Advances in Infectious Diseases
Vol. 3 No. 2 (2013) , Article ID: 32863 , 12 pages DOI:10.4236/aid.2013.32021
Other Possible Causes of a Well-Publicized Outbreak of Pseudomonas aeruginosa Following Arthroscopy in Texas*
![]()
Philadelphia, USA.
Email: Larry@myendosite.com
Copyright © 2013 Lawrence F. Muscarella. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 12th, 2013; revised March 28th, 2013; accepted April 28th, 2013
Keywords: Pseudomonas aeruginosa; Arthroscopy; Disease Transmission; Healthcare-Associated Infection; Root Cause Analysis; Instrument Reprocessing; Bacterial Outbreak; Sterilization; Sterile Technique
ABSTRACT
Background: Seven patients at a hospital in Houston, TX, were diagnosed during a two-week period in 2009 with joint space infection of pansusceptible P. aeruginosa following arthroscopic procedures of the knee or shoulder. Tosh et al. (2011), who investigated and published the principal report discussing this bacterial outbreak, conclude that its most likely cause was the improper reprocessing of certain reusable, physically-complex, heat-stable arthroscopic instruments used during these arthroscopic procedures. These reusable instruments reportedly remained contaminated with remnant tissue, despite diligent efforts by the hospital to clean their internal structures. This retained bioburden presumably shielded the outbreak’s strain of embedded P. aeruginosa from contact with the pressurized steam, reportedly resulting in ineffective sterilization of these arthroscopic instruments and bacterial transmission. Objectives: First, to clarify which specific sterilization methods, in addition to steam sterilization, Methodist Hospital employed to process its reusable arthroscopic instrumentation at the time of its outbreak, in 2009; second, to evaluate Tosh et al.’s (2011) conclusion that ineffective steam sterilization due to inadequate cleaning was the most likely cause of this hospital’s outbreak; third, to consider whether any other hitherto unrecognized factors could have plausibly contributed to this outbreak; and, fourth, to assess whether any additional recommendations might be warranted to prevent disease transmission following arthroscopic procedures. Methods: The medical literature was reviewed; some of the principles of quality assurance, engineering and a root-cause analysis were employed; and Tosh et al.’s (2011) findings and conclusions were reviewed and compared with those of other published reports that evaluated the risk of disease transmission associated with the steam sterilization of physically-complex, heat-stable, soiled surgical instruments. Results and Conclusion: Reports documenting outbreaks of P. aeruginosa or another vegetative bacterium associated with the steam sterilization of inadequately cleaned surgical or arthroscopic instruments are scant. This finding—coupled with a number of published studies demonstrating the effective steam sterilization of complex instruments contaminated with vegetative bacteria mixed with organic debris, or, in one published series of tests, with resistant bacterial endospores coated with hydraulic fluid—raises for discussion whether Methodist Hospital’s outbreak might have been due to one or more factors other than, or in addition to, that which Tosh et al. (2011) conclude was its most likely cause. An example of such a factor not ruled out by Tosh et al. (2011) findings would be the re-contamination of the implicated arthroscopic instruments after sterilization. The specific methods that Methodist Hospital employed at the time of its outbreak to sterilize some of its arthroscopic instrumentation remain unclear. A number of additional recommendations are provided to prevent disease transmission following arthroscopic procedures.
1. Introduction
Seven patients at Methodist Hospital in Houston, Texas (USA), were infected during a two-week period, between April 22, 2009, and May 7, 2009, with pansusceptible P. aeruginosa following arthroscopic procedures of the knee (n = 6) or shoulder (n = 1) [1,2]. This outbreak was reported by Tosh et al. (2011), whose authors include representatives of Methodist Hospital, the Texas Department of State Health Services and the Centers for Disease Control and Prevention (CDC). Among other risk factors, these researchers investigated the potential for these seven joint-space infections to be due to contaminated arthroscopic equipment [1]. During this outbreak, Methodist Hospital used certain reusable, physically-complex arthroscopic shaver handpieces and arthroscopic inflow/outflow cannulae, as well as rigid arthroscopes, to perform the procedures on these seven patients [1,2]. Tosh et al.’s (2011) report, which is a case-control study, concludes that the complex physical designs of these shaver handpieces and inflow/outflow cannulae were the primary factor responsible for this outbreak.
More specifically, these researchers conclude that the most likely cause of Methodist Hospital’s P. aeruginosa outbreak was the inadequate reprocessing of these arthroscopic handpieces and inflow/outflow cannulae, which are heat-stable (i.e., not damaged by a steam autoclave) and whose internal structures apparently do not facilitate thorough cleaning. These arthroscopic instruments reportedly remained contaminated with “remnant tissue” [1] (and, in the case of the shaver handpiece’s suction channel, also with brush bristles) that was “not evident to the naked eye,” [3] despite the shaver handpiece’s suction channel reportedly having been cleaned in accordance with its manufacturer’s instructions [1-3]. This retained tissue, which Tosh et al. (2011) suggest provided a “sanctuary for bacterial contamination,” [1] presumably shielded and protected the outbreak’s strain of P. aeruginosa, resulting in ineffective steam sterilization. As a direct consequence, these contaminated instruments apparently transmitted P. aeruginosa to these seven patients during these invasive procedures [1,2].
In addition to Tosh et al.’s (2011) investigation of this hospital’s outbreak of P. aeruginosa [1], several other reports discuss the potential for disease transmission due to infectious remnant tissue remaining within the internal structures of these specific arthroscopic shaver handpieces [3,4] and inflow/outflow cannulae after their reprocessing [5]. These reports include a notice issued by the U.S. Food and Drug Administration (FDA), a report filed in the FDA’s MAUDE1 database, and a device recall [3-5]. Further underscoring its potential impact on public health, Methodist Hospital’s bacterial outbreak was also the focus of the popular national news media (including NBC’s Nightly News and Fox News) [2,6,7]. The completeness of a study like Tosh et al.’s (2011) important investigation of the possible causes of Methodist Hospital’s P. aeruginosa outbreak may be better assured not only by performing a root cause analysis of this outbreak but also by providing, as warranted, additional recommendations to prevent infections of the same or of a similar etiology. Indeed, the medical literature’s inadvertent omission of every possible cause of, or factor contributing to, a healthcare facility’s bacterial outbreak (or a similar type of adverse event) could hinder or prevent the development and implementation of crucial corrective actions, boding the possibility of the outbreak’s recurrence.
2. Objectives
This article:
1) aims to clarify which specific sterilization methods, in addition to steam sterilization, Methodist Hospital employed to process its reusable arthroscopic instruments at the time of its bacterial outbreak, in 2009;
2) evaluates Tosh et al.’s (2011) conclusion that the most likely cause of this hospital’s outbreak was the ineffective steam sterilization of reusable, heat-stable arthroscopic shaver handpieces and inflow/outflow cannulae, due to bioburden that was retained within the internal structures of these physically-complex instruments after their apparent thorough cleaning;
3) considers whether one or more hitherto unrecognized deviations, non-conformances, or factors—both unrelated to the ineffective sterilization of inadequately cleaned arthroscopic instruments and not ruled out by Tosh et al.’s (2011) data—might have contributed to or have been responsible for this hospital’s outbreak; and 4) assesses whether any additional recommendations (not included in the Tosh et al. [2011] report) might be indicated to prevent bacterial infections following arthroscopic procedures of the knee and shoulder.
3. Methodology
The medical literature was reviewed; some of the principles of quality assurance, engineering and a root cause analysis were employed; and Tosh et al.’s (2011) data, findings and conclusions were compared with those of other published studies that evaluated the risk of disease transmission associated with the steam sterilization of physically-complex, heat-stable surgical instruments contaminated with bioburden and/or another type of soil or debris. To provide additional insight into risk factors associated with the transmission of P. aeruginosa in the healthcare setting, also reviewed were a number of other studies that discuss outbreaks not only of P. aeruginosa associated with improperly reprocessed flexible endoscopes, but also of P. aeruginosa and other bacteria linked to: poor hand hygiene or to the contaminated hands of healthcare workers; the re-contamination of instruments after sterilization (or disinfection) due to, for example, their improper handling; and contaminated environmental surfaces, including tap water and handwashing sinks.
4. Results
Tosh et al. (2011) report that, during the time of its P. aeruginosa outbreak, in 2009, Methodist Hospital processed both its arthroscopic shaver handpieces and inflow/ outflow cannulae (but not its rigid arthroscopes; see below) using either traditional steam sterilization or flash (steam) sterilization. In addition to using it as many as “6 times daily during the outbreak period” and to process arthroscopic instruments used on two of the seven infected patients [1], these authors also report that Methodist Hospital used flash sterilization “on rare occasions for routine sterilization,” [1]. Briefly, whereas traditional steam sterilization may be achieved using a gravity displacement sterilizer or a pre-vacuum sterilizer that is generally installed in a central (and often remote) reprocessing area, either type of which features an extended terminal drying phase, flash sterilization, which is a more rapid process without a terminal drying phase (note: this point-of-use process is indicated only in emergency situations, for example, to process instruments that were inadvertently dropped on the floor), may be installed in or near the operating-room suite. Further, Tosh et al. (2011) report that this hospital employed at the time of this P. aeruginosa outbreak a device that uses hydrogen peroxide gas plasma2, but reportedly only to process the hospital’s rigid arthroscopes (including their light cords and camera/power cords) [1].
This article’s review of the medical literature presents a number of additional findings. First, it identified several reports that document outbreaks (and pseudo-outbreaks) of P. aeruginosa associated with a number of different types of reusable medical instruments, including bronchoscopes, gastrointestinal endoscopes and transrectal ultrasound-guided prostate biopsy equipment [9- 17]. These instruments, however, unlike the arthroscopic shaver handpieces and inflow/outflow cannulae discussed by Tosh et al. (2011), are damaged by heat, and for each of these reported outbreaks disease transmission was linked, not to ineffective steam sterilization, but to inadequate high-level disinfection or liquid chemical sterilization. These latter two types of processes use a low-temperature liquid chemical disinfectant or sterilant to effect their outcome, and the cause of infection associated with either is often attributed to the terminal rinsing of heat-sensitive instruments with water that was contaminated with bacteria [10-15].
Second, this review found that published reports associating infections of P. aeruginosa (or another vegetative bacterium) to the use of reusable, heat-stable, physically-complex surgical or arthroscopic instruments exposed to a (properly functioning) steam sterilization process (i.e., a steam autoclave), no matter whether the instruments were inadequately cleaned, are scant and with few exceptions, such as Tosh et al.’s (2011) report. (Most types of vegetative bacteria are readily destroyed by even as limited a decontamination process as lowlevel disinfection [18]).
And, third, this review identified a number of studies that demonstrate pressurized steam’s successful sterilization of complex surgical instruments that not only were inoculated with high numbers of vegetative bacteria, or even with high numbers of resistant bacterial endospores, but that also were contaminated with a soil or organic debris and, in one instance, with hydraulic fluid, which reportedly poses a more formidable hindrance to effective sterilization than bioburden [19-22].
5. Discussion
Tosh et al. (2011) conclude that the most likely cause of Methodist Hospital’s bacterial outbreak was, in the following temporal sequence: the inadequate cleaning of certain arthroscopic shaver handpieces and inflow/outflow cannulae, resulting in remnant bioburden remaining within their suction channel and lumen, respectively; the ineffective sterilization of these instruments due to this bioburden shielding and protecting infectious bacteria; and the subsequent transmission of surviving P. aeruginosa to the seven case patients (during the arthroscopic procedures that used these implicated arthroscopic instruments). This conclusion is replete with important public-health implications, although it is also somewhat exceptional. Moreover, that the potential causes of Methodist Hospital’s bacterial outbreak have been studied by the FDA and CDC, as well as having been the focus of the national news [2,6,7], is testimony to the importance not only of Tosh et al.’s (2011) investigation, findings and conclusions, but also of developing and implementing validated corrective and preventive actions documented to prevent the recurrence of this type of bacterial outbreak (as well as of other types) [1-7].
5.1. Effectiveness of Steam Sterilization
Notwithstanding Tosh et al.’s (2011) conclusion of this bacterial outbreak’s most likely cause, this review identified a number of studies that demonstrate pressurized steam’s successful sterilization of physically-complex surgical instruments contaminated with bioburden. Voyles et al. (1995), for example, report that a 3-minute flash sterilization cycle destroyed high concentrations of vegetative bacteria (like P. aeruginosa) inside a 12-mm reusable metal laparoscopic cannula3 [20]. This outcome was reportedly achieved despite—as an additional challenge to the sterilization process’s effectiveness and for the purpose of creating a scenario that was “much worse than should ever occur in a clinical setting” [20]—the cannula both having had its openings sealed to interfere with the steam’s direct contact with the bacteria and having been packed with organic debris (i.e., hamburger meat inoculated with the bacteria) [20].
Like Voyles et al. (1995), [20] Rutala et al. (2008) [21] performed a series of similar tests designed to evaluate the effectiveness of steam sterilization during “worstcase” (i.e., most challenging) conditions. During one series of tests, these latter authors contaminated physically complex surgical instruments with high numbers of heatresistant spores of Geobacillus stearothermophilus. (These spores are significantly more resistant to pressurized steam than vegetative bacteria, and, therefore, from a probability standpoint, a steam sterilization process’s complete eradication of high numbers of spores of G. stearothermophilus assures the destruction of high numbers of P. aeruginosa.) These contaminated instruments—which featured a hinged surface, a crevice or a thumb screw, and, therefore, are each seemingly as (if not more so) physically complex and challenging to sterilize as the arthroscopic shaver handpieces and inflow/outflow cannulae used by Methodist Hospital during the time of its bacterial outbreak, in 2009—were air dried and then coated with (20 mL of) an oil-based hydraulic fluid, to further challenge the sterilization process [21]. During a second series of tests similarly designed to challenge the sterilization process’s effectiveness, Rutala et al. (2008) placed contaminated scalpel blades, which had been inoculated with more than 106 spores of G. stearothermophilus, air dried, and then coated with (20 mL of) hydraulic fluid, into the center of a relatively long and narrow lumen [21]. For both of these series of tests, these authors reported that the contaminated surgical instruments were successfully sterilized. Based on their findings, Rutala et al. (2008) conclude that their data demonstrate steam sterilization’s “robustness” and “huge margin of safety,” even of contaminated instruments that had also been coated with hydraulic fluid [21].
In their study Tosh et al. (2011) discuss a report by Belvins et al. (1999) that investigated three cases of organ/space surgical-site infections (SSIs) due to coagulase-negative Staphylococcus (“CoNS”) following arthroscopic procedures [22]. Implicating a single set of arthroscopic inflow/outflow cannulae that had been used during each of these three cases as a potential source of these SSIs, Belvins et al. (1999) identified “dried organic material” in the lumens of some of these cannulae after their reprocessing, with cultures from three of this set’s six cannulae (but not necessarily from the retained organic material itself) being “positive” for CoNS. Although these findings appear to be consistent with Tosh et al.’s (2011) conclusion (that Methodist Hospital’s outbreak was most likely due to ineffective sterilization resulting from inadequate cleaning), Belvins et al. (1999) also performed a number of experimental tests during their investigation that documented the successful steam (and flash) sterilization of arthroscopic cannulae inoculated with CoNS in the presence of blood [22]. In summary, the studies by Voyles et al. (1995) and Rutala et al. (2008), as well as Belvins et al.’s (1999) experimental tests, demonstrating the effectiveness of steam sterilization and its wide margin of safety, coupled with a dearth of reports associating the steam sterilization of physically-complex surgical instruments with bacterial outbreaks in the healthcare setting, save for Tosh et al.’s (2011) report, raise for discussion the possibility that one or more other, hitherto unrecognized causes—both unrelated to that which Tosh et al. (2011) conclude was its most likely cause and not excluded by their findings— might have contributed to or been responsible for Methodist Hospital’s P. aeruginosa outbreak.
5.2. Contaminated Surfaces, Hands
Outbreaks of P. aeruginosa have been associated with the improper reprocessing of flexible endoscopes, often due to their terminal rinsing with contaminated water following high-level disinfection or liquid chemical sterilization [9-18]. Yet, improper reprocessing of reusable medical equipment—whether of a flexible endoscope, an arthroscopic instrument, or another type of medical instrument—is but only one of many factors documented to be responsible for infections of P. aeruginosa in the healthcare setting. For example, reports also describe infections of P. aeruginosa and other bacteria associated with poor hand hygiene or the contaminated hands (and fingernails) of healthcare workers; with the re-contamination of (unwrapped) instruments during their improper handling; and with hand-washing sinks colonized with bacteria [18,23-30]. Like the studies by Voyles et al. (1995) and Rutala et al. (2008), these reports also lend credence to the possibility that, not necessarily the ineffective steam sterilization of the implicated arthroscopic instruments, but rather another factor—for example, the inadvertent failure to have sterilized the instruments using any method [31]4—might have contributed more to Methodist Hospital’s outbreak than has been recognized. Table 1 lists, along with Tosh et al.’s (2011) suggestion of its most likely cause, a number of other factors that would appear to be plausible contributors to this hospital’s bacterial outbreak in 2009.
5.3. Re-Contamination after Sterilization?
Unlike Belvins et al.’s (1999) report, which found cultures from three of the sampled cannulae to be “positive” for CoNS, Tosh et al. found (2011) that none of the cultured samples collected during their investigation from Methodist Hospital’s shaver handpieces and inflow/outflow cannulae grew P. aeruginosa or another bacterium [1]. While this null result, by itself, neither assuredly exculpates these complex arthroscopic instruments as a contributor to this outbreak nor, on the other hand, certainly refutes Tosh et al.’s (2011) claim of their culpability, cultured samples that grew the outbreak’s strain of pansusceptible P. aeruginosa would have been expected if steam sterilization of the implicated arthroscopic instruments, contaminated with remnant tissue, had been ineffective as Tosh et al. (2011) suggest [1]. Instead, these authors (2011) found, in addition to four samples of this hospital’s tap water being contaminated with Pseudomonas species isolates, twelve environmental isolates of Pseudomonas species collected from sink drains were pansusceptible P. aeruginosa. Like the studies by Hota et al. (2009) [29] and Lowe et al. (2012) [30]—both of which found one or more hand-washing sinks (or their drains) to be contaminated with, and the source [29] (or a contributing reservoir [29]) of, their respective outbreak’s strain of bacteria (presumed to have been transmitted to patients via “splashing” water [29,30])—Tosh et al. (2011) report that one of the twelve environmental isolates that was collected from the drain of the sink in the hospital’s decontamination room had pulsed-field gel electrophoresis (PFGE) patterns indistinguishable from the outbreak’s strain of P. aeruginosa [1]—a finding that suggests this sink’s drain may have been a source or reservoir of this hospital’s outbreak.
Based on this result, Tosh et al. (2011) conclude that the implicated arthroscopic instrumentation was most likely contaminated during the “gross decontamination

Table 1. Factors that may have contributed to or have been a cause of Methodist Hospital’s bacterial outbreaka.
steps.” [1] These authors also note that this strain (which may have formed a biofilm of pansusceptible P. aeruginosa on this sink’s surfaces [1]) was “likely introduced into the case patients’ joint spaces by direct insertion of (these) contaminated instruments or by infusion of fluids through the contaminated lumen [1].” While Tosh et al.’s (2011) explanation of the likely cause of Methodist Hospital’s outbreak is certainly plausible, if not entirely accurate, their investigation, nonetheless, do not rule out the possibility (and, in some instances, their report’s data and findings appear consistent with the possibility5) that one or more other unrecognized factors might have contributed to, or have been primarily responsible for, this outbreak (and/or that an unrecognized source may have been the origin of this outbreak’s bacteria). An example of such a factor would be the inadvertent re-contamination of the implicated arthroscopic instrumentation with the sink drain’s isolate (which was indistinguishable from the outbreak’s strain) after its successful sterilization (possibly, due to the splashing of contaminated water from the sink onto this instrumentation) (see: Table 1).
5.4. An Anomalous Outbreak
Based on this review, the possibility that Methodist Hospital’s cluster of seven P. aeruginosa infections in 2009 may have been due more to another, unrecognized deviation, cause, or factor unique to Methodist Hospital’s practices (see: Table 1) than (if at all) to ineffective sterilization (resulting from bioburden that remained within the internal structures of inadequately cleaned arthroscopic instrumentation) warrants consideration. Indeed, in addition to rationalizing and providing a viable alternative to Tosh et al.’s (2011) conclusion that P. aeruginosa (which is ordinarily destroyed even by low-level disinfection [18]) likely survived exposure to steam sterilization (albeit in the presence of remnant tissue), this possibility might explain another issue that Tosh et al.’s (2011) report (and, too, a communication by the FDA [3]) does not resolve: why this outbreak (and other bacterial outbreaks of the identical or similar etiology) was reported only once at this one hospital, in 2009, and has not also been reported at other times, at other medical facilities, nationwide, that also use these same arthroscopic instruments [1,2]. This apparent anomaly is all the more puzzling, because Tosh et al. (2011) report that arthroscopic shaver handpieces used at other medical facilities were inspected by officials of Methodist Hospital and were found, too, to have retained bioburden (after cleaning)—a finding that prompted these authors to conclude that “this problem is not specific to (Methodist Hospital) or to a specific manufacturer [1].”
Raising more questions, this review did not identify any other instances of bacterial infections or outbreaks associated with the inadequate cleaning and unsuccessful steam sterilization of the implicated arthroscopic shaver handpieces and inflow/outflow cannulae. During its discussions with, and an inspection of its facility by, the FDA just a few months after Methodist Hospital’s outbreak, in August, 2009, the manufacturer of this hospital’s arthroscopic shaver handpieces reported that for the previous decade it had “frequently” identified remnant “tissue-like” materials remaining within the internal structures of these handpieces returned by healthcare facilities for service, inspection and/or repair [32]. In the FDA’s report detailing its inspection of this manufacturer’s facility, the manufacturer stated that it was unaware of any bacterial outbreaks, save for Methodist Hospital’s, linked to this specific non-conformance [32]. According to this manufacturer, data indicating that bioburden retained within the internal structures of these heat-stable arthroscopic handpieces, which are routinely steam sterilized after each use in accordance with their labeling instructions, posed a substantive risk of patient harm are lacking, an assessment of risk that appears consistent with other published reports [18,20-22].
5.5. High-Level Disinfection or Sterilization?
Crucial to understanding all of the possible causes of its bacterial outbreak is the determination of each of the specific methods and processors Methodist Hospital used between April 22, 2009, and May 7, 2009, to sterilize at least some of its arthroscopic instrumentation. Tosh et al. (2011) use a number of different terms to describe the intended function of the hydrogen peroxide gas plasma processor that Methodist Hospital used at the time of its outbreak to sterilize its rigid arthroscopes. For example, the text of Tosh et al.’s (2011) report (which is distinguished from its tables) aptly describes this device (whose trade or brand name, however, their report does not mention) as achieving that for which the FDA cleared it6: low-temperature sterilization [1]. Tosh et al.’s (2011) Table 2, however, describes this same gas plasma processor as achieving high-level decontamination [1]—an unusual descriptor that is distinct from low-temperature sterilization (and high-level disinfection). Further, Tosh et al. (2011) do not define this descriptor in their report; nor does the FDA associate high-level decontamination with any legally marketed sterilizer or other type of terminal processor. Tosh et al.’s (2011) report also describes this processor as achieving a third outcome: highlevel disinfection7, the FDA having not cleared this (or any other) terminal sterilization processor for this intended use notwithstanding.
Tosh et al.’s (2011) use of the unusual term highlevel decontamination in their Table 2 to describe the outcome achieved by this hydrogen peroxide gas plasma processor (which the FDA cleared to achieve low-temperature sterilization) is seemingly inadvertent. But, their report’s use, additionally, of the term highlevel disinfection to describe this gas plasma processor’s outcome introduces for consideration whether some of Methodist Hospital’s arthroscopic (or surgical) instrumentation at the time of its outbreak was just that: exposed to a process that terminally achieves high-level disinfection (or liquid chemical sterilization8) (see: Table 1). As previously noted, these authors report that an isolate collected from the drain of the sink in this hospital’s decontamination room had PFGE patterns indistinguishable from the outbreak’s strain of P. aeruginosa [1]. If any of the implicated arthroscopic instruments were indeed high-level disinfected (accidentally, inadvertently, or unwittingly), then the microbial quality of the hospital’s water would have been necessary to determine in order to evaluate whether this water—which would have been used to rinse the instruments terminally after their chemical immersion and, if contaminated with the outbreak’s strain of P. aeruginosa, would most likely have been a source of this bacterial outbreak [10,13,15,17]— might have played more of a role in causing this outbreak than has been recognized9. No matter, Tosh et al.’s (2011) use of these three different terms to describe this gas plasma processor and its outcome leaves unclear and unresolved which specific methods Methodist Hospital used to process at least some of its arthroscopic instrumentation during the time of its bacterial outbreak, in 2009.
5.6. Additional Implications
Listed in Table 2, two additional issues, among others, arise from this review of Tosh et al.’s (2011) report, both with important implications to public health. First, that P. aeruginosa might remain viable within the internal structures of physically-complex surgical instruments, or at least of the implicated arthroscopic instruments, after their cleaning and steam sterilization—which Tosh et al. (2011) conclude is both the most likely cause of Methodist Hospital’s outbreak and a “problem” not specific to this one hospital or to any one manufacturer—is a potentially portending suggestion with concerning implications not just to instrument cleaning, sterilization, instrument design, and public health, but also to the FDA’s regulation of reusable medical equipment. As a consequence of these authors’ investigation of Methodist Hospital’s outbreak, reasonable questions may be asked of the FDA about which specific reusable, physically-complex heatstable surgical instruments used today in US healthcare facilities, including reusable arthroscopic shaver handpieces and inflow/outflow cannulae [1], may preclude thorough cleaning and, therefore, be prone to disease transmission during surgery. Such suspect instruments would seemingly warrant redesign or another corrective action to improve the effectiveness of its reprocessing and prevent patient harm.
Second, due to the acknowledged limitations of lowtemperature sterilization [18,33,34], not only is the exclusive use of steam sterilization to process surgical instruments not damaged by pressurized steam recommended, but also the use of low-temperature sterilization to process heat-sensitive surgical instruments (for which

Table 2. Several salient findings that arise from this review of the Tosh et al. (2011) report.
these processes might have been originally intended to process) featuring complex internal surfaces and structures that, like the designs of Methodist Hospital’s implicated arthroscopic instruments, hinder reprocessing and whose cleanliness (after cleaning) cannot be verified (whether by visual examination or another method) would be questioned (see: Table 2). (The ineffectiveness of a steam sterilization process would assure the failure of a low-temperature sterilization process [33,34]10).
5.7. A Root Cause Analysis
The possibility that any one of the several plausible factors, considerations, or causes listed in Table 1 might have contributed to Methodist Hospital’s outbreak underscores another of this review’s findings: that efforts to prevent of disease transmission and an outbreak’s recurrence may be advanced and optimized by the completion of a root cause analysis. During this analysis, every deviation that might have caused or contributed to a medical facility’s outbreak (or other type of adverse event) is identified and associated with one or more corresponding corrective actions whose effectiveness has been validated. Table 3 lists a number of additional recommendations (which supplement those included in Tosh et al.’s [2011] report) that are, in part, a consequence of the root cause analysis of Methodist Hospital’s outbreak that was performed during this review and are provided to prevent bacterial outbreaks (such as Methodist Hospital’s). This review’s root cause analysis is provided in Table 4 (although this analysis is not inclusive of every one of Methodist Hospital’s deviations).
6. Conclusions
Tosh et al.’s (2011) impressive report is as distinguished as its advice is prophylactic. Its conclusion that the most likely cause of Methodist Hospital’s bacterial outbreak in 2009 was due to a vegetative bacterium (e.g., P. aeruginosa), albeit in the presence of remnant tissue, that likely survived on and was transmitted by surgical instruments exposed to at least one complete and robust steam ster-

Table 3. Additional recommendations provided to prevent bacterial outbreaks like Methodist Hospital’s in 2009a.
ilization cycle (that is, one completed sterilization cycle after each of the arthroscopic instrumentation’s clinical uses, which may have been several uses and cycles during this outbreak’s two-week period11) is arguably as significant as “the discovery of retained bioburden in the suction channel of arthroscopic shaver handpieces despite reprocessing according to the manufacturer’s instructions,” [1] which Tosh et al. (2011) report is “the most consequential aspect of this outbreak [1].”
Resolution of the apparent disparity between, on the one hand, Tosh et al.’s (2011) conclusion of the most likely cause of Methodist Hospital’s outbreak and, on the other, the conflation of, first, the medical literature’s lack of more reports like Tosh et al.’s (2011) documenting P. aeruginosa outbreaks associated with the traditional steam sterilization, or even the flash sterilization, of complex surgical and arthroscopic instruments, including those that may retain remnant tissue within their internal structures; and, second, a number of studies validating steam sterilization’s robustness and effectiveness for the prevention of disease transmission under the most challenging testing conditions [19-21,33,34] on its face is difficult. Indeed, the solution could rest, however, with another of this review’s findings, which warrant reemphasis (see: Table 2): the possibility that, not ineffective steam sterilization of the implicated arthroscopic instruments due to their inadequate cleaning, but rather one or more other (unrecognized) factors, considerations, or causes, unique to Methodist Hospital’s practices at the time of its outbreak in 2009, might have contributed to, or have been primarily responsible for, this hospital’s seven cases of P. aeruginosa infection. That the outbreak’s strain of bacteria could have been transmitted, not by a contaminated arthroscopic instrument, but rather via another, undetermined mode is a possibility that Tosh et al.’s (2011) findings do not exclude (see: Table 1).
Finally, Tosh et al.’s (2011) conclusions also suggest that improvements in the federal oversight and regulation of medical devices are necessary—particularly of some reusable arthroscopic and surgical instruments that are complex in physical design and may not facilitate cleaning and sterilization—to improve the quality of health care and to prevent outbreaks of P. aeruginosa and other types of patient harms following surgery. One example of such an improvement (in addition to the re-design of reusable surgical instruments that cannot be adequately cleaned) would be the requirement by the FDA that manufacturers demonstrate with more scientific rigor the validated effectiveness of the reprocessing protocol(s) they provide in their reusable instrument’s labeling and instructions for use (“IFU”).




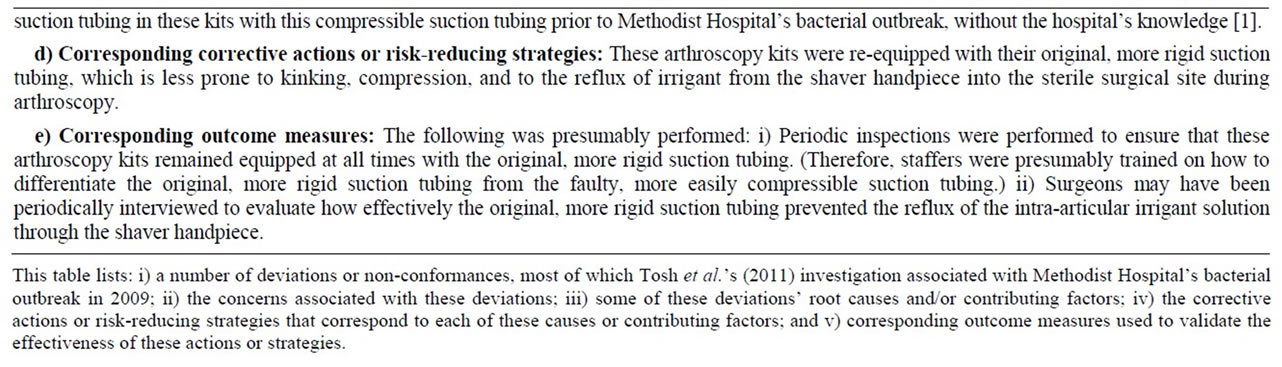
Table 4. A root cause analysis of the P. aeruginosa outbreak investigated at Methodist Hospital in 2009.
REFERENCES
- P. K. Tosh, et al., “Outbreak of Pseudomonas aeruginosa Surgical Site Infections after Arthroscopic Procedures: Texas, 2009,” Infection Control and Hospital Epidemiology, Vol. 32, No. 12, 2011, pp. 179-186. doi:10.1086/662712
- J. Eaton, “Filthy Surgical Instruments: The Hidden Threat in America’s Operating Rooms. How Dirty Medical Devices Expose Patients to Infection,” The Center for Public Integrity, Washington DC, 2012.
- Food and Drug Administration (FDA), “Ongoing Safety Review of Arthroscopic Shavers,” Safety Communications, 2009.
- Food and Drug Administration (FDA), “Formula 180 Shaver Handpeice. Manufacturer: Stryker Endoscopy,” Manufacturer and User Facility Device Experience Event, 2009.
- Stryker Endoscopy, “Class 2 Recall: Inflow/Outflow Cannula,” Medical Device Recalls, 2013.
- The NBC Nightly News, “Dirty Surgical Tools Put Patients at Risk,” 2012.
- Fox News, “Report: Dirty Surgical Tools in Hospitals Putting Patients at Risk,” 2012.
- Food and Drug Administration (FDA), “Sterrad NX Sterilizer (Manufacturer: Advanced Sterilization Products),” Manufacturer and User Facility Device Experience (MAUDE) Event, 2010.
- M. Sorin, et al., “Nosocomial Transmission of ImipenemResistant Pseudomonas aeruginosa Following Bronchoscopy Associated with Improper Connection to the Steris System 1 Processor,” Infection Control and Hospital Epidemiology, Vol. 22, No. 7, 2001, pp. 409-413. doi:10.1086/501925
- L. F. Muscarella, “Leading a Horse to Water: Are Crucial Lessons in Endoscopy and Outbreak Investigations Being Learned?” Infection Control and Hospital Epidemiology, Vol. 23, No. 7, 2002, pp. 358-360. doi:10.1086/503469
- P. Davies, “Germ Watch: Clinic Infections Put a Sterilizer of Lab Devices under Microscope,” The Wall Street Journal, 2004.
- C. J. Alvarado, S. M. Stolz and D. G. Maki, “Nosocomial Infections from Contaminated Endoscopes: A Flawed Automated Endoscope Washer. An Investigation Using Molecular Epidemiology,” The American Journal of Medicine, Vol. 91, No. 3, 1991, pp. 272S-280S. doi:10.1016/0002-9343(91)90381-7
- Centers for Disease Control and Prevention (CDC), “Pseudomonas aeruginosa Infections Associated with Transrectal Ultrasound-Guided Prostate Biopsies—Georgia, 2005,” Morbidity and Mortality Weekly Report, Vol. 55, No. 28, 2006, pp. 776-777.
- L. F. Muscarella, “Medical Errors, Infection-Control Breaches and the Use of Adulterated and Misbranded Medical Devices,” World Journal of Clinical Infectious Diseases, Vol. 2, No., 2, 2012, pp. 13-27.
- M. J. Struelen, F. Rost, A. Deplano, et al., “Pseudomonas aeruginosa and Enterobacteriaceae bacteremia after Biliary Endoscopy: An Outbreak Investigation Using DNA Macrorestriction Analysis,” The American Journal of Medicine, Vol. 95, No. 5, 1993, pp. 489-498. doi:10.1016/0002-9343(93)90331-I
- H. J. Kolmos, et al., “Pseudo-Outbreak of Pseudomonas aeruginosa in HIV-Infected Patients Undergoing Fiberoptic Bronchoscopy,” Scandinavian Journal of Infectious Diseases, Vol. 26, No. 6, 1994, pp. 653-657. doi:10.3109/00365549409008632
- L. F. Muscarella, “Contribution of Tap Water and Environmental Surfaces to Nosocomial Transmission of Antibiotic-Resistant Pseudomonas aeruginosa,” Infection Control and Hospital Epidemiology, Vol. 25, No. 4, 2004, pp. 342-345. doi:10.1086/502402
- Centers for Disease Control and Prevention (CDC), “Guideline for Disinfection and Sterilization in Healthcare Facilities,” 2008, pp. 1-158.
- Association of Perioperative Registered Nurses (AORN), “Perioperative Standards and Recommended Practices,” Denver, 2005.
- C. R. Voyles, et al., “Steam Sterilization of Laparoscopic Instruments,” Surgical Laparoscopy Endoscopy, Vol. 5, No. 2, 1995, 139-141.
- W. A. Rutala, M. F. Gergen and D. J. Weber, “Impact of an Oil-Based Lubricant on the Effectiveness of the Sterilization Processes,” Infection Control and Hospital Epidemiology, Vol. 29, No. 1, 2008, pp. 69-72. doi:10.1086/524326
- F. T. Blevins, et al., “Septic Arthritis Following Arthroscopic Meniscus Repair: A Cluster of three Cases,” Arthroscopy, Vol. 15, No. 1, 1999, pp. 35-40. doi:10.1053/ar.1999.v15.015003
- S. A. McNeil, et al., “Outbreak of Sternal Surgical Site Infections Due to Pseudomonas aeruginosa Traced to a Scrub Nurse with Onychomycosis,” Clinical Infectious Diseases, Vol. 33, No. 3, 2001, pp. 317-323. doi:10.1086/321890
- R. L. Moolenaar, et al., “A Prolonged Outbreak of Pseudomonas aeruginosa in a Neonatal Intensive Care Unit: Did Staff Fingernails Play a Role in Disease Transmission?” Infection Control and Hospital Epidemiology, Vol. 21, No. 2, 2000, pp. 80-85. doi:10.1086/501739
- A. F. Widmer, et al., “Outbreak of Pseudomonas aeruginosa Infections in a Surgical Intensive Care Unit: Probable Transmission via Hands of a Health Care Worker,” Clinical Infectious Diseases, Vol. 16, No. 3, 1993, pp. 372-376. doi:10.1093/clind/16.3.372
- X. Bertrand, et al., “Large Outbreak in a Surgical Intensive Care Unit of Colonization or Infection with Pseudomonas aeruginosa That Overexpressed an Active Efflux Pump,” Clinical Infectious Diseases, Vol. 31, No. 4, 2000, pp. E9-E14. doi:10.1086/318117
- A. M. Rogues, et al., “Contribution of Tap Water to Patient Colonisation with Pseudomonas aeruginosa in a Medical Intensive Care Unit,” Journal of Hospital Infection, Vol. 67, No. 1, 2007, pp. 72-78. doi:10.1016/j.jhin.2007.06.019
- D. Mayank et al., “Nosocomial Cross-Transmission of Pseudomonas aeruginosa between Patients in a Tertiary Intensive Care Unit,” Indian Journal of Pathology and Microbiologyl, Vol. 52, No. 4, 2009, pp. 509-513. doi:10.4103/0377-4929.56143
- S. Hota, et al., “Outbreak of Multidrug-Resistant Pseudomonas aeruginosa Colonization and Infection Secondary to Imperfect Intensive Care Unit Room Design,” Infection Control and Hospital Epidemiology, Vol. 30, No. 1, 2009, pp. 25-33. doi:10.1086/592700
- C. Lowe, et al., “Outbreak of Extended-Spectrum β-Lactamase-Producing Klebsiella oxytoca Infections Associated with Contaminated Handwashing Sinks,” Emerging Infectious Diseases, Vol. 18, No. 8, 2012, pp. 1242-1247. doi:10.3201/eid1808.111268
- D. M. Dudzinski, et al., “The Disclosure Dilemma— Large-Scale Adverse Events,” The New England Journal of Medicine, Vol. 363, No. 10, 2010, pp. 978-986. doi:10.1056/NEJMhle1003134
- Food and Drug Administration, “Establishment Inspection Report: Stryker Endoscopy,” Inspection, San Jose, 2009.
- L. F. Muscarella, “Instrument Design and Cross-Infection,” AORN Journal, Vol. 67, No. 3, 1998, pp. 552-553, 556. doi:10.1016/S0001-2092(06)62824-X
- L. F. Muscarella, “Are All Sterilization Processes Alike?” AORN Journal, Vol. 67, No. 5, 1998, pp. 966-970, 973- 976. doi:10.1016/S0001-2092(06)62622-7
- L. F. Muscarella, “The Importance of Bronchoscope Reprocessing Guidelines: Raising the Standard of Care,” Chest, Vol. 126, No. 3, 2004, pp. 1001-2; reply, pp. 1002-1003.
- L. F. Muscarella, “Application of Environmental Sampling to Flexible Endoscope Reprocessing: The Importance of Monitoring the Rinse Water,” Infection Control and Hospital Epidemiology, Vol. 23, No. 5, 2002, pp. 285- 289. doi:10.1086/502053
NOTES
*Financial disclosure: the author reports that he is not financially associated with the manufacture or sale of medical equipment, including arthroscopic instrumentation.
#The author is an independent researcher, writer and advisor.
1MAUDE refers to the FDA’s Manufacturer and User Facility Device Experience database whose data describe reports of adverse events involving medical devices.
2This processor’s reported use notwithstanding, no reports were identified in the FDA’s MAUDE database discussing its use by Methodist Hospital at the time of this bacterial outbreak, in 2009. Describing a different outbreak, however, a MAUDE report, dated 2010, associates five P. aeruginosa infections with the use of this same processor (which uses a hydrogen peroxide gas plasma) to sterilize arthroscopes [8].
3Voyles et al. (1997) also reported that exposure of the sealed cannula to steam sterilization for 7 minutes destroyed high numbers of Geobacillus stearothermophilus spores (contained within a biological indicator), although an exposure time of only 3 minutes was required to destroy these heat-resistant spores when the cannula was not sealed and one of its ports remained open [20].
4The inadvertent omission of a reprocessing step is not unprecedented. A practice associated with a self-evident risk of infection, reports document the cleaning, but not high-level disinfection or sterilization, of medical instruments prior to their clinical use [31]. Also, Tosh et al. (2011) report that Methodist Hospital’s sterilizer logs, at least those associated with the hydrogen peroxide gas plasma processor, were deficient, namely, documentation of the use of biological and chemical indicators during the time of its outbreak was incomplete, which prevented investigators from confirming that the sterilization process was properly functioning and that each load, pack or set of arthroscopic equipment had indeed been exposed to the sterilization process [1].
5An example would be, Tosh et al.’s (2011) finding that none of the cultured samples collected from the implicated shaver handpieces and inflow/outflow cannulae grew the outbreak’s strain of P. aeruginosa [1].
6Tosh et al.’s (2011) report states: “The manufacturer-recommended procedure for arthroscope reprocessing included gross decontamination with submersion in enzymatic solution for 10 - 15 minutes before low-temperature sterilization [1].”
7Tosh et al.’s (2011) report states: “The arthroscope-cleaning procedure at (Methodist Hospital) involved wiping down the instrument following a brief submersion of the instrument in enzymatic solution before highlevel disinfection [1].”
8No reports were identified during this review that would suggest that an automated device labeled to achieve “liquid chemical sterilization [9],” but considered by some healthcare facilities to achieve high-level disinfection, was used by Methodist Hospital to process any of its arthroscopic instrumentation at the time of its outbreak (although it seems plausible that this hospital might have used this specific device, in 2009, to process at least some surgical instruments).
9Tosh et al. (2011) state that due to “suboptimal water-sampling techniques,” contamination of Methodist Hospital’s tap water with Pseudomonas was “likely underestimated [1].”
10Whereas steam sterilization is associated with a SAL of 10−6, processes that achieve low-temperature sterilization are less reliable, especially for processing complex instruments soiled with organic debris, and may be associated with a greater likelihood of failure (e.g., a SAL of 10−3) [33,34].
11The individual arthroscopic instruments were not tracked at the time of this hospital’s outbreak [1], and, therefore, the number of times each might have been used and reprocessed during this time would not likely have been known.


