 Vol.2, No.2, 112-123 (2010) doi:10.4236/health.2010.22018 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ Health Comparative effects of idazoxan, efaroxan, and BU 224 on insulin secretion in the rabbit: Not only interaction with pancreatic imidazoline I2 binding sites María José García-Barrado1, María Francisca Pastor1, María Carmen Iglesias-Osma1, Christian Carpéné2, and Julio Moratinos1 1Departamento de Fisiología y Farmacología, Facultad de Medicina, Universidad de Salamanca, Salamanca, Spain; barrado@usal.es 2INSERM, U586 (Institut National de la Santé et de la Recherche Médicale), Unité de Recherches sur les Obésités, Université Paul Sabatier, Institut Louis Bugnard IFR31, Toulouse, France Received 6 November 2009; revised 1 December 2009; accepted 22 December 2009. ABSTRACT The nature of the binding site(s) involved in the insulin secretory activity of imidazoline compo- unds remains unclear. An imidazoline I2 binding site (I2BS) has been neglected since the classic I2 ligand, idazoxan, does not release insulin. Using the rabbit as an appropriate model for the study of this type of binding sites, we have tried to re-evaluate the effects of idazoxan, the selective I2 compound BU 224, and efaroxan on insulin secretion. Mimicking efaroxan, idazoxan and BU 224 potentiated insulin release from perifused islets in the presence of 8 mM glucose. In static incubation, insulin secretion induced by idazoxan and BU 224 exhibited both dose and glucose dependencies. ATP-sensitive K+ (KATP) channel blockade, though at a different site from the SUR1 receptor, with subsequent Ca2+ entry, mediates the insulin releasing effect of the three ligands. However, additional MAO independent intracellular steps in stimulus- secretion coupling linked to PKA and PKC activation are only involved in the effect of BU 224. Therefore, both an I2 related binding site at the channel level shared by the three ligands and a putative I3-intracellularly located binding site stimulated by BU 224 would be mediating insulin release by these compounds. In vivo experiments reassess the abilities of idazoxan and BU 224 to enhance glucose-induced insulin secretion and to elicit a modest blood glucose lowering response. Keywords: BU 224; Efaroxan; Idazoxan; Imidazoline Ligands; Insulin Secretion; IVGTT (Intravenous Glucose Tolerance Test); KATP Channel; PK Activity; Rabbit Pancreatic Islets 1. INTRODUCTION A number of imidazoline containing compounds have been previously shown to induce insulin release from the perifused pancreas or isolated islets [1, 2] and to improve glucose tolerance in rats [3-5] and mice [6]. In accordance with the mechanisms of their insulino- tropic effect, two groups of imidazoline compounds can be considered: classical imidazolines, i.e., imidazoline derivatives possessing both ATP-sensitive K+(KATP) channel activity and a direct effect on exocytosis, like RX871024 [7], and a new generation of compounds without effect on KATP channels though possessing a pure glucose-dependent insulinotropic effect like BL11282 [8]. The KATP channel consists of two subunits: a sulphony- lurea receptor (SUR1) and a Kir6.2 subunit. Classical imidazoline drugs bind to the transmembrane protein Kir6.2 [9] considered to be the pore-forming subunit of the channel whereas sulphonylureas bind to the SUR1 receptor. It is also established that the binding site for imidazolines and the sulphonylurea receptor are not identical since the first drugs do not displace binding from the SUR sites [10]. Additional sites located at more distal stages of the stimulus-secretion coupling pathway, mediating activation of protein kinase A (PKA) and pro- tein kinase C (PKC) have also been reported [8,11,12]. However, when trying to analyze the nature of the binding sites or receptors involved in their insulin secre- tory response a number of difficulties have emerged. An I2 imidazoline binding site (I2-site) has been discarded since the classic I2 ligand idazoxan exhibited a mild concentration independent increase in insulin release [13], failed to evoke any effect [14] or even blocked the re- sponse induced by efaroxan (an 2-adrenoceptor antago- nist) with an imidazoline structure [15]. Similarly, the monoamine oxidase A (MAO-A) inhibitor clorgyline did  M. J. García-Barrado et al. / HEALTH 2 (2010) 112-123 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ 113 not modify the insulin secretory response induced by the imidazoline compound RX871024 [16]. Radioligand bind- ing studies performed in membranes from RINm5F and MIN6 cells, in the presence of [3H]-RX821002 (meth- oxy-idazoxan an imidazoline 2-adrenoceptor antagonist) showed a low affinity non-adrenergic binding site which could be displaced by efaroxan but not by idazoxan [18,19]. Therefore, evidence for a novel putative I3 binding site involved in the insulin secretory response is being accepted. Efaroxan is tentatively considered an I3 ligand and 2-(2-ethyl-2,3-dihydro-benzofuran-2-yl)–imi- dazole (KU 14R), a close structural efaroxan analogue able to block its effect, an I3 antagonist [18,20]. However, some recent data have yielded intriguing results: even high doses of efaroxan did not increase circulating insulin in mouse [21] and the selective I2 ligand: 2-(2-benzofuranyl)-2-imidazoline (2-BFI) releases insulin from isolated rat islets [10]. Considering the het- erogeneity of imidazoline binding sites [22] and that the putative I3 binding site encompasses a nebulous group of loci, we have tried to re-evaluate the effect of imidazoline ligands on insulin release in rabbits. The rabbit was chosen as a suitable model in view of the paucity of this type of data for an animal species otherwise very rich in I2-binding sites [17,23,24]. 2-(4,5-dihydroimidazol-2-yl)- quinoline (BU 224), considered a selective I2 ligand [25-27], idazoxan (a typical 2-adrenoceptor antagonist and I2 ligand), methoxy-idazoxan (an 2-adrenoceptor antago- nist) and efaroxan (I3 putative ligand) were employed in both in vitro and in vivo experiments to delineate insulin secretion and glycaemic control. 2. MATERIALS AND METHODS 2.1. Chemicals and Solutions Forskolin, diazoxide, tolbutamide, methoxy-idazoxan, nimodipine, yohimbine, 3-isobutyl-1-methylxanthine (IB MX), chelerythrine and pargyline were provided by Sigma- Aldrich (Spain); idazoxan was obtained from Rekilt- Colman Pharmaceutical Company (Germany); brimonidine (UK 14,304) came from Pfizer (UK); 2-(4,5-dihydroi- midazol-2-yl)-quinoline (BU 224 hydrochloride), efaroxan hydrochloride, and 2-(2-ethyl-2,3-dihydro-benzofuran-2-yl) -imidazole (KU 14R) were obtained from Tocris (Bristol, UK), calphostin and Rp-Adenosine-3′,5′-cyclic mono- phosphothioate triethylamine (Rp-cAMPS) were from Bionova (Spain). Forskolin and chelerythrine were pre- pared in DMSO, and final concentrations of DMSO were 0.1% or less in each case. 2.2. Animals The experiments were performed using male New Zea- land white rabbits aged 7-12 months (body weight be- tween 2.5-3.5 kg). The animals were maintained in a 12 h light-dark cycle and were provided with free access to food and water. The study was conducted in accordance with the European Communities Council Directives for experimental animal care. 2.3. In Vitro Experiments 2.3.1. Islet Isolation and Incubation The rabbits were sacrificed after the induction of general anaesthesia with 30 mg kg-1 i.v. of sodium pentobarbital (Abbott, Spain). The pancreas was removed and disten- ded with bicarbonate-buffered physiological salt solution. The islets were isolated by collagenase (Inmunogenetic, Spain) and hand-picked using a glass loop pipette under a stereo microscope. They were free of visible exocrine contamination. The medium used for islet isolation was a bicarbonate-buffered solution containing 120 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 5 mM HEPES and 24 mM NaHCO3. It was gassed with O2-CO2 (94: 6) to maintain a pH of 7.4 and was supplemented with 1 mg ml-1 BSA and 10 mM glucose. When the concentra- tion of KCl was increased to 30 mM, that of NaCl was decreased accordingly. The concentration of glucose was adjusted and test substances were added as required. 2.3.2. Measurements of Insulin Secretion After isolation, in the first type of experiments, the islets were pre-incubated for 60 min in a medium containing 15 mM glucose before being distributed into batches of three. Each batch of islets was then incubated for 60 min in 1 ml at 37º C of medium containing 8 mM glucose and test substances, Pargyline was added to the preincubation medium 40 min before incubation. A portion of the medi- um was withdrawn at the end of the incubation and its insulin content was measured by a double antibody-RIA (insulin CT, Schering, Spain). In the other type of experiment, the isolated islets were divided in equal batches of 45-50 and placed in a parallel perifusion chamber at 37º C and perifused for 30 min before the start of the experiment at a flow rate of 1.1 ml min-1. After a 30 min stabilisation period they were perifused with 8 mM glucose and the appropriate com- pounds as indicated in the figure legends. Effluent frac- tions collected at 2 min intervals were chilled until their insulin content was measured by RIA. 2.2.3. In Vivo Experiments The experimental design carried out on conscious 24 h fasted animals has been fully described in other publica- tions [28, 29]. Arterial blood was sampled by means of an indwelling cannula placed in the central artery of one ear. Two control samples, separated by an interval of 30 min, were taken before drug infusion started. Drug solutions were infused at a constant rate (0.15 ml min-1) for 30 min through an indwelling cannula, which was kept functional by a slow constant infusion of physiological saline (0.07 ml min-1). Plasma glucose was estimated by means of the glucose oxidase procedure using a kit from Atom (Madrid, Spain). Insulin was determined by using a radioimmu- noassay kit (Schering, Spain), with human insulin as standard.  M. J. García-Barrado et al. / HEALTH 2 (2010) 112-123 SciRes Copyright © 2010 http://www.scirp.org/journal/HEALTH/ 114 3.2. Effects of Imidazoline Ligands and 2-Adrenoceptor Antagonists on Insulin Release from Isolated Islets. Glucose Dependency 2.2.4. Statistics Statistical significance was determined using the Stu- dent’s t test for unpaired data or analysis of variance in conjunction with the Newman-Keuls test for unpaired data. A P value ≤0.05 was taken as significant. Values presented in the Figures and Results represent means ± s.e.m. of at least 6 observations. When islets were incubated in 8 mM glucose, meth- oxy-idazoxan in the range 10 µM to 1 mM was unable to evoke insulin release. However, idazoxan at the same concentration range induced a clear dose dependent in- crease in insulin secretion (Figure 2A). Similar results were found when islets were incubated in the presence of efaroxan and BU 224 (1-100 µM, Figure 2B). As a marked significant increase in insulin release was observed 3. RESULTS 3.1. Effects of Imidazoline Ligands on Insulin Release in Perifused Islets The time course of the effects of imidazoline ligands on at 100 µM of either ligand, this particular imidazoline drug concentration was used for further studies. insulin release was studied in perifused islets. Idazoxan, BU 224 and efaroxan, each at the equivalent dose of 100 µM, potentiated the insulin secretory response induced by 8 mM glucose (Figure 1). Interestingly, the inhibitory effect on glucose induced insulin release mediated by 1 µM of the selective 2-adre- noceptor agonist brimonidine (BRM, 55% reduction) was Time (min) Gluc8 mMGluc8 mM 30 36 4248 5460 66 7278 8490 0 100 200 300 400 500 Insulin Secretion (pIU per islet min -1 ) A 0 100 200 300 400 32 3640 44 4852 5660 64 68 72 768030 B Time (min) Gluc8 mM -- BU 224 100µM Gluc8 mM InsulinSecretion (pIU perisletmin -1 ) -▲- IDZ 100µM --EFX 100µM Gluc8 mMGluc8 mM Figure 1. Effects of imidazolines on insulin release from rabbit perifused islets. Groups of 40 islets were perifused throughout the experiment with a medium containing 8 mM glucose (-X-). Test substances were introduced between 40 and 70min: (A) 100 µM idazoxan (-▲-) or 100 µM efaroxan (--); (B) 100 µM BU224 (--). Values are mean±s.e.m. for four to six experiments. Openly accessible at  M. J. García-Barrado et al. / HEALTH 2 (2010) 112-123 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ 115 B ** *** 0 100 200 300 400 500 G8mM10µM100µM1 mM InsulinRelease(% basal) IDZ Methoxyidz A ** *** * 0 100 200 300 400 InsulinRelease(% basal) EFX BU 224 G8mM 1µM10µM100µM Figure 2. Dose-dependent effects of idazoxan (IDZ), methoxy-idazoxan (Metho- xyidz) in (A); efaroxan (EFX) and BU 224 in (B) on insulin release from rabbit islets incubated in the presence of 8 mM glucose in static condition. Each value represents the mean±s.e.m. from at least 10 observations. *P<0.05, **P<0.01, and ***P<0.001 indicate statistically significant percentage increase relative to 8 mM glucose. Basal insulin release at this particular nutrient concen tration was: 9.65±1.1 µIU ml-1 islet-1 h-1. completely reversed by 1 µM idazoxan, thus demonstrat- ing the dual nature (I2 ligand and 2-adrenoceptor antago- nist) of this compound. However, the 2-adrenoceptor agonist clearly blunted the response to BU 224 (Figure 3). As expected, neither yohimbine nor methoxy-idazoxan affected the response to glucose, though the latter an- tagonist blocked the inhibitory response to brimonidine. It is known that efaroxan is able to potentiate glucose induced insulin release over the range of 4-10 mM glu- cose [30]. Therefore, the effects of idazoxan and BU 224 (100 µM) were also investigated at different glucose concentrations. Both ligands, mimicking efaroxan, en- hanced insulin secretion from 3-15 mM glucose (Figure 4). In the absence of nutrient these imidazolines failed to release insulin and they did not modify the maximal secretory response to 30 mM glucose. 3.3. Interaction with KATP Channels The effects of the three ligands on glucose induced insulin secretion were tested in the presence of 250 µM diazoxide. As expected, the KATP channel opener inhibited the re- sponse to glucose (a 42.5% reduction) and completely suppressed the effect of idazoxan. However, both efar- oxan and BU 224 significantly reversed the inhibitory effect mediated by the channel agonist (Figure 5A). Since imidazoline compounds and sulphonylureas block the KATP channel, though interacting with different and specific binding sites, the effect of the simultaneous addition of BU 224 plus tolbutamide on glucose mediated insulin secretion was also studied. When added alone BU 224 or 200 µM of the sulphonylurea compound both drug induced significant increases in insulin release (80% and  M. J. García-Barrado et al. / HEALTH 2 (2010) 112-123 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ 116 G 8mMMethoxyidzYOHBRMIDZIDZ+BRM 0 50 100 150 200 Insulin Release (% basal) A * * ++ 0 50 100 150 200 250 300 350 Insulin Release (% basal) G8mM BRM BU 224 BU+BRM B * *** Figure 3. Inhibition of glucose induced insulin release by the α2-adrenoceptor agonist brimonidine (BRM, 1 µM) when added alone (■) or in the presence of 100 µM of either idazoxan (, A) or BU 224 (, B). The effects of both imidazoline ligands (100 µM), the α2-adrenoceptor antagonist yohimbine (YOH, 5 µM ), and methoxy-idazoxan (Methoxyidz, 5 µM) by themselves are also shown. *P<0.05 and ***P<0.001 represent significant percentage decrease or increase relative to 8 mM glucose. ++P<0.01 when comparing to BRM alone. Basal insulin release from rabbit islets in static incubation was 12.32±1.4 µIU ml-1 islet-1 h-1. Data are from at least 12 experiments. and 51%, respectively). When added together, the insulin secretory response was clearly enhanced (=190%), thus the effect found with this drug association was signifi- cantly higher than the response induced by either drug alone (data not shown). Interestingly in fresh isolated rabbit islets, idazoxan did not block the response to efaroxan. However neither synergism nor antagonism was found when two imida- zoline ligands (either efaroxan-idazoxan, efaroxan-BU 224) were added together (Figure 5B). 3.4. Role of Ca2+ on Insulin Release Induced by Imidazoline Ligands The calcium channel blocker nimodipine (5 µM) at- tenuated the insulin secretory response to 8 mM glucose (by 36.5%, P<0.05) and significantly reduced the Insulin Release (µIU/islet.ml -1 .h -1 ) Glucose +IDZ +BU 224 0 5 10 15 20 25 30 35 G 0mMG 3mMG 8mMG 15mMG 30mM *** *** ** ** Figure 4. The effect of idazoxan or BU 224 (100 µM, ◆ each) on insulin release in isolated rabbit pancreatic islets in the presence of different glucose (G) concentrations. Results are mean±s.e.m. from at least 14 experiments. **P<0.01 and ***P<0.001, values significantly different relative to their corresponding glucose concentration. stimulatory effect of BU 224. Interestingly, nimodipine abolished the response to idazoxan (inhibitory degree: 67.6%, Figure 6A). The effects of both ligands were also explored in a low calcium medium (1 mM Ca2+), but in the presence of a higher glucose concentration (15 mM). Ca2+ re- duction significantly attenuated the responses to glucose and BU 224 (from 11.90±2.10 to 5.35±0.35 µIU ml-1 and from 23.25±4.30 to 12.45±0.65 µIU ml-1, respectively, P<0.01), though, interestingly, the per-centage increase in insulin secretion mediated by the ligand was of similar magnitude in both media (Figure 6A). Idazoxan elicited a tiny effect. 3.5. Role of the KATP-Independent Pathway on Insulin Release Mediated by Imidazolines The experimental approach followed was as has been described [31]. The response to 8 mM glucose was al- ready enhanced when islets were incubated in a medium containing 30 mM potassium and 250 µM diazoxide (64.7% increase). BU 224, but neither idazoxan or efar- oxan, still induced a significant insulin secretory effect (=80%, P<0.05, Figure 6B). 3.6. Intracellular Sites Involved in the Insulin Secretory Activity of Imidazolines Since true I2 ligands are reported to be linked to MAO enzymes, it was necessary to test the effect of a non- selective MAO inhibitor on insulin release in the presence of the three ligands. Pargyline (10 µM) did not modify the  M. J. García-Barrado et al. / HEALTH 2 (2010) 112-123 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ 117 response to either glucose or any of the ligands under investigation (Figure 9B). When either BU 224 or efaroxan was assayed in the presence of forskolin (10 µM), drug interaction led to an enhanced insulin secretory response (percentage in- creases with BU 224, forskolin and both together were: 135.5, 149.7 and 438.2, respectively; similarly, in the case of efaroxan, the results were: 100.9, 149.7, and 407.9). In the same way, the stimulatory response to BU 224 was potentiated by 100 µM of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX, 312%). Again, the increase derived from drug combination (association with either forskolin or IBMX) was significantly higher than the effect found with any drug alone (Figure 7, Δ to IBMX alone=177.3±24.5 %). Neither glucose nor efaroxan and idazoxan induced insulin release were affected by the presence of Rp- 0 50 100 150 200 250 300 G8mMIDZBU 224EFXEFX+IDZEFX+BU Insulin Release (% Basal) B * ** * 0 50 100 150 200 250 300 350 G8mMDzIDZ+DzBU+Dz EFX+Dz Insulin Release (% Basal) A * +++ +++ Figure 5. (A): Reversal of diazoxide induced inhibition of secretion by imidazoline ligands. 250 µM of the channel opener were added to islets incubated in 8 mM glucose in the absence (■) or presence () of 100µM of the three different ligands: idazoxan, BU 224 and efaroxan. *P<0.05 statistically signifi- cant percentage reduction relative to glucose; +++P<0.001 when comparing to diazoxide. Basal insulin release was 6.7±0.8 µIU ml-1 islet-1 h-1. Each value represents the mean±s.e.m. from at least 12 experiments. (B): Insulin release from isolated islets in the presence of the different imidazoline ligands added either separately (■) or together () as shown. 100 µM of each ligand was always applied. Basal insulin release in the presence of 8 mM glucose was: 7.45±0.55 µIU ml-1 islet-1 h-1. Data represent the mean from at least 17 observations. Ca +2 1mM 0 50 100 150 200 250 300 0 50 100 150 200 250 300 Insulin Release (% Basal) G 8mMNimoBU 224 BU+NimoIDZIDZ+NimoG 15mMBU 224IDZ Insulin Release (% Basal) * * * + + * A B 0 50 100 150 200 250 300 G 8mMBU 224IDZEFX Insulin Release (% basal) With K + 30mM+Dz250µM * + *** ** ** Figure 6. (A): Effects of BU 224 and idazoxan on insulin release in the absence (■) and presence () of 5 µM nimodipine *P<0.05 represents statistically significant percentage inhibition relative to glucose; +P<0.05, percentage when comparing to either ligand. Basal insulin release was: 7.6±0.6 µIU ml-1 islet-1 h-1. On the right: rabbit islets were incubated in a low calcium medium (1 mM) but with a higher glucose concentration (15 mM). In these experimental conditions insulin release was 5.35±0.3 µIU ml-1 islet-1 h-1. The effects of BU 224 and IDZ are shown. (B): The insulin secretory responses induced by BU 224, idazoxan and efaroxan in the presence of 30 mM KCl and 250 µM diazoxide (■). The effect found in normal medium (□) is also presented for comparison. *P<0.05, significant percentage increase relative to normal medium; +P<0.05, percentage in- crease when compare to insulin release from the medium en- riched whit KCl and diazoxide. Basal insulin secretion in normal medium: 9.1±0.9 µIU ml-1 islet-1 h -1. Data from these different experimental designs come from at least 14 observations. Adenosine-3′,5′-cyclic monophosphothioate triethylamine (Rp-cAMPS, 200 µM). This PKA inhibitor did attenuate the effect of BU 224, though a residual significant in- crease of 34% above basal levels was still observed with this ligand (Figure 8A). The PKC inhibitor chelerytrine 1µM [32] did not alter glucose induced insulin release though significantly blocked the response to BU 224 Figure 8B). However insulin secretion in the presence of idazoxan or efaroxan was not modified by chelerytrine. Similarly, calphostin (1 µM), another Protein-Kinase C inhibitor, completely suppressed the effect of (BU 224 (from 21.32.9 to 11.91.7 µIU ml-1 islet–1 h -1, insulin secretion in the presence of 8 mM glucose being= 11.61.4 µIU ml-1 islet –1 h-1), the response to efaroxan (22.24.7 and 22.04.4 µIU ml-1 islet–1 h -1 remaining unaltered in the absence and presence of the inhibitor. Data are from at least 12 experiments). 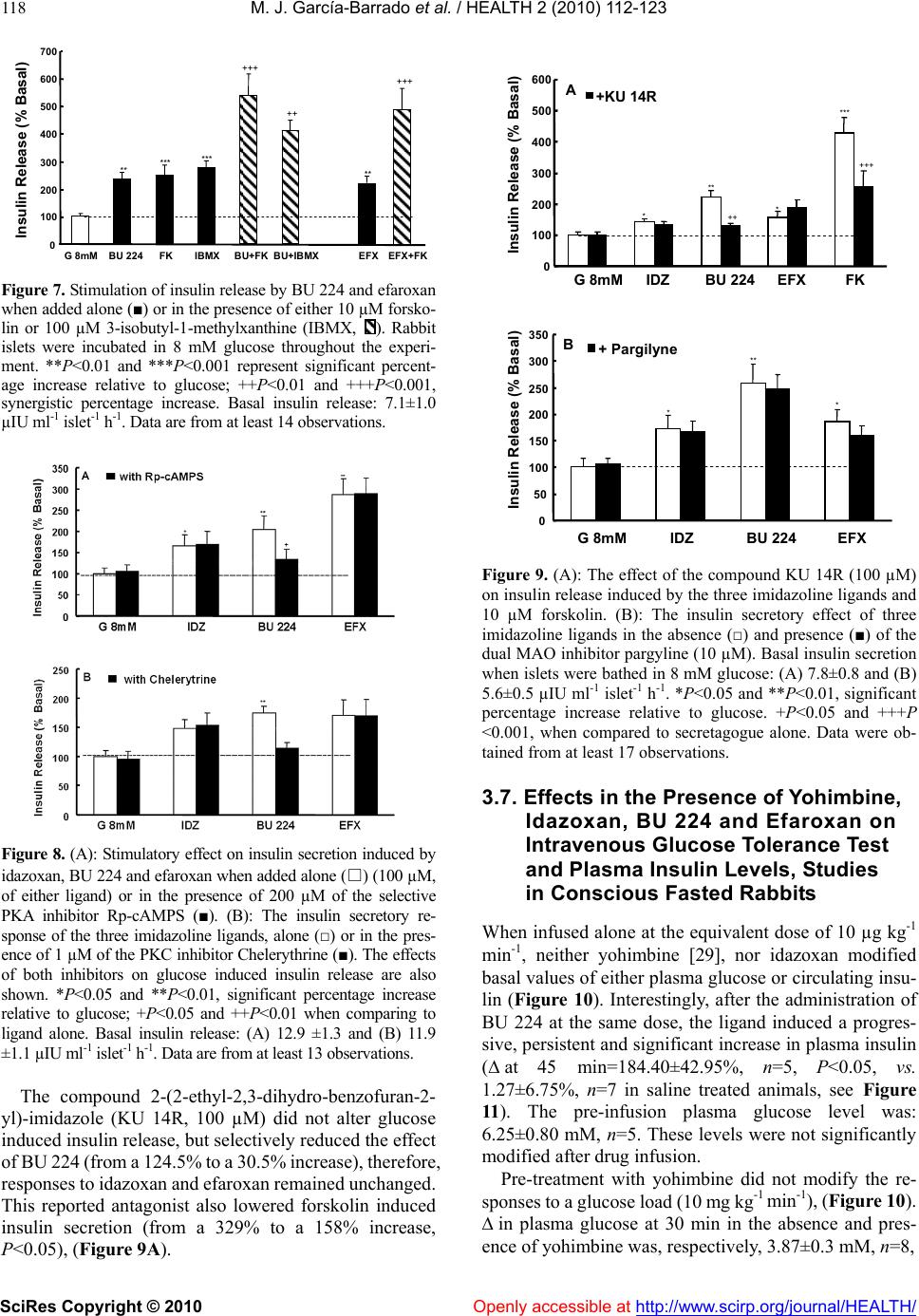 M. J. García-Barrado et al. / HEALTH 2 (2010) 112-123 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ 118 0 100 200 300 400 500 600 700 G 8mMBU 224FKIBMXBU+FKBU+IBMXEFXEFX+F Insulin Release (% Basal) *** *** +++ ++ +++ ** ** Figure 7. Stimulation of insulin release by BU 224 and efaroxan when added alone (■) or in the presence of either 10 µM forsko- lin or 100 µM 3-isobutyl-1-methylxanthine (IBMX, ). Rabbit islets were incubated in 8 mM glucose throughout the experi- ment. **P<0.01 and ***P<0.001 represent significant percent- age increase relative to glucose; ++P<0.01 and +++P<0.001, synergistic percentage increase. Basal insulin release: 7.1±1.0 µIU ml-1 islet-1 h-1. Data are from at least 14 observations. Figure 8. (A): Stimulatory effect on insulin secretion induced by idazoxan, BU 224 and efaroxan when added alone (□) (100 µM, of either ligand) or in the presence of 200 µM of the selective PKA inhibitor Rp-cAMPS (■). (B): The insulin secretory re- sponse of the three imidazoline ligands, alone (□) or in the pres- ence of 1 µM of the PKC inhibitor Chelerythrine (■). The effects of both inhibitors on glucose induced insulin release are also shown. *P<0.05 and **P<0.01, significant percentage increase relative to glucose; +P<0.05 and ++P<0.01 when comparing to ligand alone. Basal insulin release: (A) 12.9 ±1.3 and (B) 11.9 ±1.1 µIU ml-1 islet-1 h-1. Data are from at least 13 observations. The compound 2-(2-ethyl-2,3-dihydro-benzofuran-2- yl)-imidazole (KU 14R, 100 µM) did not alter glucose induced insulin release, but selectively reduced the effect of BU 224 (from a 124.5% to a 30.5% increase), therefore, responses to idazoxan and efaroxan remained unchanged. This reported antagonist also lowered forskolin induced insulin secretion (from a 329% to a 158% increase, P<0.05), (Figure 9A). Insulin Release (% Basal) G 8mMIDZBU 224EFXFK 0 50 100 150 200 250 300 350 G 8mMIDZBU 224EFX Insulin Release (% Basal) + Pargilyne +KU 14R A B +++ ++ + * ** * *** * ** * 0 100 200 300 400 500 600 Figure 9. (A): The effect of the compound KU 14R (100 µM) on insulin release induced by the three imidazoline ligands and 10 µM forskolin. (B): The insulin secretory effect of three imidazoline ligands in the absence (□) and presence (■) of the dual MAO inhibitor pargyline (10 µM). Basal insulin secretion when islets were bathed in 8 mM glucose: (A) 7.8±0.8 and (B) 5.6±0.5 µIU ml-1 islet-1 h-1. *P<0.05 and **P<0.01, significant percentage increase relative to glucose. +P<0.05 and +++P <0.001, when compared to secretagogue alone. Data were ob- tained from at least 17 observations. 3.7. Effects in the Presence of Yohimbine, Idazoxan, BU 224 and Efaroxan on Intravenous Glucose Tolerance Test and Plasma Insulin Levels, Studies in Conscious Fasted Rabbits When infused alone at the equivalent dose of 10 µg kg-1 min-1, neither yohimbine [29], nor idazoxan modified basal values of either plasma glucose or circulating insu- lin (Figure 10). Interestingly, after the administration of BU 224 at the same dose, the ligand induced a progres- sive, persistent and significant increase in plasma insulin (at 45 min=184.40±42.95%, n=5, P<0.05, vs. 1.27±6.75%, n=7 in saline treated animals, see Figure 11). The pre-infusion plasma glucose level was: 6.25±0.80 mM, n=5. These levels were not significantly modified after drug infusion. Pre-treatment with yohimbine did not modify the re- sponses to a glucose load (10 mg kg-1 min-1), (Figure 10). in plasma glucose at 30 min in the absence and pres- ence of yohimbine was, respectively, 3.87±0.3 mM, n=8, 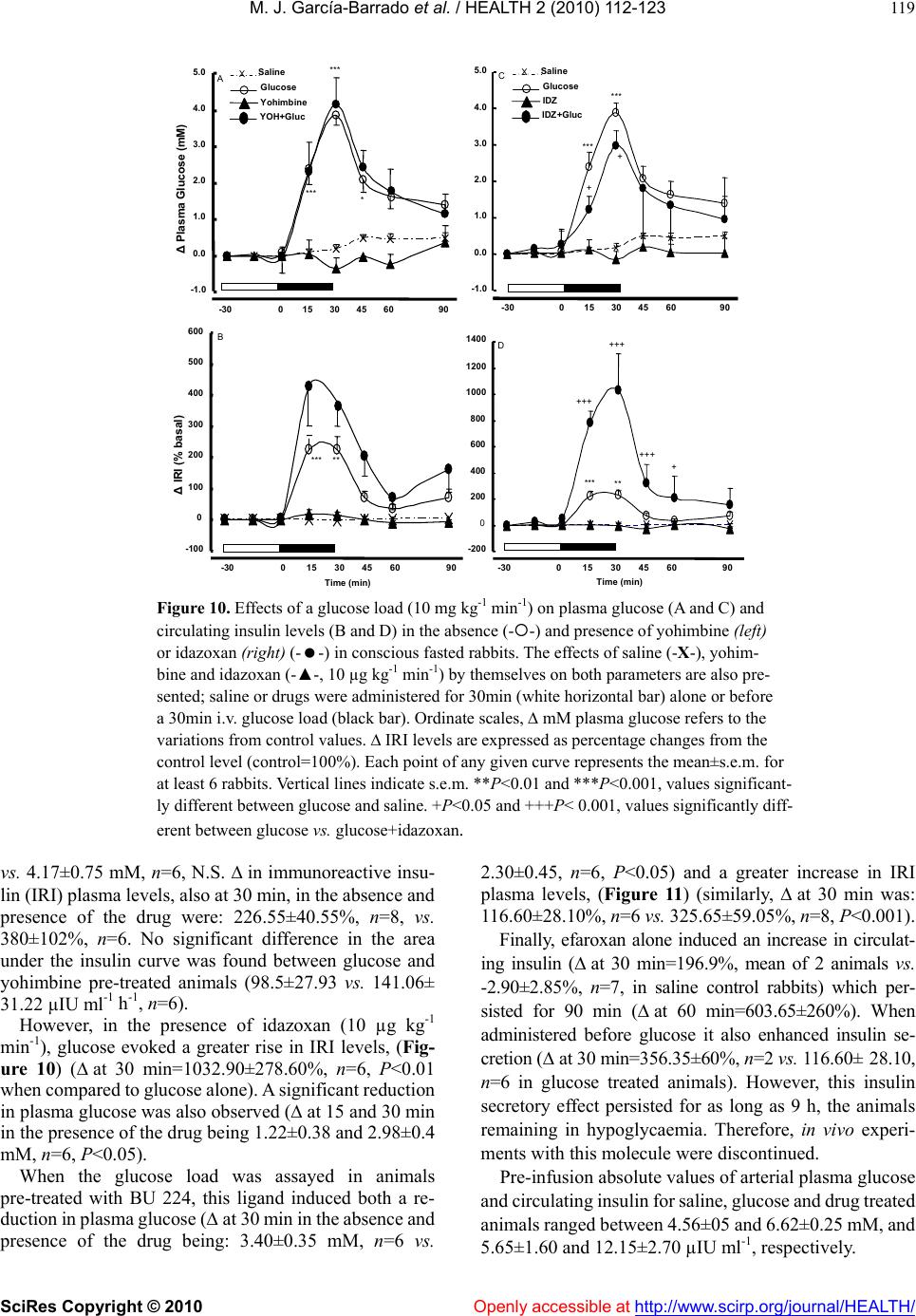 M. J. García-Barrado et al. / HEALTH 2 (2010) 112-123 SciRes Copyright © 2010 http://www.scirp.org/journal/HEALTH/Openly accessible at 119 -1.0 0.0 1.0 2.0 3.0 4.0 5.0 ΔPlasma Glucose (mM) -30015 3045 6090 A Yohimbine Glucose YOH+Gluc Saline X -100 0 100 200 300 400 500 600 ΔIRI (% basal) -30015 30 45 6090 Time (min) B *** ** *** *** * -1.0 0.0 1.0 2.0 3.0 4.0 5.0 C *** *** + + -3001530 456090 IDZ Glucose IDZ+Gluc Saline X -200 0 200 400 600 800 1000 1200 1400 -3001530 45 6090 Time (min) D *** ** +++ +++ +++ + Figure 10. Effects of a glucose load (10 mg kg-1 min-1) on plasma glucose (A and C) and circulating insulin levels (B and D) in the absence (--) and presence of yohimbine (left) or idazoxan (right) (--) in conscious fasted rabbits. The effects of saline (-X-), yohim- bine and idazoxan (-▲-, 10 µg kg-1 min-1) by themselves on both parameters are also pre- sented; saline or drugs were administered for 30min (white horizontal bar) alone or before a 30min i.v. glucose load (black bar). Ordinate scales, ∆ mM plasma glucose refers to the variations from control values. ∆ IRI levels are expressed as percentage changes from the control level (control=100%). Each point of any given curve represents the mean±s.e.m. for at least 6 rabbits. Vertical lines indicate s.e.m. **P<0.01 and ***P<0.001, values significant- ly different between glucose and saline. +P<0.05 and +++P< 0.001, values significantly diff- erent between glucose vs. glucose+idazoxan. vs. 4.17±0.75 mM, n=6, N.S. in immunoreactive insu- lin (IRI) plasma levels, also at 30 min, in the absence and presence of the drug were: 226.55±40.55%, n=8, vs. 380±102%, n=6. No significant difference in the area under the insulin curve was found between glucose and yohimbine pre-treated animals (98.5±27.93 vs. 141.06± 31.22 µIU ml-1 h-1, n=6). However, in the presence of idazoxan (10 µg kg-1 min-1), glucose evoked a greater rise in IRI levels, (Fig- ure 10) (at 30 min=1032.90±278.60%, n=6, P<0.01 when compared to glucose alone). A significant reduction in plasma glucose was also observed (at 15 and 30 min in the presence of the drug being 1.22±0.38 and 2.98±0.4 mM, n=6, P<0.05). When the glucose load was assayed in animals pre-treated with BU 224, this ligand induced both a re- duction in plasma glucose (at 30 min in the absence and presence of the drug being: 3.40±0.35 mM, n=6 vs. 2.30±0.45, n=6, P<0.05) and a greater increase in IRI plasma levels, (Figure 11) (similarly, at 30 min was: 116.60±28.10%, n=6 vs. 325.65±59.05%, n=8, P<0.001). Finally, efaroxan alone induced an increase in circulat- ing insulin (at 30 min=196.9%, mean of 2 animals vs. -2.90±2.85%, n=7, in saline control rabbits) which per- sisted for 90 min (at 60 min=603.65±260%). When administered before glucose it also enhanced insulin se- cretion (at 30 min=356.35±60%, n=2 vs. 116.60± 28.10, n=6 in glucose treated animals). However, this insulin secretory effect persisted for as long as 9 h, the animals remaining in hypoglycaemia. Therefore, in vivo experi- ments with this molecule were discontinued. Pre-infusion absolute values of arterial plasma glucose and circulating insulin for saline, glucose and drug treated animals ranged between 4.56±05 and 6.62±0.25 mM, and 5.65±1.60 and 12.15±2.70 µIU ml-1, respectively.  M. J. García-Barrado et al. / HEALTH 2 (2010) 112-123 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ 120 -1. 0 0.0 1.0 2.0 3.0 4.0 5.0 -30015 30 456090 plasma glucose(mM) saline glucose BU 224 BU+glucose A *** *** -100 0 100 200 300 400 500 600 -30015 30 456090 IRI (% basal) Time (min) B *** *** * * +++ +++ + ++ Figure 11. Changes in plasma glucose (A) and in immunoreactive insulin (IRI) (B) levels in conscious fasted rabbits, measured after the i.v. infusion of physiological saline (-X-), glucose alone (--, 10 mg kg-1min-1), BU 224 (-▲- 10 µg kg-1min-1) and BU 224+glucose (--); the BU 224 was infused for 30 min (open bar) just before a 30min glucose infusion (black horizontal bar). Ordinate scales, mM plasma glucose refers to the variations from control values. ∆ IRI levels are expressed as percentage changes from the control level (control=100%). Each point of any given curve represents the mean±s.e.m. for at least 7 rabbits. *P<0.05 and ***P<0.001, values significantly different be- tween glucose or BU 224 vs. saline. +P<0.05, ++P<0.01, and +++P<0.001, values significantly different between glucose vs. glucose+BU 224. 4. DISCUSSIONS Studies on insulin secretion have mainly been carried out using mouse and/or rat isolated islets. However, there is a lack of experimental data for other animal species, rabbit included. Since rabbit tissues are very rich in imidazoline binding sites [17,24] this animal was chosen for the present work. The results reported in this study assess the validity of our model: insulin release from isolated islets was glu- cose-dependent and very sensitive to changes in the ex- tracellular concentrations of Ca2+ and K+ ions. Similarly, conventional stimulatory and/or inhibitory responses (i.e., to forskolin, diazoxide) were also confirmed. Idazoxan induced a very clear dose-dependent insulin secretory response. These are rather paradoxical results, since it has been reported that idazoxan is unable to re- lease insulin in the rat, or it has a weak concentration independent effect in mouse [13,14]. An even more I2-selective ligand, BU 224 [27], also evoked a dose- dependent secretory response, as did the well known and studied ligand efaroxan [1]. In this model methoxy-idazoxan assayed over a wide range failed to elicit insulin release, thus corroborating its nature as a true 2-adrenoceptor antagonist. This property was also shared by idazoxan and it was unmasked when tested against the 2-adrenoceptor agonist brimonidine (UK 14,304). In this way, idazoxan reflected its established dual behaviour. However, no inter- action with 2-adrenoceptors could be found with BU 224. The mode of action of imidazolines is complex. Clas- sical insulinotropic compounds inhibit KATP channels in the ß-cell, but, in addition, they exert a direct effect on exocytosis [7,32]. The three ligands studied behaved as KATP channel blockers. Interaction of efaroxan with KATP channels could be inferred from perifusion studies and when incubating the islets in the presence of diazoxide, since efaroxan alleviated the suppressive effect on insulin release induced by the potassium channel opener. Similar results have been reported for rat islets [30] using other imidazoline drugs (RX871024, S-22068 [4, 34] and in the present work with the selective I2-ligand BU 224. How- ever, diazoxide abolished the response to idazoxan, but not to BU 224. It is necessary to consider at this time that in mouse islets idazoxan induces a partial inhibition of KATP channels, sufficient to depolarize the plasma mem- brane and to open voltage-dependent Ca2+ channels with a subsequent modest increase in intracellular calcium [13]. In the presence of partial channel inhibition, dia- zoxide, by reducing the binding affinity of the ligand, could easily suppress the response. It is also noteworthy that nimodipine similarly abolished the effect of idazoxan and that just simple reduction in the Ca2+ concentration in the extracellular medium severely attenuated the ligand response. An α-1 partial agonist effect of idazoxan has been recently reported [35]. However there is no evidence for a α-1 adrenoceptor involvement in insulin secretion in isolated islets [36]. When rabbit islets were incubated in the presence of the selective α-1 adrenoceptor agonist, amidephrine, no insulin secretory response was found (glucose 8 mM 11.61.4 vs amidefrine 10.52.2 µIU ml-1 islet–1 h-1). In the same experimental situations, BU 224 was still able to increase insulin release. It is also accepted that this kind of imidazoline com- pounds block the KAT P channel at the level of the Kir6.2 pore [9]. Our experimental results, studying drug interac- tions among themselves and in the presence of tolbutamide, trend to support this notion. Simultaneous addition of efaroxan and idazoxan, or efaroxan and BU 224, did not lead to synergism or antagonism, probably considering that the three ligands shared a common drug binding site at the pore level [37]. On the other hand, an additive effect was found when BU 224 and tolbutamide were administered together. Indeed, the imidazoline could not displace the  M. J. García-Barrado et al. / HEALTH 2 (2010) 112-123 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ 121 sulphonylurea from its SUR1 receptor. The ensuing en- hanced response could result from additive effects at the KATP channel, or by BU 224 activation of a signal- transduction pathway (see below). Similar results have been reported with other imidazolines [14,38]. It is also well established that insulin secretion in the presence of these compounds exhibited glucose depend- ency [8,38]. Identical results, expressing the requirement for a high energy state of the cell (high ATP/ADP ratio) have been found with the I2-ligands used in this work. Studies with permeabilised islets and HIT T15 cells have revealed a direct effect of imidazolines (RX871024, BL11282) on exocytosis independent of KAT P channel activity. PKA and PKC, with subsequent activation of protein phosphorylation/dephosphorylation steps, would play a central role in the regulation of this process [8]. However, these kinase inhibitors failed to alter the re- sponse to efaroxan and idazoxan [12]. Our results, using either PKA/PKC inhibitors or excess K+, confirm that efaroxan and idazoxan induced insulin secretion is de- pendent of KAT P channel activity, whereas the effect of BU 224 requires, in addition, PK activation. A synergism be- tween the effect of BU 224 and either forskolin or IBMX on insulin secretion was also evident, suggesting the per- missive role of PKA activity on this particular response. Interestingly, the compound KU 14R, known as an efaroxan antagonist [39], did not alter, in the present work, the response to this ligand, though it blocked the effect of BU 224, significantly attenuating the response to forsko- lin [40]. A lack of antagonism between efaroxan and KU 14R has also been reported recently in mouse islets [41]. Consequently an association among BU 224-PKA-KU 14R could be inferred in our model. It is noteworthy that at the concentrations used in the present work BU 224 behaved as a reversible inhibitor of MAO A and B, pre- venting hydrogen peroxidase production in adipose tissue [42]. However, in our model MAO inhibition did not modify glucose or BU 224 mediated insulin release. At this point it is interesting to note that the total capacity of the pancreas to oxidise MAO substrates was limited compared with the overall mass and amine oxidase ac- tivities of muscular and adipose tissue [43]. Therefore these results reassess the true nature of BU 224 as an I2 ligand, though the response under study seems to be independent of MAO binding sites. It has been reported recently that selective I2-ligands can bind creatine kinase [44,45], a key enzyme important for ATP synthesis. This additional interaction would help to understand the mechanism(s) of BU 224 induced insulin release, con- sidering the importance of ATP for exocytosis even at stages distal to an increase in [Ca+2]i (see experiments at high concentrations of K+). The presence of I2 binding sites (IBS) mediating the ef- fects of these ligands has not been accepted on the basis of the failure of idazoxan to elicit insulin secretion, lack of data with more selective I2-ligands and binding studies with methoxy-idazoxan. Results presented in this work refute these premises. In addition the ligand BTS 67582 can bind to the I2 imidazoline receptor with potency con- sistent with its effect on insulin secretion [46]. The drug could regulate insulin release by an interaction with the KATP channel or by exerting a direct effect in the process of exocytosis [46,47]. Curiously indeed, idazoxan and BU 224 also increase insulin release, blocking KATP channel activity at a site shared by the third imidazoline ligand efaroxan. Therefore, considering that a number of I2-ligands can bind a common site on the channel, this binding site, independent of MAO activity, might be con- sidered as a variant or subtype of the classic I2-binding site. It is also known that the presence of I2 sites on MAO en- zyme can not satisfactorily represent the diverse biological targets of I2-ligands [48,49]. Intracellular binding sites linked to protein kinase(s) activation (I3-receptor?) would also be involved in the amplifying effect of BU 224. In vivo studies reassess in vitro data. Idazoxan and BU 224, but not yohimbine, enhanced the insulin secretory response to a glucose load. Temporal patterns of insulin secretion when BU 224 was infused alone or in the pres- ence of glucose showed the complex behaviour of this molecule: its glucose dependency as well as its interaction with KATP dependent and independent mechanisms. When comparing circulating levels of insulin after a glucose challenge in animals pretreated with any of the three drugs, idazoxan was able to induce the maximal response. The dual nature of this ligand should be borne in mind: its ability to block: 1) pre and post-synaptic 2 adrenoceptors and thus increase plasma catecholamine levels [50], with a subsequent -adrenoceptor mediated effect; and 2) KATP channels as an I-ligand (like BU 224). Combined mecha- nisms would be responsible for such an effect. Though BU 224 showed a greater antihyperglycaemic effect than idazoxan, the effect was lower than expected considering its ability to release insulin and to restrain lipolysis [42]. However this molecule, being an MAO inhibitor, should prevent metabolic inactivation of en- dogenous catecholamines, thus enhancing intrinsic sympathomimetic activity. Non-selective blocking of Kir6.2 affecting channels at several locations could also unmask compensatory responses able to attenuate the blood glucose lowering response. 5. CONCLUSIONS The imidazoline ligands interacting with either I2 or I3-binding sites would mediate in vitro, as well as in vivo, insulin secretion. However, additional extrapancreatic sites of action would attenuate their antihyperglycaemic effect. In conclusion, the administration of BU 244 in-  M. J. García-Barrado et al. / HEALTH 2 (2010) 112-123 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ 122 duces extended insulin release that would produce po- tential hypoglycaemia. This could be an adverse effect whether this molecule is used as antidiabetic drug. 6. ACKNOWLEDGEMENTS The authors express gratitude to Mr. G.H. Jenkins for his help with the English version of the manuscript. We are very grateful for the fi- nancial support from Junta de Castilla y León (JCYL), grant nº SA42/00B (Spain). REFERENCES [1] Chan, S.L., Brown, C.A., and Morgan, N.G. (1993) Stimulation of insulin secretion by the imidazoline alpha 2-adrenoceptor antagonist efaroxan is mediated by a novel, stereoselective, binding site. Eur J Pharmacol, 230(3), 375-8. [2] Schulz, A. and Hasselblatt, A. (1989) An insulin-releasing property of imidazoline derivatives is not limited to compounds that block alpha-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol, 340, 321-7. [3] Berdeu, D., Puech, R., Ribes, G., Loubatieres-Mariani, M.M., and Bertrand, G. (1997) Antazoline increases insu- lin secretion and improves glucose tolerance in rats and dogs. Eur J Pharmacol, 324, 233. [4] Pele-Tounian, A., Chan, S.L., Rondu, F., Le Bihan, G., Giroix, M.H., Lamouri, A., Touboul, E., Pfeiffer, B., Manechez, D., Renard, P., Guardiola-Lemaitre, B., God- froid, J.J., Penicaud, L., Morgan N.G., and Ktorza A. (1999) Effect of the new imidazoline derivative S-22068 (PMS 847) on insulin secretion in vitro and glucose turnover in vivo in rats. Eur J Pharmacol, 377, 81-87. [5] Wang, X., Rondu, F., Lamouri, A., Dokhan, R., Marc, S., Touboul, E., Pfeiffer, B., Manechez D., Renard, P., Guar- diola-Lemaitre, B., Godfroid, J.J., Ktorza, A., and Peni- caud, L. (1996) Effect of S-21663 (PMS 812), an imida- zoline derivative, on glucose tolerance and insulin secre- tion in a rat model of type II diabetes. J Pharmacol Exp Ther, 278, 82-89. [6] Williams, C.A., Shih, M.F., and Taberner, P.V. (2000) Effect of acute and sub-chronic administration of the imidazoline compound S22068 on in vivo glucose and insulin responses in normal lean CBA/Ca mice. Gen. Pharmacol., 34, 183-91. [7] Efendic, S., Efanov, A.M., Berggren, P.O., and Zaitsev, S.V. (2002) Two generations of insulinotropic imidazoline compounds. Diabetes, 51 (3), S448-S547. [8] Efanov, A.M., Zaitsev, S.V., Mest, H.J., Raap, A., Appel- skog, I.B., Larsson, O., Berggren, P.O., and Efendic, S. (2001) The novel imidazoline compound BL11282 po- tentiates glucose-induced insulin secretion in pancreatic beta-cells in the absence of modulation of KATP channel activity. Diabetes, 50, 797-802. [9] Proks, P. and Ashcroft, F.M. (1997) Phentolamine block of KAT P channels is mediated by Kir6.2. Proc Natl Acad Sci USA, 94, 1716-20. [10] Morgan, N.G., Chan, S.L., Mourtada, M., Monks, L.K., and Ramsden, C.A. (1999) Imidazolines and pancreatic hormone secretion. Ann N Y Acad Sci, 881, 217-28. [11] Farret, A., Lugo-Garcia, L., Galtier, F., Gross, R., and Petit, P. (2005) Pharmacological interventions that di- rectly stimulate or modulate insulin secretion from pan- creatic beta-cell: Implications for the treatment of type 2 diabetes. Fundam Clin Pharmacol, 19, 647-656. [12] Mourtada, M., Smith, S.A., and Morgan, N.G. (1998) Effector systems involved in the insulin secretory re- sponses to efaroxan and RX871024 in rat islets of Langerhans. Eur J Pharmacol, 350, 251-8. [13] Rustenbeck, I., Leupolt, L., Kowalewski, R., and Hassel- blatt, A. (1999) Heterogeneous characteristics of imida- zoline-induced insulin secretion. Naunyn Schmiedebergs Arch Pharmacol, 359, 235-42. [14] Mourtada, M., Brown, C.A., Smith, S.A., Piercy, V., Chan, S.L., and Morgan, N.G. (1997) Interactions between imi- dazoline compounds and sulphonylureas in the regulation of insulin secretion. Br J Pharmacol, 121, 799-805. [15] Berdeu, D., Gross, R., Puech, R., Loubatieres-Mariani, M.M., and Bertrand, G. (1995) Evidence for two different imidazoline sites on pancreatic B cells and vascular bed in rat. Eur J Pharmacol, 275, 91-8. [16] Zaitsev, S.V., Efanov, A.M., Raap, A., Efanova, I.B., Schloos, J., Steckel-Hamann, B., Larsson, O., Ostenson, C.G., Berggren, P.O., Mest, H.J., and Efendic, S. (1999) Different modes of action of the imidazoline compound RX871024 in pancreatic beta-cells: Blocking of K+ channels, mobilization of Ca2+ from endoplasmic reticu- lum, and interaction with exocytotic machinery. Ann N Y Acad Sci, 881, 241-52. [17] Boronat, M.A., Olmos, G., Miller, D.D., Patil, P.N., and Garcia-Sevilla, J.A. (1998) Isothiocyanatobenzyl imida- zoline is an alkylating agent for I2-imidazoline binding sites in rat and rabbit tissues. Naunyn Schmiedebergs Arch Pharmacol, 357, 351-57. [18] Chan, S.L., Brown, C.A., Scarpello, K.E., and Morgan, N.G. (1994) The imidazoline site involved in control of insulin secretion: Characteristics that distinguish it from I1- and I2-sites. Br J Pharmacol, 112, 1065-70. [19] Le Brigand, L., Virsolvy, A., Manechez, D., Godfroid, J.J., Guardiola-Lemaitre, B., Gribble, F.M., Ashcroft, F.M., and Bataille, D. (1999) In vitro mechanism of action on insulin release of S-22068: A new putative antidiabetic compound. Br J Pharmacol, 128, 1021-6. [20] Cooper, E.J., Hudson, A.L., Parker, C.A., and Morgan, N.G. (2003) Effects of the beta-carbolines, harmane and pinoline: On insulin secretion from isolated human islets of Langerhans. Eur J Pharmacol, 482, 189-96. [21] Mayer, G. and Taberner, P.V. (2002) Effects of the imi- dazoline ligands efaroxan and KU14R on blood glucose homeostasis in the mouse. Eur J Pharmacol, 454, 95-102. [22] Ernsberger, P. (1992) Heterogeneity of imidazoline bind- ing sites: Proposed I1 and I2 subtypes. Fund Clin Phar- macol., 6(1), 55S. [23] Coupry, I., Podevin, R.A., Dausse, J.P., and Parini, A. (1987) Evidence for imidazoline binding sites in baso- lateral membranes from rabbit kidney. Biochem Biophys Res Commun, 147, 55-60. [24] Hudson, A.L, Husbands, S., Lewis, J.W., and Nutt, D.J. (1994) Affinity and selectivity of BU224 and BU239 for rabbit brain non-adrenoceptor idazoxan binding sites (I2-sites). Br J Pharmacol, 112, (Proceedings Suppl), 320. [25] Jou, S.B., Liu, I.M., and Cheng, J.T. (2004) Activation of  M. J. García-Barrado et al. / HEALTH 2 (2010) 112-123 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ 123 imidazoline receptor by agmatine to lower plasma glucose in streptozotocin-induced diabetic rats. Neurosci Lett, 358, 111-4. [26] MacInnes, N. and Handley, S.L. (2002) Characterization of the discriminable stimulus produced by 2-BFI: Effects of imidazoline I(2)-site ligands, MAOIs, beta-carbolines, agmatine, and ibogaine. Br J Pharmacol, 135, 1227-34. [27] Nutt, D.J., French, N., Handley, S., Hudson, A., Husbands, S., Jackson, H., Jordan, S., Lalies, M.D., Lewis, J., Lione, L., et al. (1995) Functional studies of specific imidazoline-2 receptor ligands. Ann N Y Acad Sci, 763, 125-39. [28] García-Barrado, M.J., Sancho, C., Iglesias-Osma, M.C., and Moratinos, J. (2001) Effects of verapamil and el- godipine on isoprenaline-induced metabolic responses in rabbits. Eur J Pharmacol., 415, 105-115. [29] Moratinos, J., Carpene, C., De Pablos, I., and Reverte, M. (1988) Role of alpha 1- and alpha 2-adrenoceptors in catecholamine-induced hyperglycaemia, lipolysis and insulin secretion in conscious fasted rabbits. Br J Phar- macol, 94, 299-310. [30] Chan, S.L., Dunne, M.J., Stillings M.R., and Morgan, N.G. (1991) The alpha 2-adrenoceptor antagonist efaroxan modulates K+ ATP channels in insulin-secreting cells. Eur J Pharmacol, 204, 41-8. [31] Gembal, M., Gilon, P., and Henquin, J.C. (1992) Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest, 89, 1288-95. [32] Cuchillo-Ibanez, I., Lejen, T., Albillos, A., Rose, S.D., Olivares, R., Villarroya, M., et al. (2004) Mitochondrial calcium sequestration and protein kinase C cooperate in the regulation of cortical F-actin disassembly and secre- tion in bovine chromaffin cells. J Physiol, 560, 63-76. [33] Henquin, J.C. (2004) Pathways in beta-cell stimulus- secretion coupling as targets for therapeutic insulin se- cretagogues. Diabetes, 53 (3), S48-S58. [34] Zaitsev, S.V., Efanov, A.M., Efanova, I.B., Larsson, O., Ostenson, C.G., Gold, G., Berggren, P.O., and Efendic, S. (1996) Imidazoline compounds stimulate insulin release by inhibition of KAT P channels and interaction with the exocytotic machinery. Diabetes, 45, 1610-1618. [35] Serban, D.N., Serban, I.L., and Nechifor, M. (2004) Ida- zoxan effects upon contractile activity in the rat aorta are related to alpha adrenoceptors and L-type channels. Fundan Clin Pharmacol, 18, 635-41. [36] Malaisse, W.J. and Moratinos, J. (1986) Are pancreatic beta-cells equipped with alpha-1 adrenoceptors? IRCS Med Sci, 1194-1195. [37] Ugedo, L., Pineda, J., Ruiz-Ortega, J.A., and Martin-Ruiz, R. (1998) Stimulation of locus coeruleus neurons by non-I1/I2-type imidazoline receptors: An in vivo and in vitro electrophysiological study. Br J Pharmacol, 125, 1685-1694. [38] Efanov, A.M., Zaitsev, S.V., Efanova, I.B., Zhu, S., Os- tenson, C.G., Berggren, .PO., and Efendic, S. (1998) Signalling and sites of interaction for RX-871024 and sulfonylurea in the stimulation of insulin release. Am J Physiol, 274, E751-E757. [39] Mourtada, M., Chan, S.L., Smith, S.A., and Morgan, N.G. (1999) Multiple effector pathways regulate the insulin secretory response to the imidazoline RX871024 in iso- lated rat pancreatic islets. Br J Pharmacol, 127, 1279- 1287. [40] Chan, S.L., Mourtada, M., and Morgan, N.G. (2001) Characterization of a KAT P channel-independent pathway involved in potentiation of insulin secretion by efaroxan. Diabetes, 50, 340-347. [41] Bleck, C., Wienbergen, A., and Rustenbeck, I. (2005) Essential role of the imidazoline moiety in the insulino- tropic effect but not the KATP channel-blocking effect of imidazolines: A comparison of the effects of efaroxan and its imidazole analogue, KU14R. Diabetologia, 48, 2567-75. [42] Bour, S., Iglesias-Osma, M.C., Marti, L., Duro, P., Gar- cia-Barrado, M.J., Pastor, M.F., Prevot, D., Visentin, V., Valet, P., Moratinos, J., and Carpene, C. (2006) The imi- dazoline I2-site ligands BU 224 and 2-BFI inhibit MAO-A and MAO-B activities, hydrogen peroxide production, and lipolysis in rodent and human adipocytes. Eur J Pharmacol, 552, 20-30. [43] Iglesias-Osma, M.C., Garcia-Barrado, M.J., Visentin, V., Pastor-Mansilla, M.F., Bour, S., Prevot, D., Valet, P., Moratinos, J., and Carpene, C. (2004) Benzylamine ex- hibits insulin-like effects on glucose disposal, glucose transport, and fat cell lipolysis in rabbits and diabetic mice. J Pharmacol Exp Ther, 309, 1020-1028. [44] Kimura, A., Tyacke, R.J., Minchin, M.C., Nutt, D.J., and Hudson, A.L. (2003) Identification of an I(2) binding protein from rabbit brain. Ann N Y Acad Sci, 1009, 364-366. [45] Paterson, L.M., Robinson, E.S., Nutt, D.J., and Hudson, A.L. (2003) In vivo estimation of imidazoline(2) binding site turnover. Ann N Y Acad Sci, 1009, 367-370. [46] Dickinson, K., North, T.J., and Jones, R.B. (1999) BTS 67 582 may mediate insulin secretion via the putative pancre- atic islet imidazoline receptor. Br J Pharmacol, 126, 133. [47] Page, T.B.C.J. (1997) Glucose-lowering effect of BTS 67 582. Br J Pharmacol, 122, 1464-8. [48] Tesson, F., Limon-Boulez, I., Urban, P., Puype, M., Vandekerckhove, J., Coupry, I., Pompon, D., and Parini, A. (1995) Localization of I2-imidazoline binding sites on monoamine oxidases. J Biol Chem, 270, 9856-61. [49] Marti, L., Morin, N., Enrique-Tarancon, G., Prevot, D., Lafontan, M., Testar, X., Zorzano, A., and Carpene, C. (1998) Tyramine and vanadate synergistically stimulate glucose transport in rat adipocytes by amine oxidase- dependent generation of hydrogen peroxide. J Pharmacol Exp Ther, 285, 342-9. [50] Portillo, M., Reverte, M., Langin, D., Senard, J.M., Tran, M.A., Berlan, M., and Montastruc, J.L. (1991) Effect of a 7-day treatment with idazoxan and its 2-methoxy deriva- tive RX 821002 [correction of RX 821001] on alpha 2-adrenoceptors and non-adrenoceptor idazoxan binding sites in rabbits. Br J Pharmacol, 104, 190-194.
|