Paper Menu >>
Journal Menu >>
 Vol.2, No.2, 97-100 (2010) Health doi:10.4236/health.2010.22016 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ Comparative study of haemagglutination inhibition, Agar gel precipitation test, Serum neutralization and Enzyme linked immunosorbent assay for detection to avian influenza viruses Shahid Faraz, Muhammad Abubakar 1, Mohammad Farooque2, Sarfaraz Ali Fazlani, Ghluam Hussain Jaffar Faculty of Veterinary and Animal Sciences, Lasbela University of Agriculture, Water and Marine Sciences, Uthal. 1National Veterinary Laboratory, Park Road, Islamabad. 2Quarantine Officer, Animal Quarantine Department, Islamabad. Received 22 October 2009; revised 7 December 2009; accepted 9 December 2009. ABSTRACT The sensitivity, specificity and reproducibility of the serological tests for detection of avian in- fluenza viruses were carried-out by using Ham- agglutination inhibition (HI), Agar gel precipita- tion test (AGPT), and Enzyme linked immu- nosorbent assay (ELISA) and Serum neutraliza- tion test. The geometric mean titre (GMT) of hae- magglutination inhibition antibodies recor- ded as log2 indicated that the post vaccination titres in the field were on higher side i.e., 7.9 for H7 and 5.9 for H9. The correlation between HI titre and AGPT affirmed that for the AGPT test need high antibody titre for positive reaction. The pooled sera were also used to correlate the se- rum neutralization test and enzyme linked im- muno-sorbent assay. The serial two fold dilute- ons were tested for the serum neutralization ac- tivity and concluded that the HI titre log2 4 pro- vided 100% protection, than 52% and 45% pro- tection in 1:2 and 1:4 dilution was recorded, respectively. Similarly, the ELISA test showed positive results up to 1:16 HI titre, i.e. log2 4 and confirmed the linear relation between these two serological tests. In HI test, the concentration of antigen can influence the result. It also needs careful preparation of concentration of eryth- rocyte suspension. Agar Gel immuno-diffusion is basically a qualitative test as it can not de- termine the quantity of antigen or antibody with the help of this test. It lacks the level of sensi- tivity as offered by other test. If serum neutrali- zation test is performed on a pooled serum sam- ples, then it could lead to a false conclusion on antibodies status. ELISA is most sensitive, spe- cific and accurate as compare to all other sero- logical tests. Keywords: Serology; HI; ELISA and Avian Influ- enza Virus. 1. INTRODUCTION Infectious viral diseases are a major threat to poultry. Avian influenza is one of most important among them which inflicts heavy economical losses. It is caused by a virus that belongs to family Orthomixoviridae, genus influenza A virus [1]. Avian influenza virus is classified into subtypes on the basis of antigenic differences in their surface glycopro- tein hemagglutinin (HA; H) and neuraminidase, (NA; N). To date 16H subtypes (H1-H16) and 9N subtypes (N1- N9) have been recognized [2]. It rapidly infects the poul- try when heavy outbreak occurs. Many species of birds are found to be susceptible to avian influenza virus. Avian influenza viruses are circulating periodically among the domestic poultry over 100 years [3]. Wild waterfowl and shorebirds are considered to be reservoir of influenza A virus because the species harbor all 16 HA subtypes [4]. Although chicken and turkeys are not natural host species for avian influenza but these viruses routinely cross over from wild bird’s reservoir to infect poultry birds [5]. During 2003 and 2004, there were quite a few out- breaks of avian influenza throughout the Asia, including South Korea, Japan, Indonesia, Vietnam, Thailand and China. The outbreak resulted not only tremendous eco- nomic loses in the poultry industry but also claimed dea- th in human in Vietnam and Thailand in 2004. 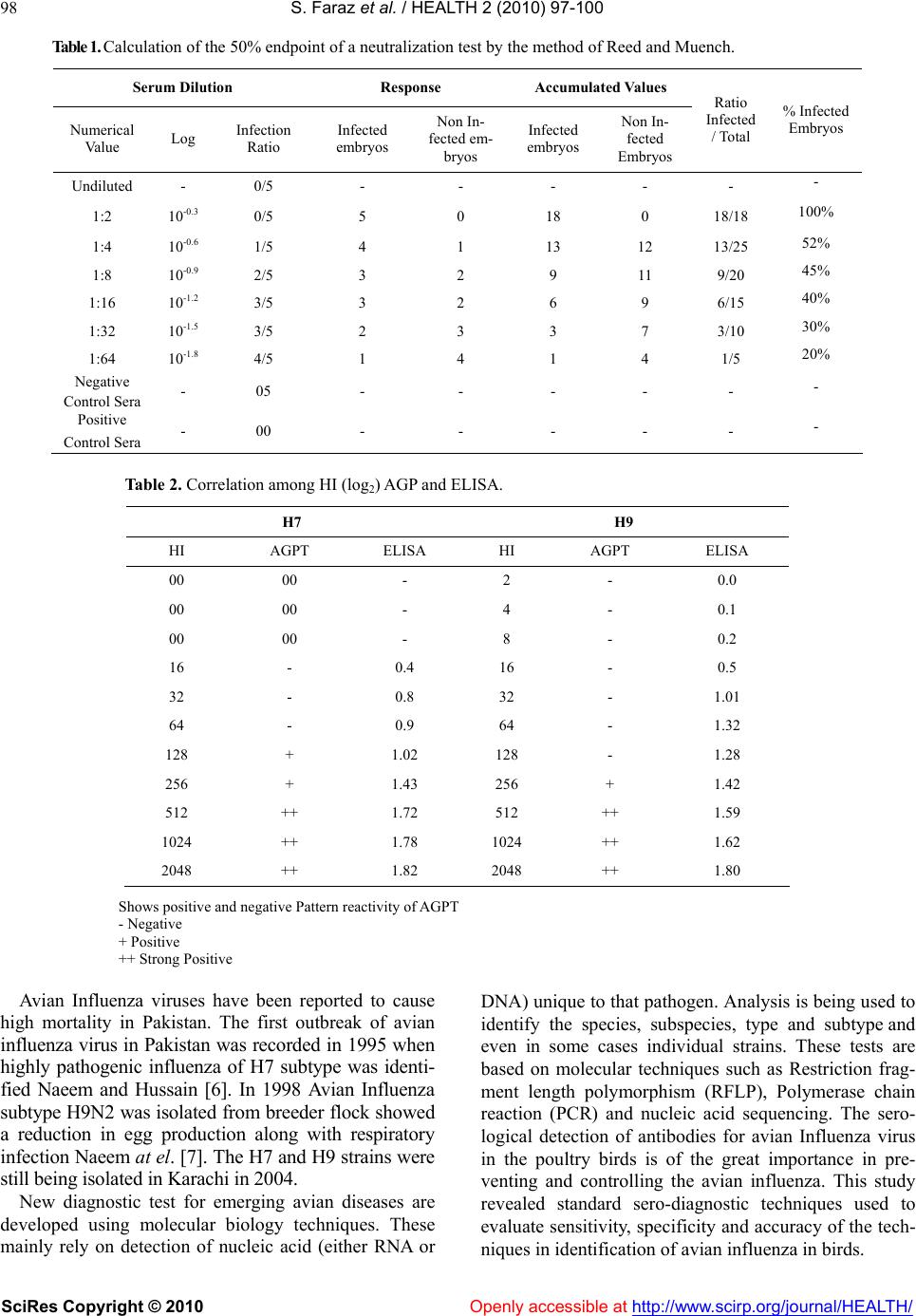 98 S. Faraz et al. / HEALTH 2 (2010) 97-100 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ Table 1. Calculation of the 50% endpoint of a neutralization test by the method of Reed and Muench. Serum Dilution Response Accumulated Values Numerical Value Log Infection Ratio Infected embryos Non In- fected em- bryos Infected embryos Non In- fected Embryos Ratio Infected / Total % Infected Embryos Undiluted - 0/5 - - - - - - 1:2 10-0.3 0/5 5 0 18 0 18/18 100% 1:4 10-0.6 1/5 4 1 13 12 13/25 52% 1:8 10-0.9 2/5 3 2 9 11 9/20 45% 1:16 10-1.2 3/5 3 2 6 9 6/15 40% 1:32 10-1.5 3/5 2 3 3 7 3/10 30% 1:64 10-1.8 4/5 1 4 1 4 1/5 20% Negative Control Sera - 05 - - - - - - Positive Control Sera - 00 - - - - - - Table 2. Correlation among HI (log2) AGP and ELISA. H7 H9 HI AGPT ELISA HI AGPT ELISA 00 00 - 2 - 0.0 00 00 - 4 - 0.1 00 00 - 8 - 0.2 16 - 0.4 16 - 0.5 32 - 0.8 32 - 1.01 64 - 0.9 64 - 1.32 128 + 1.02 128 - 1.28 256 + 1.43 256 + 1.42 512 ++ 1.72 512 ++ 1.59 1024 ++ 1.78 1024 ++ 1.62 2048 ++ 1.82 2048 ++ 1.80 Shows positive and negative Pattern reactivity of AGPT - Negative + Positive ++ Strong Positive Avian Influenza viruses have been reported to cause high mortality in Pakistan. The first outbreak of avian influenza virus in Pakistan was recorded in 1995 when highly pathogenic influenza of H7 subtype was identi- fied Naeem and Hussain [6]. In 1998 Avian Influenza subtype H9N2 was isolated from breeder flock showed a reduction in egg production along with respiratory infection Naeem at el. [7]. The H7 and H9 strains were still being isolated in Karachi in 2004. New diagnostic test for emerging avian diseases are developed using molecular biology techniques. These mainly rely on detection of nucleic acid (either RNA or DNA) unique to that pathogen. Analysis is being used to identify the species, subspecies, type and subtype and even in some cases individual strains. These tests are based on molecular techniques such as Restriction frag- ment length polymorphism (RFLP), Polymerase chain reaction (PCR) and nucleic acid sequencing. The sero- logical detection of antibodies for avian Influenza virus in the poultry birds is of the great importance in pre- venting and controlling the avian influenza. This study revealed standard sero-diagnostic techniques used to evaluate sensitivity, specificity and accuracy of the tech- niques in identification of avian influenza in birds.  S. Faraz et al. / HEALTH 2 (2010) 97-100 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ 99 2. MATERIALS AND METHODS Day-old broiler chicks were purchased and used for the experiment. The broiler chickens were divided into three groups A, B and C. Group A and B were vaccinated with H7N3 and H9N2, respectively, while group C was kept as control. Before vaccination, the blood samples were collected randomly from 5% birds prior to vaccination at 3rd week of age to determine the status of maternal anti- bodies in the sera of chicks. 2.1. Serum Samples To assess the susceptibility of chickens the serum sam- ples were checked prior to vaccinate and found zero titre against avian influenza. Blood samples were allowed to coagulate than serum was collected, marked, centrifuged and stored at 20 oC prior to use. 2.2. Source Virus A local virus isolate chicken/Pakistan/23/99 (H9N2) virus was used with ELD 50 of 109.26/0.1ml. Another vi- rus isolate H7N3 was also received from Sindh Poultry Vaccine Centre (SPVC) with ELD 50 of 107.56/0.1ml. Both serotypes were confirmed from southeast poultry research institute Georgia. 2.3. Haemagglutination Inhibition Test The (HI) test was performed by adopting the technique described in (OIE) manual of diagnostic tests using 4HA units of H7 and H9 viruses with 1% suspension of washed chicken RBCs. Serum was diluted while antigen was constant. 2.4. Agar Gel Precipitation (AGP) Test This test was performed to adopt the method described by Beard in 1998. Agar was prepared in borate buffer, than allowed to solidify. Cylinder of gel were cut with a gel cutter, than antigen was placed in the central wells while the sera were in the peripheral wells than the Petri plates were kept in moist chamber to avoid drying of gel. 2.5. Virus Neutralization Test The virus neutralization test was performed to titrate the antibodies against avian influenza virus; both strains were checked and confirmed for sterility. Test was done by using the embryo inoculation, ß-method (constant virus diluted serum) and followed the technique de- scribed by Beard [8], while endpoint titre was calculated as log2 exponent that was 50% neutralization endpoint or PD50 by the formula of Reed and Munch. 2.6. ELISA Test The ELISA test was run in accordance as described by Terry and Tony in 1991 [9] and as described in the monograph of Australian Centre for International Agri- cultural Research (ACIAR 1995), in which plates were read at 405nm absorbancy in a microplate ELISA reader. 3. RESULTS The geometric mean titer (GMT) in log2 of serotype H7 was recorded as 7.9±0.23 (66) and for H9 was cal- culated as 5.7±0.29 (66). The mean H7 titre was sig- nificantly (p<0.05) higher than H9. 3.1. Agar Gel Precipitation Test The pattern of reactivity of both strains is similar and no remarkable difference has been observed. However, the precipitation line can be seen in those samples which ha- ve HI titres more than 7 log2 in H9 subtype. Moreover, there is a linear relation between precipitates of antibod- ies and antigen. Results also showed that H7 subtype was not positive till the HI titre was 9 log2. 3.2. Virus Neutralization Test Before the using of strains for serum Neutralization Test, the ELD 50 was determined and recorded as 1011.26/ml for H9 and 108.31/ml for H7subtype. The results in (Table 1) indicated that undiluted and 1:2 dilution of serum showed 100% protection and than gradually decreased i-e 52% and 45% protection in 1:4 and 1:8 dilution respectively. However, negative control sera showed no protection while positive showed 100%. 3.3. ELISA Test The average optical density value (OD405nm) of posi- tive sera showed 0.4 OD up to 1:16 dilution, i.e, 4 on log 2. Both serotypes showed positive correlation between HI titre and ELISA at GMT Log24. However there is a linear relation between HI titre, ELISA and AGPT shown in Table 2. 4. DISCUSSION Present study revealed that ELISA is better than HI in identifying sera with low antibody titer. HI titer within range of 1:2 to 1:4 are considered as suspected whereas 1:8 may be considered as positive and according to the manufacturer of ELISA kit, 0.2 value considered as a suspected positive value if value was recorded more than 0.6 considered as positive Ewing et al.[10]. Meilinjin et al. [11] conducted the comparative study on newly developed ELISA technique, in which they used nucleoprotein as antigen for detecting the antibod- ies to Avian Influenza Virus. They compared this techni- que with (HI) and ELISA test. Comparative study indi- cated that these two tests had a high agreement ratio and  S. Faraz et al. / HEALTH 2 (2010) 97-100 SciRes Copyright © 2010 Openly accessible at http://www.scirp.org/journal/HEALTH/ 100 no statistically significant difference. Haem-agglutination inhibition test and titration of an- tibodies by geometric mean titer (GMT) is the convenient and best technique to measure the level of protection in vaccinated chickens as well as to check the efficacy of vaccine, such types of results were also reported by Meu- lemans, et al. [12] who carried out a study to run HI, AGP and ELISA for measuring the antibodies against avian influenza virus infection. His studies showed a lin- ear relation among these three techniques, when the chic- kens were exposed with Avian Influenza Virus the anti- body status was measured 157 day post infection. It was suggested that AGP is a type-specific; the HI detects only haemagglutinin subtypes, however only ELISA is most sensitive test to detect the antibodies. The findings of present study showed a degree of cor- relation between HI, AGP, ELISA and virus Neutraliza- tion test, however the degree of accuracy depends on the standardization of reagents, buffers and all other pa- rameters related to the standard procedures of the test. This statement correlates with the availability of equip- ments and expertise in different laboratories as they per- form the same test. That’s why the repeatability and re- producibility are two key features that a test must have to be accepted for different laboratories. REFERENCES [1] Swayne, D.E., Senne, D.A. and Beard, C.W. (1998) Avi- an Influenza in: A laboratory manual for the isolation and identification of avian pathogens, 4, 150-155. [2] Micheal L.P. and David E.S. (2005) Public health risk from Avian Influenza viruses. Avian Dis. 49, 317-327. [3] Swayne, D.F. and Suarez, D.L. (2000) Highly pathogenic avian influenza. Rey. Sci. Tech. Off. Int. Epizoot. 19, 463 -482. [4] Suss, J., Schafer, J., Sinnecker, H. and Webster, R.G. (1994) Influenza virus subtypes in aquatic birds of east- ern Germany. Arch. Virol. 135, 101-114. [5] Suarez, D.L. (2000). Evolution of avian influenza viruses. Vet. Microbiol., 74, 15-27. [6] Naeem, K, and Hussain, M. (1995) An outbreak of avian influenza in poultry in Pakistan Vet. Rec., 137, 439. [7] Naeem, K, Ullah, A., Manvell, R.J. and Alexander, D.J. (1999) Avian influenza subtype H9N2 in poultry in Paki- stan, Vet. Rec., 145, 560. [8] Beard, C.W. (1998) Isolation and identification of avian pathogens. In: Hitchner, S.B., Domermuth, C.H., Pur- chase, H.G. and Williams, J.E. (ed.). 67-69. [9] Terry, S. and Tony, D. (1991) Australian center for inter- national agricultural research (ACIAR). University of Pertanian Malaysia. [10] Ewing, M.L., Kleven S.H. and Brown, M.B. (1994) Comparison of enzyme linked immunosorbent assay (ELISA) and hemagglutination-inhibition for detection of antibody to Mycoplasma gallisepticum in commercial broiler. Avian Diseases, 40, 13-22. [11] Meilinjin, A.B, Wang, G., Zhang, A.R., Zhao, S., Li, H., Tan, Y. and Chen, H. (2004) Development of enzyme- linked immunosorbent assay with nucleoprotein as anti- gen detection of antibodies to avian influenza virus. Lab or animal infectious diseases. [12] Meulemans, M, Carlier, C., Gonza, M. and Petit (1987) Comparasion of hemagglutination inhibition, agar gel precipitation, Enzyme linked Immunosorbent assay for measuring the antibodies against influenza virus in chicken. Avian Dis. 31, 560-563. |

