post-bypass, after 3monthes and 1, 3, 5 years postoperative.
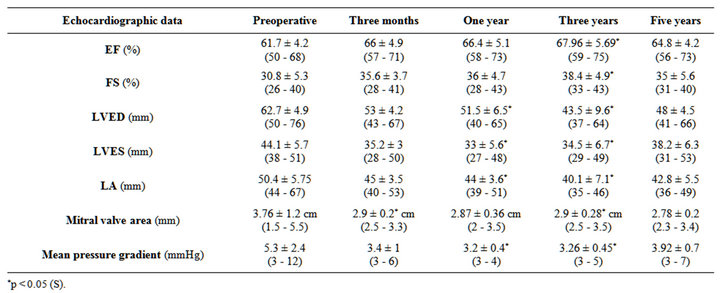
Table 10. Echocardiographic data; preoperative, 3 months postoperative and after 1, 3, 5 years post operative.
reoperation in the rheumatic patients.
In our series, the severity of fibrous retraction (more prominent in type III), mixed lesions resulting in a mitral orifice area under 1.5 cm2 at the time of initial repair, and recurrent rheumatic fever episodes were risk factors for late reoperation.
Late morbidity; reoperation was carried out in 5 patients with different interval from initial surgery. Causes of reoperation were restrictive valve motion in one patient, left atrial thrombus formation in 1 patient and sever mitral regurge in 3 patients. Freedom from reoperation was 93% at 5 years. The reoperation rate was 1.6% per patient-year (Table 11).
Most reoperations were performed because progressive rheumatic process. In our patients, we found that there was slight increase in the degree of regurgitation during follow-up.
Incidence of thromboembolic complications in this study was 0.9% per patient-year. Thrombo embolism may not relate to the type of repair. Risk factor for thromboembolism was atrial fibrillation; therefore, the patient who had a risk factor should receive an anticoagulant.
5. Discussion
Mitral valve repair preserves the mitral valve apparatus and this has been shown to enhance and maintain left ventricular function [6-8]. Rheumatic mitral valve repair provides stable actuarial survival with fewer thromboemboliccomplications in a pediatric population noncompliant to anticoagulation. Similarly, despite the greater rate of reoperation as compared with mitral valve replacement with mechanical prosthesis, rheumatic mitral valve repair as a non thrombogenic surgical procedure remains an attractive alternative to mitral valve replacement whenever feasible [3].
This avoids the necessity of chronic anticoagulation and its associated sequela when a mechanical valve is inserted. Furthermore, mitral valve replacement is known to alter the geometry of the left ventricle [6,7].
We use autologous gluteraldehyde preserved pericardium to avoid problems encountered by use of fresh pericardium as calcification, progressive contracture, thickening fibrosis, loss of pliability, early degeneration, and endocarditis.
It has been applied successfully for anterior and posterior leaflet augmentation in cases of rheumatic mitral valve disease [9,10]. The scientific procedure of using autologous tissue treated with a brief immersion in gluteraldehyde solution was established by Chauvaud et al. in 1991 [2].
There were several studies on repair of severe mitral regurge by pericardial augmentation of anterior and posterior mitral leaflet. Edward et al. in 2004 described this technique in repair of ischemic mitral regurge [11].
Late morbidity; re operation was carried out in 5 patients with different interval from initial surgery.
Incidence of thromboembolic complications in this study was 0.9% per patient-year. Some reports showed thromboembolic rates of 0.6% to 2.52% per patient-year [12,13]. Therefore, thromboembolism may not relate to the type of repair. Risk factor for thrombo embolism was atrial fibrillation; therefore, the patient who had a risk factor should receive an anticoagulant. Causes of reoperation were restrictive valve motion in one patient, left atrial thrombus in 1 patient and sever mitral regurge in 3 patients. Freedom from reoperation was 87.5% at 5 years In our patients, we found that there was slight increase in the degree of regurgitation during the follow-up.
At 5 years, freedom from thrombo-embolic complications and reoperation were 97.9% and 87.5%, respectively. Thirty nine patients (92.9%) were in New York.
Heart Associationfunctional class I, 3 patients (7.1%) were in class II.
Echocardiography at follow-up showed satisfactory mitral valve function.
At 5 years of follow-up, freedom from cardiac death and mitral valve reoperation were 98% ± 2% and 89.6% ± 3%, respectively. Freedom from significant mitral regurgitation was 92.9%.
Anterior and posterior leaflet augmentation with autologous pericardium is a useful adjunct to compensate leaflet and chordae retraction [14,15]. None of the patients in our series required revision to a mechanical valve at over 2 years of follow-up.
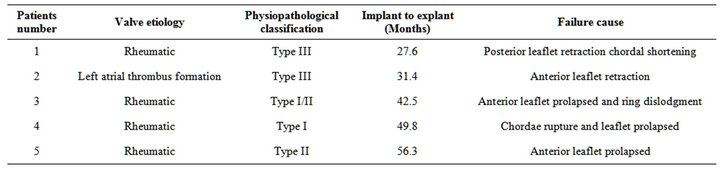
Table 11. Causesof mitral valve reconstruction failure.
Clearly, these midterm results using leaflet augmentation provide substantial evidence that mitral valve reconstruction can be accomplished with low hospital morbidity and mortality rates. The low incidence of reoperation and late cardiac events suggests that leaflet augmentation, offers a definite advantage in increasing cooptation line between anterior and posterior leaflet succeeding process of repair in fibrotic and shrinked rheumatic valves and may positively influence mid-term results. Also redundant MV leaflet tissue after augmentation reduces mechanical stress on the noncoapted leaflets, the extent of coaptation or frictional interleaflet interaction does not independently influence leaflet stresses. Repair techniques that increase or preserve noncoapted leaflet area may decrease mechanical stresses and thereby enhance repair durability [15-17].
These findings support the continued use of leaflet augmentation in patients presenting with mitral insufficiency secondary to rheumatic disease of the mitral valve with shrunked one or both leaflets or chordae retraction.
We didn’t see stiffening of the patch with follow-up echocardiography.
Further information and follow-up is needed to determine the extended long-term durability of patch augmentation of the posterior and anterior leaflet. However, these results demonstrate excellent utility of repair and this technique should be considered for these patients.
In conclusion, posterior and anterior leaflet augmentation is a simple and reproducible method of valve repair for rheumatic MR with good midterm result.
REFERENCES
- A. Carpentier, S. Chauvaud and J. N. Fabiani, “Reconstructive Surgery of Mitral Valve Incompetence. TenYear Appraisal,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 79, No. 3, 1980, pp. 338-348.
- S. Chauvaud, V. Jebara, J. C. Chachques, B. el Asmar, S. Mihaileanu, P. Perier, G. Dreyfus, J. Relland, J. P. Couetil and A. Carpentier, “Valve Extension with Glutaraldehyde-Preserved Autologous Pericardium: Results in Mitral Valve Repair,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 102, 1991, pp. 171-178.
- R. Zegdi, Z. Khabbaz, S. Chauvaud, C. Latremouille, J.-N. Fabiani and A. Deloche, “Posterior Leaflet Extension with an Autologous Pericardial Patch in Rheumatic Mitral Insufficiency,” The Annals of Thoracic Surgery, Vol. 84, No. 3, 2007, pp. 1043-1044. doi:10.1016/j.athoracsur.2006.12.058
- A. S. Kumar, S. Talwar and A. Saxena, “Results of Mitral Valve Repair in Rheumatic Mitral Regurgitation,” Interactive Cardiovascular and Thoracic Surgery, Vol. 5, No. 4, 2006, pp. 356-361.
- U. Bortolotti, A. D. Milano and R. W. M. Frater, “FRCSc: Mitral Valve Repair with Artificial Chordae,” The Annals of Thoracic Surgery, Vol. 93, No. 2, 2012, pp. 684-691.
- M. E. Goldman, F. Mora, T. Guarino, V. Fuster and B. P. Mindich, “Mitral Valvuloplasty Is Superior to Valve Replacement for Preservation of Left Ventricular Function: An Intraoperative Two-Dimensional Echocardiographic Study,” Journal of the American College of Cardiology, Vol. 10, No. 3, 1987, pp. 568-575. doi:10.1016/S0735-1097(87)80199-7
- M. D. Tischler, K. A. Cooper, M. Rowen and M. M. LeWinter, “Mitral Valve Replacement versus Mitral Valve Repair: A Doppler and Quantitative Stress Echocardiographic Study,” Circulation, Vol. 89, No. 1, 1994, pp. 132-137. doi:10.1161/01.CIR.89.1.132
- S. F. Bolling, F. D. Pagani, G. M. Deeb and D. S. Bach, “Intermediate-Term Outcome of Mitral Reconstruction in Cardiomyopathy,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 115, No. 2, 1998, pp. 381-388. doi:10.1016/S0022-5223(98)70282-X
- S. Aubert, E. Flecher, S. Rubin, C. Acar and I. Gandjbakhch, “Anterior Mitral Leaflet Augmentation with Autologous Pericardium,” The Annals of Thoracic Surgery, Vol. 83, No. 4, 2007, pp. 1560-1561.
- A. Matthew, Romano, J. Himanshu, Patel and D. Francis, “Anterior Leaflet Repair with Patch Augmentation for Mitral Regurgitation,” The Annals of Thoracic Surgery, Vol. 79, No. 5, 2005, pp. 1500-1504.
- E. H. Kincaid, R. D Riley and M. H. Hines, “Anterior Leaflet Augmentation for Ischemic Mitral Regurgitation,” The Annals of Thoracic Surgery, Vol. 78, No. 2, 2004, pp. 564-568.
- S. F. Bolling, F. D. Pagani, G. M. Deeb and D. S. Bach, “Intermediate-Term Outcome of Mitral Reconstruction in Cardiomyopathy,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 115, No. 2, 1998, pp. 381-388. doi:10.1016/S0022-5223(98)70282-X
- S. N. Omeroglu, K. Kirali, D. Mansuroglu, D. Goksedef, M. Balkanay, G. Ipek, O. Isik and C. Yakut, “Is Posterior Leaflet Extension and Associated Commissurotomy Effective in Rheumatic Mitral Valve Disease? Long-Term Outcome,” Texas Heart Institute Journal, Vol. 31, No. 3, 2004, pp. 240-245.
- S. Aubert, E. Flecher, S. Rubin, C. Acar and I. Gandjbakhch, “Anterior Mitral Leaflet Augmentation with Autologous Pericardium,” The Annals of Thoracic Surgery, Vol. 83, No. 4, 2007, pp. 1560-1561. doi:10.1016/j.athoracsur.2006.05.083
- A. Gupta, P. Gharde and A. Sampath Kumar, “Anterior Mitral Leaflet Length: Predictor for Mitral Valve Repair in a Rheumatic Population,” The Annals of Thoracic Surgery, Vol. 90, No. 6, 2010, pp. 1930-1933. doi:10.1016/j.athoracsur.2010.07.035
- C. Xu, S. Arminder, Jassar, et al., “Augmented Mitral Valve Leaflet Area Decreases Leaflet Stress: A Finite Element Simulation,” The Annals of Thoracic Surgery, Vol. 93, No. 4, 2012, pp. 1141-1145. doi:10.1016/j.athoracsur.2012.01.069
- B. El Oumeiri, M. Boodhwani, D. Glineur, et al., “Extending the Scope of Mitral Valve Repair in Rheumatic Disease,” The Annals of Thoracic Surgery, Vol. 87, No. 6, 2009, pp. 1735-1740. doi:10.1016/j.athoracsur.2009.03.009
NOTES
*Corresponding author.

