Advances in Biological Chemistry
Vol.3 No.1(2013), Article ID:28113,7 pages DOI:10.4236/abc.2013.31013
Spectral properties of LH2 exhibit very similar even when heterologously express LH2 with β-subunit fusion protein in Rhodobacter sphaeroides
![]()
1School of Chemistry and Pharmaceutical Engineering, Sichuan University of Science & Engineering, Zigong, China
2Bioengineering College, Chongqing University, Chongqing, China
Email: *zhipingzhao@suse.edu.cn
Received 7 November 2012; revised 8 December2012; accepted 14 December 2012
Keywords: LH2; Spectral Property; FT-IR; Photopigment
ABSTRACT
Interactions between the light-harvesting subunits and the non-covalently bound photopigments attribute considerably to the spectral properties of photosynthetic bacteria light-harvesting complexes. In our previous studies, we have constructed a novel Rhodobacter sphaeroides expression system. In the present study, we focus on the spectral properties of LH2 when heterologously express LH2 with β-subunitGFP fusion protein in Rb. sphaeroides. Near infra-red spectrum of LH2 remained nearly unchanged as measured by spectroscopy. Fluorescence spectrum suggested that the LH2 with β-subunit-GFP fusion protein complexes still possessed normal activity in energy transfer. However, photopigments contents were significantly decreased to a very low level in the LH2 with β-subunit-GFP fusion protein complexes compared to that of LH2. FT-IR spectra indicated that interactions between photopigments and LH2 α/β- subunits appeared not to be changed. It was concluded that the LH2 spectral properties exhibited very similar even when heterologously expressed LH2 (-subunit fusion protein in Rb. sphaeroides. Our present study may supply a new insight into better understand the interactions between light-harvesting subunits and photopigments and bacterial photosynthesis and promote the development of the novel Rb. sphaeroides expression system.
1. INTRODUCTION
The photosynthetic apparatus of photosynthetic bacteria such as Rb. sphaeroides commonly composed of three pigment-protein complexes located in the intracytoplasmic membrane (ICM) system, namely light-harvesting 1 complexes (LH1), LH2 and reaction center (RC) [1]. The LH1 is usually closely associated with the RC and both form the so-called LH1-RC core complexes [2], which are surrounded by closely connected LH2 [3]. The LH1 is present in a fixed stoichiometry to the RC at approximately 15:1, whereas the ratio of LH2 to the LH1-RC core complexes is variable according to the growth conditions such as light intensity and temperature [4]. The LH2 absorbs light energy during photosynthesis and subsequently transfers the energy to the RC where charge separations take place through the LH1.
The LH1 possesses the absorption band at approximately 875 nm, whereas the LH2 absorbs maximally at approximately 800 nm and 850 nm [5]. LH1 and LH2 are constructed in a remarkably similar fashion and the primary component is the α/β-subunit. It has been well characterized that the LH1 is made up of 16 α/β-subunits [6]. However, the LH2 is comprised of 8 or 9 α/β-subunits depending on bacterial species as revealed by electron microscopy or other techniques [7]. Both LH1 and LH2 non-covalently bind carotenoids (Crt) and Bacteriochlorophyll (BChl) which play crucial roles in light absorption, the assembly of the two pigment-proteins and the process of photosynthesis [8].
LH2 from Rb. sphaeroides is a nonamer [7]. The inner ring is formed by 9 α-subunits, while the outer ring is made up of 9 β-subunits. Generally, each α/β heterodimer non-covalently binds a total of three BChl and one or two Crt molecules, giving a total of 27 BChl and 9 or 18 Crt molecules in the specific LH2 [9]. BChl serves as the dominant chromophore taking part in light capture, transition and reaction. The 27 BChl molecules are subdivided into two discrete pools. Eighteen BChl molecules are arranged near the periplasmic surface of LH2 as a closely coupled dimer and these molecules have an absorption maximum at 850 nm and are identified as BChl- 850. The rest 9 BChl molecules lie toward the cytoplasmic side of LH2 and they absorb in the near infra-red at 800 nm and are thus termed BChl-B800 [10]. Interactions between the BChls and within the protein binding pocket studies indicate the structural arrangement of the BChls and the central Mg2+ atoms plays important roles in the binding of the pigment to α/β-subunits [11]. It has been proposed that protein environment exerts significant effects on the spectral properties of the BChl molecules of the LH2 [12]. Moreover, protein-induced macrocycle distortion will contribute significantly to spectral properties of the BChl bound in purple bacterial lightharvesting complex [13]. Interestingly, absorbance appears to be entirely changed in purple bacteria arises primarily due to growth conditions. Fowler and coworkers have pointed out that the two highly conserved aromatic residues, α-Tyr-44 and α-Tyr45, in LH2 are involved in the interactions of BChl and LH2, and mutagenesis of the two residues give rise to the breakage of the hydrogen bond and consequent blue shift of the LH2 absorption [14]. The residue βArg-10 has been proposed to be involved in an H-bond with the acetyl carbonyl of the B800-BChl absorbing proved by siteselected mutagenesis [15]. Hydrogen bonding environment largely influences LH2 absorbance [16] and the H-bonding was also revealed to drive the assembly of LH2 in Rb. sphaeroides [17,18]. Similarly, the LH1 αTrp43 was proposed to be located near the putative binding site for BChl molecule [19]. Dissimilar to the purple non-sulfur bacteria, calcium ions were demonstrated to be involved in the unusual red shift of the LH1 Qy transition of the core complex in Thermochromatium tepidum [20]. Hydrogen bonds were intensively studied in the binding of BChl molecules to RC [21]. On the other hand, Crt molecules exerts an effect on the structure of the BChl binding site in LH2 [22].
In photosynthetic bacteria, Crts appear not be exist within the cells in a free state. Instead, they are non-covalently attached to the three pigment-proteins. In LH2 and LH1, the non-covalently bound Crts molecules serve a number of functions. They absorb the visible region sunlight that is not absorbed by the BChl molecules [23]. In addition, the Crts serve as photo-protecting pigment to protect light-harvesting complexes against photo-oxidative damage and dissipate excess radiant energy and which thus is crucial for the survival of the photosynthetic organisms [24]. It appears that the assembly and stability of LH2 requires the presence of the Crts molecule as the carotenoid-deficient purple non-sulfur bacteria such as Rb. sphaeroides generally is incapable of forming LH2 [8]. Moreover, blue shifts of light-harvesting complexes will be observed in carotenoid-deficient strains of purple photosynthetic bacteria [25]. However, the mode of Crts binding to LH2 subunits is as yet largely unknown. It is postulated that the two phenylalanine residues, α-Phe12 and β-Phe8, are two key factors for the binding of Crts molecules to LH2 subunits [26].
Although, several independent explanations have been put forward to explain the mechanisms how the two important photopigments BChl and Crt bound by pigmentproteins and consequently influence the spectral properties of light harvesting complexes through mutagenesis and other effective techniques, the relationships between two pigments and the spectral properties of light-harvesting complexes are still a matter of debate. Furthermore, literatures on whether the LH2 spectral properties will be changed when heterologously express LH2 b-subunit fusion protein in Rb. sphaeroides have not been reported. In our previous study, we constructed a novel Rb. sphaeroides expression system which could be employed to evaluate heterologous protein expression levels in real-time using LH2 characteristic absorption at near infra-red regions [27-29]. In the present study, we lie emphasis on the spectral properties of LH2 when heterologously express LH2 with b-subunit fusion protein in Rb. sphaeroides and the interactions between photopigments and light-harvesting subunits. Our present study may supply a new insight into better understand the close interactions between light-harvesting subunits and photopigments and bacterial photosynthesis and promote the development of the novel Rb. sphaeroides expression system.
2. MATERIALS AND METHODS
2.1. Bacterial Strains Growth Conditions
Rb. sphaeroides CQU68 mutant strains (genomic deletions of pufBALMX, puc1BA and puc2BA, could be formed the photosynthetic apparatus including LH2, LH1 and RC) were grown at 34˚C in M22+ medium supplemented with 0.1% casamino acids for growth in liquid culture [29,30]. Antibiotics were added to the growth media at the following concentrations: 1 μg/ml tetracycline, 20 μg/ml neomycin, 5 μg/ml streptomycin, and 30 μg/ml gentamycin.
2.2. Expression and Purification of LH2 and LH2 with β-Subunit-GFP Fusion Protein Complexes
Plasmid DNA constructed to express LH2 subunits and LH2 with β-subunit-GFP fusion protein complexes were constructed as revealed in Figure 1 and transferred into Rb. sphaeroides CQU68 as described in our previous study [29]. Rb. sphaeroides CQU68 cell cultures expressing LH2 or LH2 with the β-subunit-GFP fusion protein complexes were shifted from aerobic conditions to semi-aerobic conditions at an OD600 of 0.5 - 1.0, and continuously incubated for about 8 hours after adding the isopropyl β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 1.0 mM [27]. LH2 and LH2 with the β-subunit-GFP fusion protein complexes were one-step
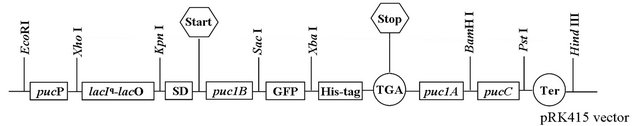
Figure 1. Construction of the LH2 and LH2 with β-subunit-GFP fusion protein expression vector containing pucP-lacIq-lacO hybrid promoter based on pRK415 vector. puc1B, puc1A and pucC encoded the LH2 β-subunit, α-subunit and PucC protein, respectively. LH2 β-subunit and GFP protein formed the fusion protein.
purified as described in our previous literatures, respectively [28,29]. Rb. sphaeroides cells were harvested and resuspended with resuspension buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl). DNase I and RNase A were added to the suspension to final concentrations of 50 μg/ml and 10 μg/ml, respectively. The cells were subsequently broken through high-pressure homogenizer. Cell debris were removed by centrifugation at 17.000 g and membranes were pelleted by ultra-centrifugation at 160.000 g. The membrane pellets were resuspended with resuspension buffer and further solubilized with 1% (v/v) lauryldimethylamine N-oxide along with phenyl methyl sulfonyl fluoride at 1 mM. LH2 and LH2 with β-subunit-GFP fusion protein complexes were purified from the solubilized solution by Ni-IDA resin. For Western blot analysis, proteins were separated on 15% - 20% SDS-PAGE gradient gel and transferred to PVDF membrane. Anti-His antibody was used as primary antibody. Immunoblots were visualized using D-AB and recorded on a GS-800 Calibrated Densitometer (Bio-Rad).
2.3. Spectroscopy
Near infra-red and fluorescence spectral of cell cultures were recorded on a PerkinElmer lambda 900 UV/VIS Spectrometer (USA) and PerkinElmer lambda 900 Fluorescence Spectrometer (USA), respectively. FT-IR spectra were recorded on a FT-IR Spectrometer (Nicolet, 5DX/550II, USA). A suitable blank of growth medium or buffer was used in each case.
2.4. Photopigments Quantification
Photopigments were extracted with acetone-methanol (7:2, v/v) from purified membranes. The BChl and Crts were calculated by using extinction coefficients of 76 mM−1∙cm−1 at 770 nm and 128 mM−1∙cm−1 at 484 nm, respectively [31].
3. RESULTS
3.1. Near Infra-Red Spectral Properties of LH2 and LH2 with β-Subunit-GFP Fusion Protein Complexes
The LH2 and LH2 with β-subunit-GFP fusion protein complexes were subsequently extracted from cell cultures after correct expression, respectively. The two spectral peaks of LH2 were observed at 846.59 nm and 799.92 nm (solid line), as revealed in Figure 2. On the other hand, the LH2 with β-subunit-GFP fusion protein complexes possessed its absorption at 845.53 nm and 799.89 nm under the same conditions (dot line). Apparently, the two complexes exhibited very similar spectral properties, just slight differences were observed at the second spectral peak position. Moreover, the LH2 exhibited higher spectral peaks compared to that of the LH2 with β-subunit-GFP fusion protein complexes under the same protein (LH2 β-subunit) concentrations. For further demonstration, Western blotting analysis was performed, as shown in Figure 3. The LH2 β-subunit (~8 KDa) and LH2 with β-subunit-GFP (~34 KDa) bands were expectedly observed in the related lanes, which demonstrated the expression of LH2 with β-subunit-GFP fusion protein.
3.2. Fluorescence Spectral Properties of LH2 and LH2 with β-Subunit-GFP Fusion Protein Complexes
The fluorescence spectrum suggested that energy transfer activity of LH2 appeared to be not significantly influenced by the heterologously expressed GFP protein, as shown in Figure 4, which showed the ability of the 800 nm-absorbing pigments to transfer energy to the 850 nm pigments, from which fluorescence is detected at 900 nm, indicating the LH2 with β-subunit-GFP fusion protein complexes was still functional and efficient in energy transfer. Obviously, energy transfer in LH2 (solid line) was somewhat more efficient than that in LH2 with β-subunit-GFP fusion protein complexes (dot line), indicating the introduction of GFP protein exerted effects on the energy transfer from 800 nm-absorbing pigments to the 850 nm pigments. However, the LH2 and LH2 with β-subunit-GFP fusion protein complexes possessed similar fluorescence spectrum.
3.3. Photopigments Contents in the LH2 and LH2 with β-Subunit-GFP Fusion Protein Complexes
As differences of the spectral peaks of LH2 and LH2
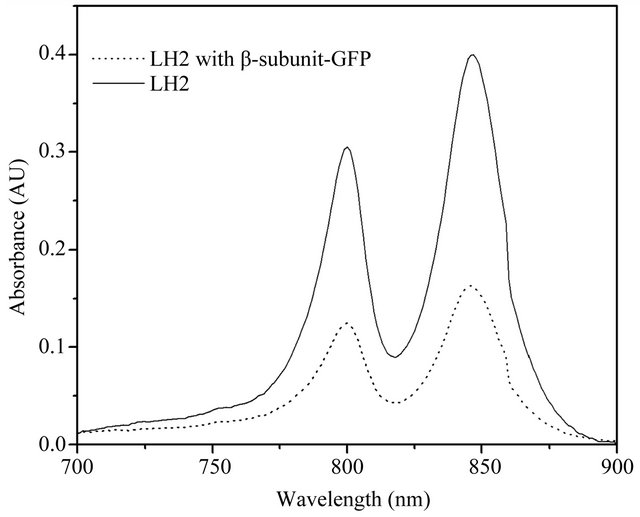
Figure 2. Near infra-red spectra of LH2 and LH2 with β-subunit-GFP fusion protein complexes. The spectral bands of LH2 were 846.59 nm and 799.92 nm (solid line). Whereas, the spectral bands of LH2 with β-subunit-GFP fusion protein complex were 845.53 nm and 799.89 (dot line).
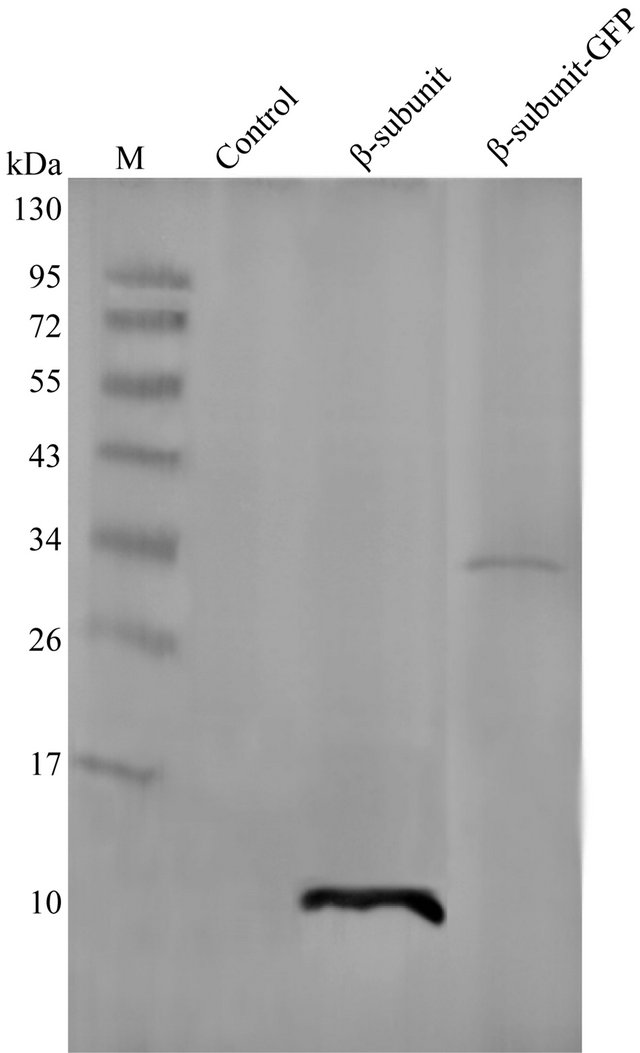
Figure 3. Identification of LH2 and LH2 with β-subunit-GFP fusion protein complex by Western blotting analysis. The LH2 β-subunit band and the LH2 with β- subunit-GFP were observed at ~8 KDa and ~34 KDa. Extraction from Rb. sphaeroides CQU68 mutant strain was used as control.
with β-subunit-GFP fusion protein complexes were occurred and the energy transfer from 800 nm-absorbing pigments to the 850 nm pigments was slightly influenced, BChl and Crt were consequently extracted from the puri-
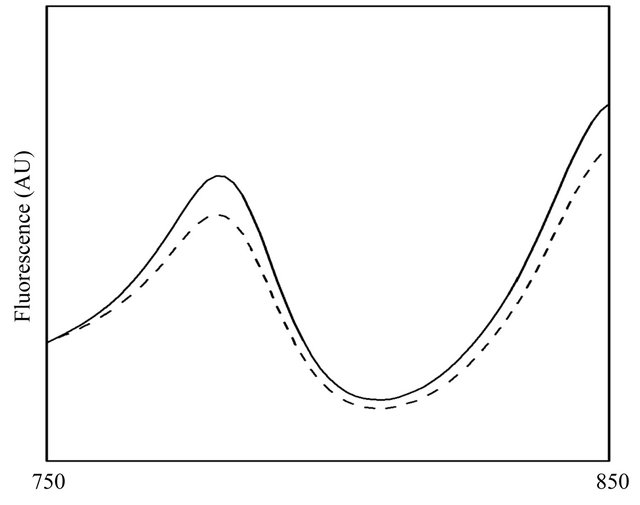
Figure 4. Fluorescence spectrum of LH2 (solid line) and LH2 with β-subunit-GFP fusion protein complex (dash line). Energy transfer in LH2 was slightly more efficient than that in LH2 with β-subunit-GFP fusion protein complexes.
fied LH2 and LH2 with β-subunit-GFP fusion protein complexes, as shown in Figure 5. Clearly, BChl and Crt extracted from LH2 was considerably more than that of BChl and Crt extracted from LH2 with β-subunit-GFP fusion protein complexes. BChl extracted from LH2 with the concentration of 0.0952 mM was great more than that of Crt with the concentration of 0.0353 mM extracted from LH2, at nearly a ratio of 3:1. However, different results were observed in their contents in LH2 with β- subunit-GFP fusion protein complexes. 0.0130 mM BChl and 0.0141 mM Crt were extracted from LH2 with β- subunit-GFP fusion protein complexes. It could be concluded based on the above experimental results that the introduction of the GFP protein caused much lower photopigments bound in LH2 α/β-subunits and which may thus slightly change the near infra-red spectrum and fluorescence spectrum.
3.4. FT-IR Spectral Analysis
It has been well studied that BChl molecules bound by LH2 play crucial roles in the determination of LH2 spectral properties. To further elucidate the differences of photopigments contents in LH2 and LH2 with β-subunitGFP fusion protein complexes, FT-IR spectroscopy was employed to analyze the LH2 and LH2 with β-subunitGFP fusion protein complexes, respectively, as can been seen in Figure 6. The FT-IR spectrum of LH2 with β-subunit-GFP fusion protein complexes (dash line) was similar to the spectrum of LH2 (dot line), bands where observed in LH2 spectra also appeared in LH2 with β-subunit-GFP fusion protein complexes. However, the most significant difference was that spectral peaks in LH2 were much higher than that in LH2 with β-subunitGFP fusion protein complexes. It has been suggested that
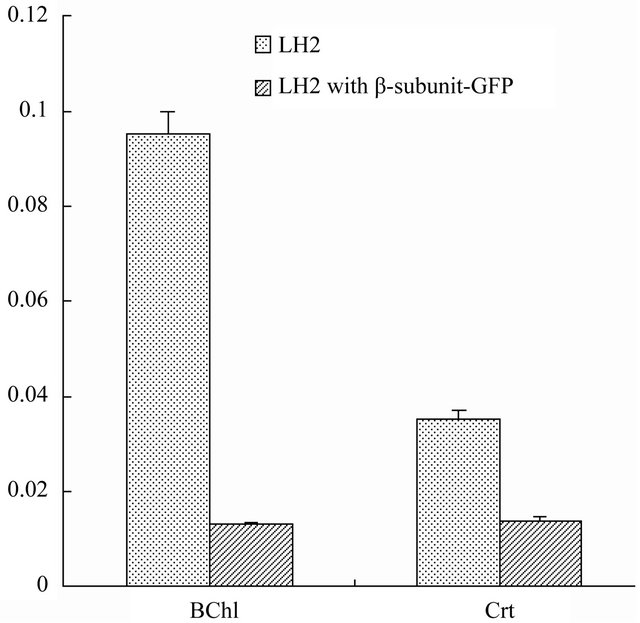
Figure 5. BChl and Crt contents analysis in purified LH2 and LH2 with β-subunit-GFP fusion protein complexes. The pigments content exhibited in LH2 complexes were much higher than that in LH2 with β-subunit-GFP fusion protein complexes.
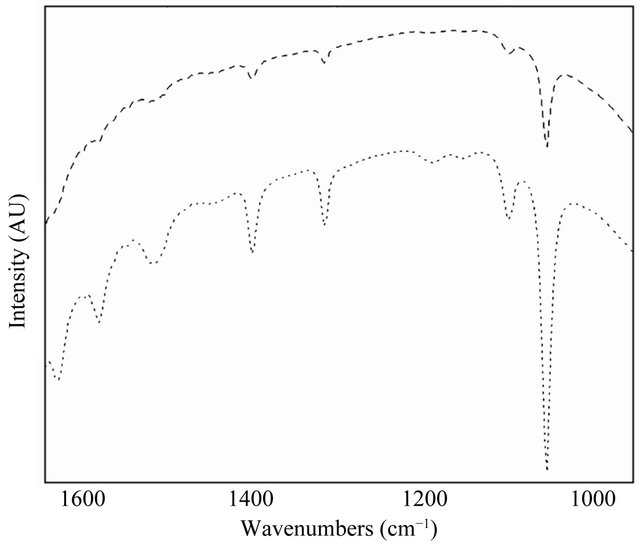
Figure 6. FT-IR spectrum of LH2 (dot line) and LH2 with β-subunit-GFP fusion protein complexes (dash line). The two spectrum displayed similar properties and the interactions between photopigments and LH2 subunits changed.
signals between 1620 and 1700 cm−1 arise from the stretching modes of either the 2-acetyl or 9-keto carbonyl substituents found in the dihydrophorbin macrocycles of the BChl pigments in LH2. The similarity in the FT-IR spectrum indicated that the introduction of the GFP protein exerted slight effects on the organization of LH2 and interactions between α/β-subunit and their bound pigments. The lower peaks revealed in LH2 with β-subunitGFP fusion protein complexes probably implied the interactions between BChl and α/β-subunit were changed.
The differences of photopigments contents in LH2 and LH2 with β-subunit-GFP fusion protein complexes could result from changed interactions between BChl and LH2 as we found in the present study.
4. DISCUSSION
In the present study, we reported that the Rb. sphaeroides LH2 spectral properties remained unchanged when heterologously expressed LH2 β-subunit-GFP fusion protein. Photopigments bound by LH2 were decreased to very low levels because of the introduction of foreign protein. Furthermore, interactions between BChl and LH2 α/β- subunits could not be significantly influenced by the GFP protein, as indicated by the FT-IR spectrum.
Functions of photopigments bound by LH2 have been well defined [4]. The type and number of BChl molecules non-covalently bound by LH2 α/β-subunits attribute greatly to the LH2 spectrum. In the purple photosynthetic bacterium Rb. sphaeroides, three BChl and one or two Crt(s) molecules are non-covalently bound by each α/β-subunit [9]. In this study, BChl and Crt were extracted from LH2 and LH2 with β-subunit-GFP fusion protein complexes and quantified, respectively (Figure 5). It is already well known that LH2 α/β-subunits are the predominant and primary acceptors of BChl and Crt, thus it is necessary and correct to measure the photopigments under the same LH2 β-subunit or LH2 α-subunit concentrations. On the other hand, GFP should not be considered as the acceptor of the photopigments. As can be seen in Figure 5, photopigments were considerably decreased to very low levels in LH2 with β-subunit-GFP fusion protein complexes compared to that extracted from LH2, which could be the main reasons caused low spectral peaks in LH2 with β-subunit-GFP fusion protein complexes. In addition, the introduction of GFP in LH2 appeared not exert effects on the organization of LH2, since the two separate complexes are still functional in energy transfer, as observed in Figure 4. It has been demonstrated that Crt is involved in the assembly of LH2 and play important roles in the protection of LH2 against photo-oxidative damage and dissipating excess radiant energy [24,32]. Consequently, the considerable decrease of Crt in LH2 with β-subunit-GFP fusion protein complexes may influence the stability of the complex and thus lead to the minor changes of the near infra-red spectral properties.
It is worth of being figured out that interactions between photopigments and LH2 α/β-subunits predominantly determines the spectral properties, which could be the primary approaches to elucidate changes of spectrum of light harvesting complexes. On the other hand, major advances have been made to better understand the factors that determine membrane protein folding and assembly in recent years [33]. Hydrogen bond exerts effects on the electronic structures of LH1 and also is applicable for LH2 and LH3, it may change the disorder of the environment of the BChl [34]. Hydrogen bonding interactions have been proposed to be closely related to the stability of membrane protein and intermolecular hydrogen bonding probably be involved in the assembly of transmembrane helices in detergent and biological membranes [35]. As we described in Figure 6, no significant differences has been observed in the FT-IR spectrum of LH2 and LH2 with β-subunit-GFP fusion protein complexes, suggesting the interactions between BChl and LH2 α/β- subunit could not be greatly influenced by the introduction of GFP protein. However, their interactions probably might be changed, as the spectral peaks are lowered in the spectrum of LH2 with β-subunit-GFP fusion protein complexes. However, experimental findings in our present study may help to better understand the interactions between photopigments and LH2 α/β-subunits and provide a novel aspect to elucidate bacterial photosynthesis.
5. ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No. 31100089), Scientific Research Foundation of the Education Department of Sichuan Province, China (No. 11ZB102) and the Talent Project of Sichuan University of Science & Engineering (No. 2011RC12).
REFERENCES
- Tucker, J.D., Siebert, C.A., Escalante, M., et al. (2010) Membrane invagination in Rhodobacter sphaeroides is initiated at curved regions of the cytoplasmic membrane, then forms both budded and fully detached spherical vesicles. Molecular Microbiology, 4, 833-847. doi:10.1111/j.1365-2958.2010.07153.x
- Pugh, R.J., McGlynn, P., Jones, M.R., et al. (1998) The LH1-RC core complex of Rhodobacter sphaeroides: Interaction between components, time-dependent assembly, and topology of the PufX protein. Biochimica et Biophysica Acta, 3, 301-316.
- Olsen, J.D., Tucker, J.D., Timney, J.A., et al. (2008) The organization of LH2 complexes in membranes from Rhodobacter sphaeroides. Journal of Biological Chemistry, 45, 30772-30779. doi:10.1074/jbc.M804824200
- Zeilstra-Ryalls, J., Gomelsky, M., Eraso, J.M., et al. (1998) Control of photosystem formation in Rhodobacter sphaeroides. Journal of Bacteriology, 11, 2801-2809.
- Boonstra, A.F., Visschers, R.W., Calkoen, F., et al. (1993) Structural characterization of the B800-850 and B875 light-harvesting antenna complexes from Rhodobacter sphaeroides by electron microscopy. Biochimica et Biophysica Acta, 50, 181-188.
- Hu, X., Damjanovic, A., Ritz, T., et al. (1998) Architecture and mechanism of the light-harvesting apparatus of purple bacteria. Proceedings of the National Academy of Sciences of the USA, 11, 5935-5941. doi:10.1073/pnas.95.11.5935
- Walz, T., Jamieson, S.J., Bowers, C.M., et al. (1998) Projection structures of three photosynthetic complexes from Rhodobacter sphaeroides: LH2 at 6 Å, LH1 and RC-LH1 at 25 A. Journal of Molecular Biology, 4, 833-845. doi:10.1006/jmbi.1998.2050
- Lang, H.P. and Hunter, C.N. (1994) The relationship between carotenoid biosynthesis and the assembly of the light-harvesting LH2 complex in Rhodobacter sphaeroides. Biochemical Journal, 298, 197-205.
- McDermott, G., Prince, S.M., Freer, A.A., et al. (1995) Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature, 374, 517-521. doi:10.1038/374517a0
- Braun, P., Gebhardt, R., Kwa, L., et al. (2005) High pressure near infrared study of the mutated light-harvesting complex LH2. Brazilian Journal of Medical and Biological Research, 8, 1273-1278.
- Law, C.J., Roszak, A.W., Southall, J., et al. (2004) The structure and function of bacterial light-harvesting complexes. Molecular Membrane Biology, 3, 183-191. doi:10.1080/09687680410001697224
- Urboniene, V., Vrublevskaja, O., Trinkunas, G., et al. (2007) Solvation effect of bacteriochlorophyll excitons in light-harvesting complex LH2. Biophysical Journal, 6, 2188-2198. doi:10.1529/biophysj.106.103093
- Pandit, A., Buda, F., van Gammeren, A.J., et al. (2010) Selective chemical shift assignment of bacteriochlorophyll a in uniformly [13C-15N]-labeled light-harvesting 1 complexes by solid-state NMR in ultrahigh magnetic field. Journal of Physical Chemistry B, 18, 6207-6215. doi:10.1021/jp100688u
- Fowler, G.J., Sockalingum, G.D., Robert, B., et al. (1994) Blue shifts in bacteriochlorophyll absorbance correlate with changed hydrogen bonding patterns in light-harvesting 2 mutants of Rhodobacter sphaeroides with alterations at alpha-Tyr-44 and alpha-Tyr-45. Biophysical Journal, 299, 695-700.
- Gall, A., Fowler, G.J., Hunter, C.N., et al. (1997) Influence of the protein binding site on the absorption properties of the monomeric bacteriochlorophyll in Rhodobacter sphaeroides LH2 complex. Biochemistry, 51, 16282- 16287. doi:10.1021/bi9717237
- Braun, P., Vegh, A.P., von Jan, M., et al. (2003) Identification of intramembrane hydrogen bonding between 13(1) keto group of bacteriochlorophyll and serine residue alpha27 in the LH2 light-harvesting complex. Biochim Biophys Acta, 1, 19-26.
- Garcia-Martin, A., Kwa, L.G., Strohmann, B., et al. (2006) Structural role of (bacterio)chlorophyll ligated in the energetically unfavorable beta-position. Journal of Biological Chemistry, 15, 10626-10634. doi:10.1074/jbc.M510731200
- Kwa, L.G., Garcia-Martin, A., Vegh, A.P., et al. (2004) Hydrogen bonding in a model bacteriochlorophyll-binding site drives assembly of light harvesting complex. Journal of Biological Chemistry, 15, 15067-15075. doi:10.1074/jbc.M312429200
- Olsen, J.D., Sockalingum, G.D., Robert, B., et al. (1994) Modification of a hydrogen bond to a bacteriochlorophyll a molecule in the light-harvesting 1 antenna of Rhodobacter sphaeroides. Proceedings of the National Academy of Sciences of the USA, 15, 7124-7128. doi:10.1073/pnas.91.15.7124
- Kimura, Y., Hirano, Y., Yu, L.J., et al. (2008) Calcium ions are involved in the unusual red shift of the lightharvesting 1 Qy transition of the core complex in thermophilic purple sulfur bacterium Thermochromatium tepidum. Journal of Biological Chemistry, 20, 13867- 13873. doi:10.1074/jbc.M800256200
- Allen, J.P., Artz, K., Lin, X., et al. (1996) Effects of hydrogen bonding to a bacteriochlorophyll-bacteriopheophytin dimer in reaction centers from Rhodobacter sphaeroides. Biochemistry, 21, 6612-6619. doi:10.1021/bi9528311
- Gall, A., Cogdell, R.J. and Robert, B. (2003) Influence of carotenoid molecules on the structure of the bacteriochlorophyll binding site in peripheral light-harvesting proteins from Rhodobacter sphaeroides. Biochemistry, 23, 7252-7258. doi:10.1021/bi0268293
- Sundstrom, V. and Pullerits, T. (1999) Photosynthetic light-harvesting: Reconciling dynamics and structure of purple bacterial LH2 reveals function of photosynthetic unit. The Journal of Physical Chemistry, 13, 2327-2346. doi:10.1021/jp983722+
- Wormit, M., Harbach, P.H., Mewes, J.M., et al. (2009) Excitation energy transfer and carotenoid radical cation formation in light harvesting complexes—A theoretical perspective. Biochimica et Biophysica Acta, 6, 738-746.
- Moskalenko, A.A., Makhneva, Z.K., Fiedor, L., et al. (2005) Effects of carotenoid inhibition on the photosynthetic RC-LH1 complex in purple sulphur bacterium Thiorhodospira sibirica. Photosynthesis Research, 1-2, 71- 80. doi:10.1007/s11120-005-4473-9
- Garcia-Martin, A., Pazur, A., Wilhelm, B., et al. (2008) The role of aromatic phenylalanine residues in binding carotenoid to light-harvesting model and wild-type complexes. Journal of Molecular Biology, 1, 154-166. doi:10.1016/j.jmb.2008.07.002
- Hu, Z., Zhao, Z., Pan, Y., et al. (2010) A powerful hybrid puc operon promoter tightly regulated by both IPTG and low oxygen level. Biochemistry, 4, 519-512.
- Zhao, Z., Hu, Z., Liang, Y., et al. (2010) One-step purification of functional light-harvesting 2 complex from Rhodobacter sphaeroides. Protein & Peptide Letters, 4, 444-448. doi:10.2174/092986610790963663
- Zhao, Z., Hu, Z., Nie, X., et al. (2011) A novel Rhodobacter sphaeroides expression system for real-time evaluation of heterologous protein expression levels. Protein & Peptide Letters, 6, 568-572. doi:10.2174/092986611795222722
- Hunter, C.N. and Turner, G. (1988) Transfer of genes coding for apoproteins of reaction center and light-harvesting LH1 complexes to Rhodobacter sphearoides. Journal of General Microbiology, 6, 1471-1480.
- Clayton, R.K. and Clayton, B.J. (1981) B850 pigmentprotein complex of Rhodopseudomonas sphaeroides: Extinction coefficients, circular dichroism, and the reverseble binding of bacteriochlorophyll. Proceedings of the National Academy of Sciences of the USA, 9, 5583-5587. doi:10.1073/pnas.78.9.5583
- Bailey, S. and Grossman, A. (2008) Photoprotection in cyanobacteria: Regulation of light harvesting. Photochemistry and Photobiology, 6, 1410-1420. doi:10.1111/j.1751-1097.2008.00453.x
- DeGrado, W.F., Gratkowski, H. and Lear, J.D. (2003) How do helix-helix interactions help determine the folds of membrane proteins? Perspectives from the study of homo-oligomeric helical bundles. Protein Science, 4, 647- 665. doi:10.1110/ps.0236503
- Uyeda, G., Williams, J.C., Roman, M., et al. (2010) The influence of hydrogen bonds on the electronic structure of light-harvesting complexes from photosynthetic bacteria. Biochemistry, 6, 1146-1159. doi:10.1021/bi901247h
- Gratkowski, H., Lear, J.D. and DeGrado, W.F. (2001) Polar side chains drive the association of model transmembrane peptides. Proceedings of the National Academy of Sciences of the USA, 3, 880-885. doi:10.1073/pnas.98.3.880
NOTES
*Corresponding author.

