Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2008, 1,182-189 Published Online November 2008 in SciRes. http://www.srpublishing.org/journal/jbise JBiSE Temperature and Magnetic Resonance Characteristics of Zinc, Manganese, Gadolinium, Gold, Iron Magnetic Nanoparticles and Cytokine Synergy in Hyperthermia Saleh S. Hayek1, Rakesh Sharma1, Soonjo Kwon2, Avdhesh Sharma3, Ching J.Chen1 1College of Engineering, King Faisal University, Al-Hasa, P.O. Box 380, Zip 31982. 2Department of Biological Engineering, Utah State University, Logan, UT 84322. 3Nanotechnology Division, Electrical Engineering Department, MP University of Agriculture and Technology, Udaipur, Rajasthan, India. Correspon- dence should be addressed to Rakesh Sharma (rksz2004@gmail.com) Received June 30, 2008; revised October 18, 2008; accepted October 18, 2008 ABSTRACT The temperature sensitive magnetic resonance dependence for assessing localized heating ef- fect of Manganese (Mn), Zinc (Zn), Gadolin- ium(Gd), Gold(Au) and Iron(Fe) magnetic nanoparticles was compared. These particles showed heating effect when subjected to alter- nating filed. The relationship between tempera- ture and magnetic nanoparticle moment is spe- cific in imaging. The art of imaging temperature in a tumor at various locations is emerging as the selective approach of hyperthermia to moni- tor temperature and treat the tumor. Two un- solved issues are related with tumor tempera- ture rise in the presence of magnetized nanoparticles. First, the relationship of tumor energy changes as a result of cytokine synergy after tumor magnetization. The second issue is linear attenuation after magnetic field exposure with tissue temperature increase due to inflam- mation and lysosomal enzyme action in tumor. In present paper, a new approach of heating tumor is analyzed without spot heating by polymer coated particles at controlled Curie temperature of less than 44oC. The study re- ports a comparison of Mn, Zn, Gd, Fe, Au nanoparticles designed for imaging purpose using chemical co-precipitation technique. The possibility of nanoparticle stimulated tempera- ture treatment (hypothermia effect) is hypothe- sized to recover the tumor metabolic integrity and inflammatory status of tumor cells pre- sumably associated with the depleted intracellu- lar energy (low ATP) and the inflammation (ele- vated cytokines, interleukins) and lysosomal enzymes initially. The multimodal imaging tech- niques were compared using nanoparticles for their sensitivity. The art of the nanoparticle in- duced hyperthermia does have a great impact on public health as alternative therapeutic on- cology. Keywords: hyperthermia, nanoparticles, temperature imaging, cytokine 1. INTRODUCTION Heating tumors by nanoparticles and resistance in hy- poxic tumor cells to a high temperature is emerging as an effective tool in therapeutic oncology [1]. Heating of organs and tissues in cancer treatment was first reported [2]. The introduction of nanoparticles enhanced the diag- nosis and localization of specific tumor characteristics by multimodal imaging techniques including optical, mag- netic resonance, positron emission tomography, com- puted tomography and X-ray techniques. With rapid de- velopment, feasible clinical therapeutic applicators and hyperthermia equipment were designed. In this direction, colloidal gold-thiol preparations were first reported as effective staining agents to label proteins in both diag- nostics such as imaging, blotting, flow cytometry, hy- bridization assays and gold-thiol hyperthermia agents. Other potential hyperthermic particles are silver, iron, zinc and lanthanum nanoparticles [3-10]. At sites of tumor, hyperthermia is a state of therapeu- tic temperature induced recovery of metabolic integrity and enough defense by cytokines and lysosomes to com- pensate the damage perhaps increase in oxygenated blood circulation across tumors due to stimulus of heat. In normal tumor, the cells experience loss of metabolic integrity, hypoxia, inflammation, stimulated lysosomal enzymes due to phagocytosis. As a result tumor cells get overactive and overgrown. The hyperthermia can be pro- duced by the interaction of tumor cells with energy such as alpha-, beta- or gamma radiation (Therapeutic Nuclear Medicine), X-Ray (radiotherapy), ultrasound (Therapeu- tic Ultrasonography) and magnetization (magnetother- apy). However, these interactions are not risk free. Alter- SciRes Co py ri g ht © 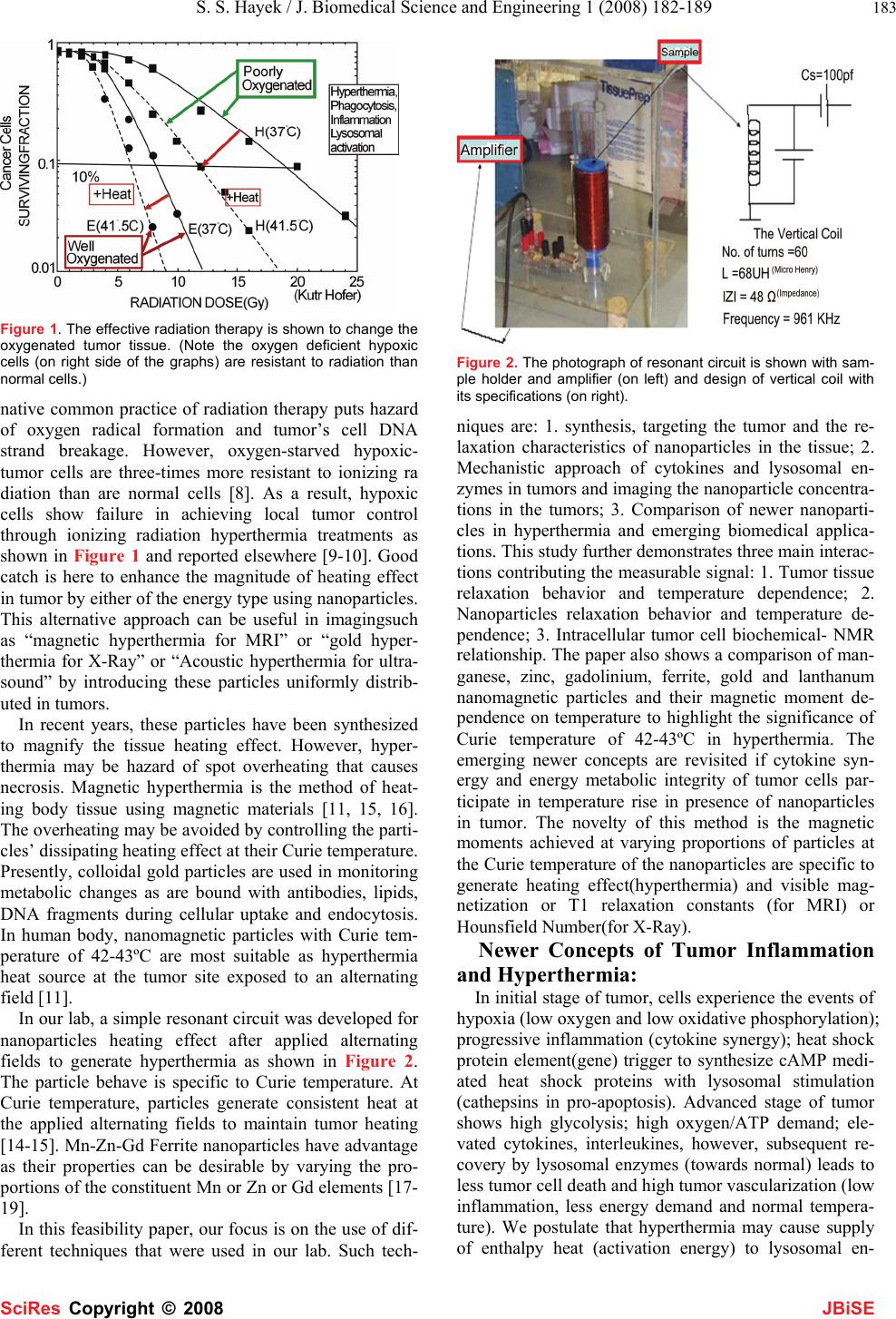 S. S. Hayek / J. Biomedical Science and Engineering 1 (2008) 182-189 183 SciRes Copyright © 2008 JBiSE Figure 1. The effective radiation therapy is shown to change the oxygenated tumor tissue. (Note the oxygen deficient hypoxic cells (on right side of the graphs) are resistant to radiation than normal cells.) native common practice of radiation therapy puts hazard of oxygen radical formation and tumor’s cell DNA strand breakage. However, oxygen-starved hypoxic- tumor cells are three-times more resistant to ionizing ra diation than are normal cells [8]. As a result, hypoxic cells show failure in achieving local tumor control through ionizing radiation hyperthermia treatments as shown in Figure 1 and reported elsewhere [9-10]. Good catch is here to enhance the magnitude of heating effect in tumor by either of the energy type using nanoparticles. This alternative approach can be useful in imagingsuch as “magnetic hyperthermia for MRI” or “gold hyper- thermia for X-Ray” or “Acoustic hyperthermia for ultra- sound” by introducing these particles uniformly distrib- uted in tumors. In recent years, these particles have been synthesized to magnify the tissue heating effect. However, hyper- thermia may be hazard of spot overheating that causes necrosis. Magnetic hyperthermia is the method of heat- ing body tissue using magnetic materials [11, 15, 16]. The overheating may be avoided by controlling the parti- cles’ dissipating heating effect at their Curie temperature. Presently, colloidal gold particles are used in monitoring metabolic changes as are bound with antibodies, lipids, DNA fragments during cellular uptake and endocytosis. In human body, nanomagnetic particles with Curie tem- perature of 42-43ºC are most suitable as hyperthermia heat source at the tumor site exposed to an alternating field [11]. In our lab, a simple resonant circuit was developed for nanoparticles heating effect after applied alternating fields to generate hyperthermia as shown in Figure 2. The particle behave is specific to Curie temperature. At Curie temperature, particles generate consistent heat at the applied alternating fields to maintain tumor heating [14-15]. Mn-Zn-Gd Ferrite nanoparticles have advantage as their properties can be desirable by varying the pro- portions of the constituent Mn or Zn or Gd elements [17- 19]. In this feasibility paper, our focus is on the use of dif- ferent techniques that were used in our lab. Such tech- Figure 2. The photograph of resonant circuit is shown with sam- ple holder and amplifier (on left) and design of vertical coil with its specifications (on right). niques are: 1. synthesis, targeting the tumor and the re- laxation characteristics of nanoparticles in the tissue; 2. Mechanistic approach of cytokines and lysosomal en- zymes in tumors and imaging the nanoparticle concentra- tions in the tumors; 3. Comparison of newer nanoparti- cles in hyperthermia and emerging biomedical applica- tions. This study further demonstrates three main interac- tions contributing the measurable signal: 1. Tumor tissue relaxation behavior and temperature dependence; 2. Nanoparticles relaxation behavior and temperature de- pendence; 3. Intracellular tumor cell biochemical- NMR relationship. The paper also shows a comparison of man- ganese, zinc, gadolinium, ferrite, gold and lanthanum nanomagnetic particles and their magnetic moment de- pendence on temperature to highlight the significance of Curie temperature of 42-43ºC in hyperthermia. The emerging newer concepts are revisited if cytokine syn- ergy and energy metabolic integrity of tumor cells par- ticipate in temperature rise in presence of nanoparticles in tumor. The novelty of this method is the magnetic moments achieved at varying proportions of particles at the Curie temperature of the nanoparticles are specific to generate heating effect(hyperthermia) and visible mag- netization or T1 relaxation constants (for MRI) or Hounsfield Number(for X-Ray). Newer Concepts of Tumor Inflammation and Hyperthermia: In initial stage of tumor, cells experience the events of hypoxia (low oxygen and low oxidative phosphorylation); progressive inflammation (cytokine synergy); heat shock protein element(gene) trigger to synthesize cAMP medi- ated heat shock proteins with lysosomal stimulation (cathepsins in pro-apoptosis). Advanced stage of tumor shows high glycolysis; high oxygen/ATP demand; ele- vated cytokines, interleukines, however, subsequent re- covery by lysosomal enzymes (towards normal) leads to less tumor cell death and high tumor vascularization (low inflammation, less energy demand and normal tempera- ture). We postulate that hyperthermia may cause supply of enthalpy heat (activation energy) to lysosomal en-  184 R. Sharma / J. Biomedical Science and Engineering 1 (2008) 182-189 SciRes Copyright © 2008 JBiSE zymes at normal rate; keeping normal cytokines and hu- moral immunity as most important event. The heating power area under loop enclosed by hys- teresis was calculated by heating power dissipated by particles in magnetic field, H as following: P = f. M.dH (1) where f is frequency of AC magnetic field and M.dH is hysteresis loop area as shown in Figure 3. 2. MATERIALS AND METHODS The batch method of co-precipitation was adopted as previously described by Saleh et al.[12] The particles in the form of complexes were synthesized by chemical co- precipitation method. We present here a model ZnxGdxMn(1-x)Fe3O4 chemical co-precipitation method using different Gd proportions (x) such as Mn0.5Zn0.5GdxFe(2-x)O4. In our lab, the following method was standardized as: (1) 0.1 M solution of the metal salts FeCl3, Fe2SO4, ZnSO4 and GdCl3 (2) Added to an 8 M solution of NaOH. (3) The mixture was stirred vigorously at 90ºC for 40 minutes. (4) The synthesized Zn Gd Fe nanoparticles filtered with Size up to 10 nm. (5) Washed 3 times with distilled water and 3 times with acetone. (6) The particles allowed to dry in nitrogen gas at room temperature. 2.1. Polymer PEG Encapsulation for Nano- spheres A following batch process was developed using above steps for preparing composite particles as described ini- tially elsewhere [12]. Poly ethylene glycol PEG was used to encapsulate Mn.Zn.Fe and Zn.Gd.Fe nanoparticles to improve their biocompatibility. Encapsulation of Mn.Zn.Fe and Zn.Gd.Fe nanoparticles was performed using polymer emulsion method patented by modified solvent evaporation method. The ingredients were used: Polyethylene glycol (PEG) MW: 1,540: 2 gm (polymer), Methylene Chloride: 10 ml, 13.2 gm (solvent), Water: 40 ml (Aqueous medium), Sodium dodecyl sulphate: 0.33 gm (Emulsifying agent), 1-Octanol: 1.1 ml, 1.32 gm (In- hibitor compound) and Mn.Zn.Fe and Zn.Gd.Fe particles: 50 mg. The magnetic particle: polymer ratio was ap- proximately 1:40. The sodium dodecyl sulphate and 1- octanol were dissolved in 40 ml of distilled water using a magnetic stirrer. Later 50 mg of Mn.Zn.Fe / Zn.Gd.Fe was added. The polymer phase was prepared by dissolv- ing 2 gm of PEG into 10 ml of methylene chloride. A crude emulsion was formed by adding the polymer phase to the aqueous medium phase. It was sonicated using an ultrasonicator 5 times in steps of 3 minutes and stirred inside a round bottom flask for 12 hours at 700 rpm. The solvent was then removed using vacuum evaporation method. The polymer encapsulated particles formed were washed with acetone and stored under PBS buffer solu- tion. These Mn-Zn-ferrite particles and Gd substituted Mn- Zn-Ferrite particles were obtained via chemical co- precipitation and ferritization. First the metal salts were co-precipitated into hydroxides. This was done by addi- tion of aqueous solution of metal salts in water to the co precipitating base (e.g. NaOH, CH3NH3OH etc.). For the case of Mn-Zn Ferrite particles the reaction occurs as follows: (1-x)Mn2+ + xZn2+ + 2Fe3+ + 8OH- (1-x)Mn(OH)2. xZn(OH)2.2Fe(OH)3 Soon after, this precipitate was transformed into ferrite by heating it in the precipitation alkaline solution (ferriti- zation). The reaction for Mn-Zn ferrite particles was as follows: (1-x)Mn(OH)2.xZn(OH)2.Fe(OH)3 Mn(1- x)ZnxFe2O4.nH2O + (4-n) H2O 2.2. Measurement of Reaxation Constants in Nanometals and Tumor Tissues The measurement of inverse longitudinal (1/T1) and inverse transverse relaxation (1/T2) constants of nanometals in soplutions and tumor excised tissues was performed on Bruker-Spec 60 desktop model as described elsewhere [15]. Different chemicals FeCl3.6H2O, GdCl3.6H2O, MnCl2.4H2O and ZnSO4.7H2O were used to obtain Fe3+, Gd3+, Mn2+ and Zn2+ions in the aqueous solution. This salt solution at 90°C was added to 8M NaOH solution at 90°C followed by vigorous stirring. The stirring and heating at 90°C was continued for a minimum of 40 mins. It has been previously reported elsewhere that heating for over 40 mins does not produce any significant changes in the particles properties [17-19]. The product was then fil- tered, washed with distilled water and finally washed and dried with acetone. The hysteresis curves were obtained at room tempera- ture using a vibration sample magnetometer (VSM). A Quantum Design SQUID was used to study the tempera- ture dependence of the magnetization [19]. Figure 3. A representative hysteresis loop is shown with different regions of the loop. 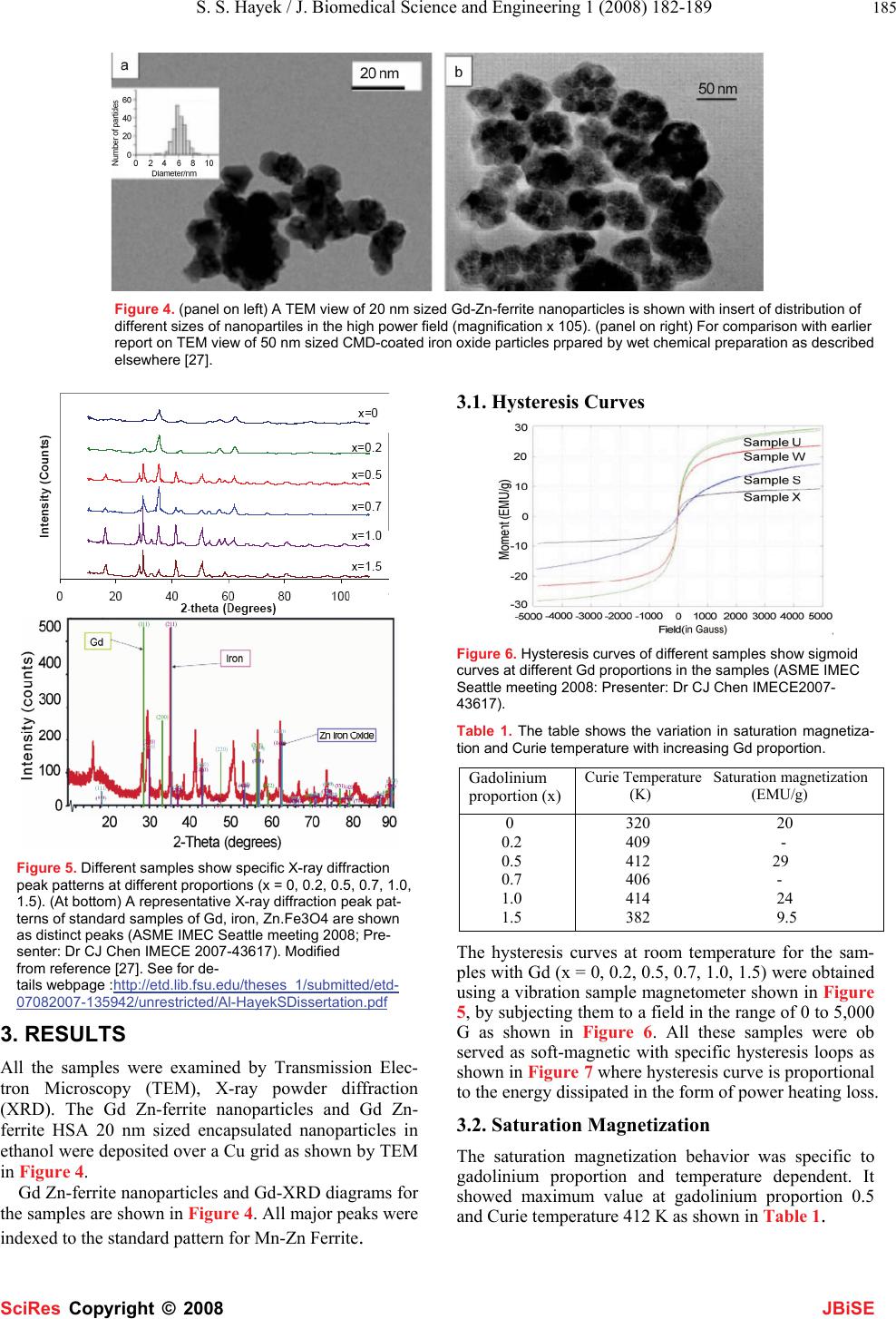 S. S. Hayek / J. Biomedical Science and Engineering 1 (2008) 182-189 185 SciRes Copyright © 2008 JBiSE Figure 4. (panel on left) A TEM view of 20 nm sized Gd-Zn-ferrite nanoparticles is shown with insert of distribution of different sizes of nanopartiles in the high power field (magnification x 105). (panel on right) For comparison with earlier report on TEM view of 50 nm sized CMD-coated iron oxide particles prpared by wet chemical preparation as described elsewhere [27]. Figure 5. Different samples show specific X-ray diffraction peak patterns at different proportions (x = 0, 0.2, 0.5, 0.7, 1.0, 1.5). (At bottom) A representative X-ray diffraction peak pat- terns of standard samples of Gd, iron, Zn.Fe3O4 are shown as distinct peaks (ASME IMEC Seattle meeting 2008; Pre- senter: Dr CJ Chen IMECE 2007-43617). Modified from reference [27]. See for de- tails webpage :http://etd.lib.fsu.edu/theses_1/submitted/etd- 07082007-135942/unrestricted/Al-HayekSDissertation.pdf 3. RESULTS All the samples were examined by Transmission Elec- tron Microscopy (TEM), X-ray powder diffraction (XRD). The Gd Zn-ferrite nanoparticles and Gd Zn- ferrite HSA 20 nm sized encapsulated nanoparticles in ethanol were deposited over a Cu grid as shown by TEM in Figure 4. Gd Zn-ferrite nanoparticles and Gd-XRD diagrams for the samples are shown in Figure 4. All major peaks were indexed to the standard pattern for Mn-Zn Ferrite. 3.1. Hysteresis Curves Figure 6. Hysteresis curves of different samples show sigmoid curves at different Gd proportions in the samples (ASME IMEC Seattle meeting 2008: Presenter: Dr CJ Chen IMECE2007- 43617). Table 1. The table shows the variation in saturation magnetiza- tion and Curie temperature with increasing Gd proportion. Gadolinium proportion (x) Curie Temperature Saturation magnetization (K) (EMU/g) 0 0.2 0.5 0.7 1.0 1.5 320 20 409 - 412 29 406 - 414 24 382 9.5 The hysteresis curves at room temperature for the sam- ples with Gd (x = 0, 0.2, 0.5, 0.7, 1.0, 1.5) were obtained using a vibration sample magnetometer shown in Figure 5, by subjecting them to a field in the range of 0 to 5,000 G as shown in Figure 6. All these samples were ob served as soft-magnetic with specific hysteresis loops as shown in Figure 7 where hysteresis curve is proportional to the energy dissipated in the form of power heating loss. 3.2. Saturation Magnetization The saturation magnetization behavior was specific to gadolinium proportion and temperature dependent. It showed maximum value at gadolinium proportion 0.5 and Curie temperature 412 K as shown in Table 1. 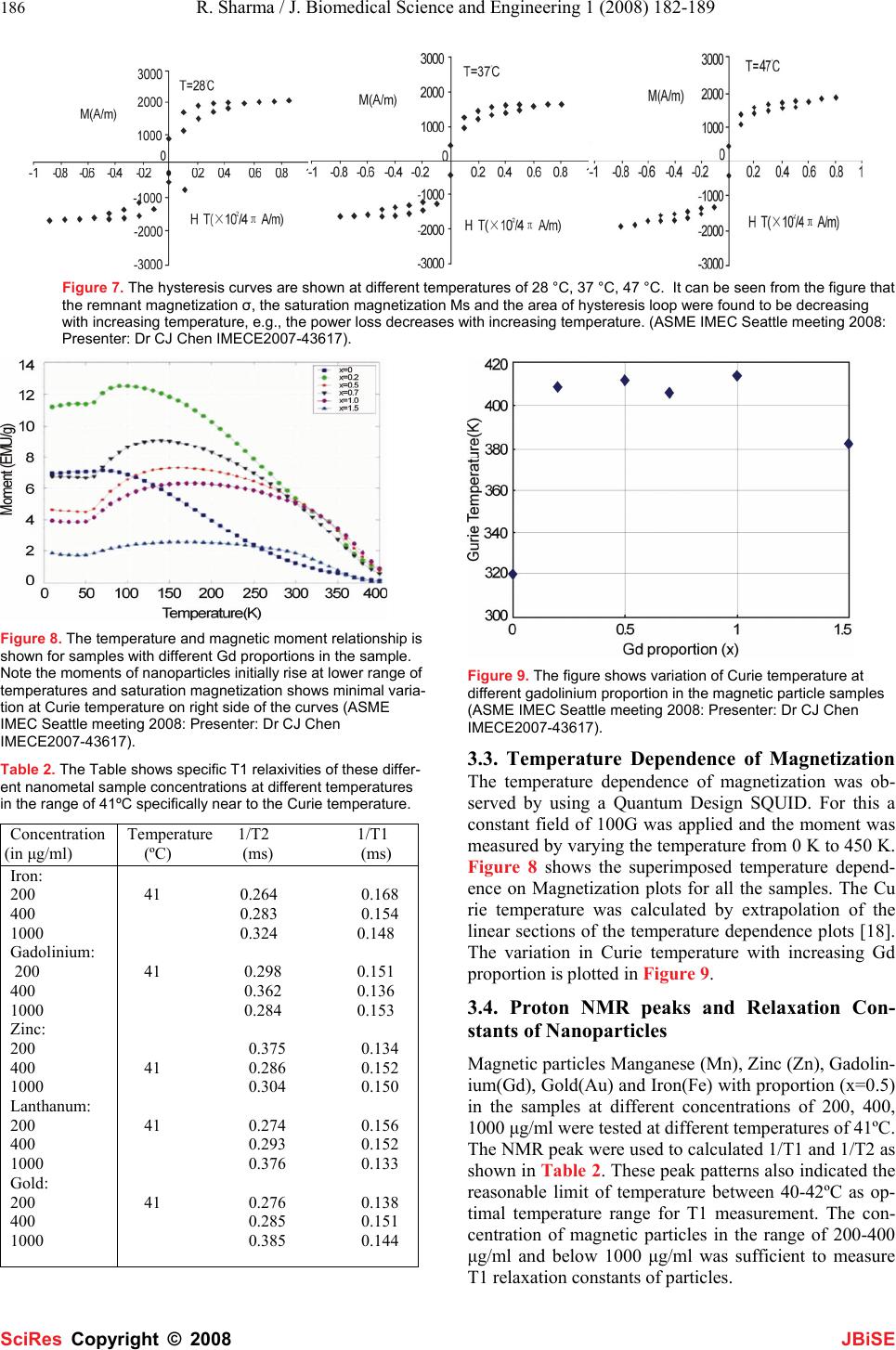 186 R. Sharma / J. Biomedical Science and Engineering 1 (2008) 182-189 SciRes Copyright © 2008 JBiSE Figure 7. The hysteresis curves are shown at different temperatures of 28 °C, 37 °C, 47 °C. It can be seen from the figure that the remnant magnetization σ, the saturation magnetization Ms and the area of hysteresis loop were found to be decreasing with increasing temperature, e.g., the power loss decreases with increasing temperature. (ASME IMEC Seattle meeting 2008: Presenter: Dr CJ Chen IMECE2007-43617). Figure 8. The temperature and magnetic moment relationship is shown for samples with different Gd proportions in the sample. Note the moments of nanoparticles initially rise at lower range of temperatures and saturation magnetization shows minimal varia- tion at Curie temperature on right side of the curves (ASME IMEC Seattle meeting 2008: Presenter: Dr CJ Chen IMECE2007-43617). Table 2. The Table shows specific T1 relaxivities of these differ- ent nanometal sample concentrations at different temperatures in the range of 41ºC specifically near to the Curie temperature. Concentration (in μg/ml) Temperature 1/T2 1/T1 (ºC) (ms) (ms) Iron: 200 400 1000 Gadolinium: 200 400 1000 Zinc: 200 400 1000 Lanthanum: 200 400 1000 Gold: 200 400 1000 41 0.264 0.168 0.283 0.154 0.324 0.148 41 0.298 0.151 0.362 0.136 0.284 0.153 0.375 0.134 41 0.286 0.152 0.304 0.150 41 0.274 0.156 0.293 0.152 0.376 0.133 41 0.276 0.138 0.285 0.151 0.385 0.144 Figure 9. The figure shows variation of Curie temperature at different gadolinium proportion in the magnetic particle samples (ASME IMEC Seattle meeting 2008: Presenter: Dr CJ Chen IMECE2007-43617). 3.3. Temperature Dependence of Magnetization The temperature dependence of magnetization was ob- served by using a Quantum Design SQUID. For this a constant field of 100G was applied and the moment was measured by varying the temperature from 0 K to 450 K. Figure 8 shows the superimposed temperature depend- ence on Magnetization plots for all the samples. The Cu rie temperature was calculated by extrapolation of the linear sections of the temperature dependence plots [18]. The variation in Curie temperature with increasing Gd proportion is plotted in Figure 9. 3.4. Proton NMR peaks and Relaxation Con- stants of Nanoparticles Magnetic particles Manganese (Mn), Zinc (Zn), Gadolin- ium(Gd), Gold(Au) and Iron(Fe) with proportion (x=0.5) in the samples at different concentrations of 200, 400, 1000 μg/ml were tested at different temperatures of 41ºC. The NMR peak were used to calculated 1/T1 and 1/T2 as shown in Table 2. These peak patterns also indicated the reasonable limit of temperature between 40-42ºC as op- timal temperature range for T1 measurement. The con- centration of magnetic particles in the range of 200-400 μg/ml and below 1000 μg/ml was sufficient to measure T1 relaxation constants of particles.  S. S. Hayek / J. Biomedical Science and Engineering 1 (2008) 182-189 187 SciRes Copyright © 2008 JBiSE Table 3. Emerging thermal mapping hybrid applications of ther- mal mapping and hyperthermia monitoring. Different techniques of temperature mapping with hyperthermia monitoring are shown with asterisk for their routine use *; research use **; and in in- fancy status*** in use. _______________________________________ Hybrid Modality of thermal Physical property Mapping with hyperthermia for thermal mapping Unit attached _______________________________________ Ultrasound-MR* Echo and moment X-ray and MRI* Attenuation/moment Optical and MRI** Ligand and moment PET and CT** SUV and attenuation PET and MRI** SUV and moment Optical/Molecular imaging*** Ligand specificity _______________________________________ 4. DISCUSSION In this study, Gd, Mn, Zn, ferrites with various Gd pro- portions were analyzed to study the effect on the mag- netic properties of these particles and also to find a com- bination which will result in particles having a Curie temperature suitable for hyperthermia application. TEM suggested the size of these particles in the range of 20 nm and polymer PEG encapsulated nanospheres were measured in the range of 50-70 nm. The particles were distinct by XRD peak patterns of zinc, Gd and iron oxide suggesting their distinct identity after PEG encapsulation in the nanospheres. In the following section different debated views are displayed for tumor temperature rise by magnetic nanoparticles Mn, Zn, Gd, Au, La, Fe us- able by multimodal imaging techniques. A simplistic approach of tumor cell metabolic integrity loss associated with ATP loss, energy depletion, hypoxia leading to inflammation by lysosomal enzyme stimula- tion and cytokine synergy was presumed as pro- inflammatory cytokines and nitric oxide (NO) responsi- ble for tumor cell killing. Under the condition of hyper- thermia, the expression of pro-inflammatory cytokine (e.g. IL-6) and inhibitory (anti-inflammatory) cyto- kine(IL-10) were regulated. These temperature- dependent changes in the expression of IL-10 may imply an important clinical marker for hyperthermia-related tumor cell killing [28]. However, the mechanistic rela- tion among temperature rise in tumor cells, energy deple- tion, and inflammation is still unclear. Heat shock treat- ment, inducing heat shock protein synthesis, also af- fected the regulation of cytosolic I-κB and translocation of NF-κB into the nucleus [29]. The hysteresis curves showed sigmoid behavior of these nanoparticles and its magnitude dependent on tem- perature rise till temperature of particles reaches Curie temperature during AC vibrating resonator application. It suggests the hysteresis as major contributor at peculiar temperature end point so called “Curie temperature” in heating up to 70% of total heat as described in said sec- tion “Heat calculation of hyperthermia”. The similar be- havior was reported earlier without much information Table 4. Potential newer nanoparticles used in thermal map- ping and hyperthermia monitoring. Different nanoparticle composites are shown with their use in thermal mapping technique and possible use in hyperthermia monitoring. The potential nanoparticles in hyperthermia use are shown with + plus sign for routine use +++, research use ++, infancy state + or not established – at present. ______________________________________ Nanoparticles Thermal mapping Hyperthermia ___________________________________________________ Zinc-Gadolinium-Ferrite MRI, CT + Gold-Ferrite MRI, CT ++ Gadolinium-Ferrite MRI, CT +++ Lanthanum-Ferrite MRI + Calcium Optical, Molecular + Gd-Mn-Zn-Ferrite MRI, CT +++ Mn-Gd-Ce MRI -- La-Sr-Mn MRI -- Nanoparticulate agents MRI -- ____________________________________________ of contribution in heating effect of tumors [15]. It was observed that the saturation magnetization of the particles drop with increasing Gd proportion. The initial increase in the saturation magnetization can be explained by considering that the Gd3+ ions have a large spin magnetic moment per atom (7µB) as compared to that of Fe3+ ion (5 µB) [20-23]. Addition of Gd3+ ions results in their occupancy of the octahedral sites. The preference for octahedral sites maybe attributed to their large ionic radii. Since the ionic radii of the Gd3+ ions are large, there is a decrease in the distance between these and the oxygen ions when adding Gd ions [24]. As a result the ions at the octahedral sites no longer have their moments parallel to each other. A part of these ions have moments aligned antiparallel to the other atoms on these octahedral sites. This results in a reduction in the net magnetic moment of the octahe- dral atoms. As the Gd substitution is increased, more and more octahedral atoms have their moments as antiparal- lel. As a result the saturation magnetization drops. Upadhyay et al. [23] have synthesized Gd substituted Mn-Zn Ferrite nanoparticles using chemical co- precipitation. They observed an increase in the pyromag- netic co-efficient (HTM)/ (∂∂) of the resultant particles. The increase in the pyromagnetic co-efficient is desirable because it results in a steeper slope of the magnetization v/s temperature plot which in turn ensures that the mag- netization decreases rapidly as the temperature ap- proaches the Curie temperature. This rapid decrease in magnetization means that the particles are heated up faster at temperatures below the Curie temperature and suddenly stop being heated near the Curie temperature which is a desirable property for Hyperthermia applica- tion. From Fig. 5 it can be seen that there is an increase in Curie temperature with Gd substitution. Magnetic nanoparticles have found utility in many biological applications, including imaging, cancer ther- apy, drug delivery, sensing and hyperthermia for tumor therapy. In general, hyperthermia raises the tissue tem- perature between 41.5 - 46 degrees Celsius to kill can-  188 R. Sharma / J. Biomedical Science and Engineering 1 (2008) 182-189 SciRes Copyright © 2008 JBiSE cerous cells while preserving the normal cells. Several nanoparticles such as gold, zinc, gadolinium, lanthanum, and calcium have emerged as potential hyperthermia agents. Recently, new composite materials such as Mn- Zn-Fe, Co-Gd-Zn and Zn-Gd-Fe nanoparticles with sta- ble magnetic behavior have replaced magnetic oxides for use in hyperthermia at our lab. These composites gener- ated sufficient heat at room temperature and stop heating at the Curie temperature Tc of the respective nanoparticle system. Gold nanoparticles (AuNP) killing the cancer cells was first reported by Hainfeld [98]. However, after in- jecting gold particles in animals and irradiation them by 250 kV, X-rays caused tumor shrinkage and enhanced survival rate by four fold. Th e major challenge was lo- calization of gold particles because of vascular leakage in the tumor but maximized particles entry in the tumor. Still, there are ample potential evidences in favor of gold enhanced x-ray hyperthermia in tumor treatment by kill- ing. The application of gold particles in nanomedicine is its promise in radiotherapy of cancer. The Au-198 (βmax = 0.96 MeV; t/2 = 2.7 days) and Au-199 (βmax = 0.46 MeV; t/2 = 3.14 days) make them suitable in radiother- apy. In addition, gold particles display gamma emissions for dosimetry and pharmacokinetic studies. Therapeutic agents derived from gold particles provide a higher ra- dioactivity dose to tumor sites. Furthermore, tumor- specific nanotherapeutic agents as a nanoparticle while tagged with peptides selective to receptors and over- expressed by tumor concentration offer another advan- tage. The gold nanoradioisotopes encapsulated within a nanocomposite device were reported as vehicles to trans- port radioactive particles to tumor sites. In this approach, particle size and number play a significant role such as nanocomposites made of monodisperse hybrid radioac- tive gold nanoparticles immobilized by dendritic poly- amidoamine matrix prepared by reaction of polymer and tetrachloroaurate HAuCl4 solution. The salt formation between these solutions ensured the effective encapsula- tion of gold within the matrix using neutron irradiation in mice B 16 melanoma, prostate DU 145, human KB squamous cell carcinoma xenograft models. The property of polymer with β emitting Au-198 enriched nano-device proved useful in tumor therapy. Moreover, the polymer enhances the stealthiness of magnetic nanoparticles by preventing macrophage recognition of particles as less toxic and resisting oxidation to make them valuable in multifunctional hyperthermia and imaging modalities. Another issue in tumor treatment is delivery of chemo-, gene-, radiotherapeutic agents within gold nanoparticles. It becomes effective as a tumor killing and targeted de- livery tool. These multicoponent particles are made of ZnxMn (1- x)Fe3O4 and MnxZnxGdxFe(2-x)O4 composites synthesized by physical and chemical co-precipitation methods. These particles displayed the increased tissue tempera- ture and hyperthermia nature. Additionally particles in the form of Ni(1-x)Crx were also formed. These particles may be encapsulated in thermo-sensitive polymer that dissolves when melted. The magnetic Mn-Zn ferrite and Gd substituted Mn-Zn ferrite particles synthesized by the chemical co-precipitation method exhibited a specific behavior applicable in hyperthermia. Paramagnetic gadolinium offers the excellent detec- tion limit using contrast injection of 15 gm/gm tissue in clinical imaging. In our lab, these particles were encap- sulated in thermosensitive polymer that dissolves after melting. These nanosized particles exhibited specific behavior of magnetic moments at Curie temperature and provide a window to evaluate their heating effect in tis- sue. Addition of Gd3+ ions up to proportions of x=0.5 results in an increase in the net moment. Further addition of the Gd3+ ions results in a decrease in the net moment or saturation magnetization (SM). To prove it, the nanoparticle complex Mn-Gd-Ce was characterized by placing particles in a tube and its temperature increase with time as shown in Figure 9. The SM increases as temperature rises close to Curie temperature initially and subsequently SM decreases at temperature higher than the Curie temperature at increasing proportion of Gado- linium. Recently, nanosize Gd substituted Mn-Zn ferrite particles have been synthesized by a chemical coprecipi- tation method. These particles were mostly soft-magnetic. Gd substituted Mn-Zn Ferrite nanoparticles using chemi- cal co-precipitation demonstrate an increase in the pyro- magnetic co-efficient (HTM)/(∂∂) of the resultant parti- cles. The increase in the pyromagnetic coefficient is de- sirable because it results in a steeper slope of the mag- netization v/s temperature plot which in turn ensures that the magnetization decreases rapidly as the temperature approaches the Curie temperature. The Silica-Coated Lanthanum-Strontium Manganite Particles were prepared suited for hyperthermia. The core-comprising LaSr–manganites with different stoichiometries, ranging from La0.5Sr0.5MnO3+δ to LaMnO3+δ, were synthesized as silica-coated magnetic particles with designable Curie temperature, offering a wide range of possibilities of adapting the material to practical instrumental setups in drug delivery and hyperthermia treatments. The relationship was based on temperature dependence of the proton chemical shift of water < 0.01 ppm/ºC at < 0.7 Hz at 1.5T for 1 ºC tem- perature change using lanthanide complexes. Malignant Hyperthermia (MH) is a hypermetabolic syndrome that results from the altered control of sar- coplasmic reticulum (SR) Ca2+ release. Recent study es- tablished the imaging of cytosolic [Ca2+] ([Ca2+]c) in sin- gle cells grown from human skeletal muscle biopsies and in H9c2 myotubes in response to a low dose of halothane on the cells derived from MH susceptible patients. The Ca2+ imaging in single cells is a promising candidate for the development of a new diagnostic and hyperthermia procedure of MH. Recent improvements in optical imaging techniques now allow these microdomains to be visualized as single channel calcium fluorescence transients (SCCaFTs),  S. S. Hayek / J. Biomedical Science and Engineering 1 (2008) 182-189 189 SciRes Copyright © 2008 JBiSE providing information about channel properties to moni- tor the activity and localization of microdomain calcium complexes. Other emerging possibilities are MR molecu- lar imaging by gadolinium based perfluorocarbons, in- tegrins, fibrins, and monoclonal antibody labeled nanoparticles. Recently remarkable progress is reported in real-time monitoring of thermal therapy and ablation using multimodal imaging techniques. The emergence of a newer class of nanoparticles as contrast agents have created the desire of localized tumor molecular imaging based monitoring of tumor hyper- thermia and molecular mapping responsible for thermal heating of a tumor as represented in Table 4. However, multimodal and multifunctional approaches of hyper- thermia monitoring and thermal mapping are still in their infancy to use them in routine. The present growing in- terest of thermal mapping and hyperthermia monitoring is to achieve a rapid thermotherapy heating effect over focused tumor areas accurately by molecular imaging techniques. Regional hyperthermia in combination with chemo- therapy or/and radiotherapy has become state of the art as effective hyperthermia for locally advanced deep- seated tumors as shown in Table 3. The thermometry using 3-dimensional US-MR-hyperthermia hybrid- system was installed and tested in phantoms and under clinical conditions in patients. The simultaneous MR- imaging and T1-relaxometry at 0.2 Tesla during RF heat- ing was performed using T1 sensitive pulse sequences to serve a basis for non-invasive MR-thermometry. The subtraction of T1 parameter maps before and during heating visualized the changes in T1. The patterns of T1 relaxation changes during hyperthermia treatment may prove to be useful for spatially resolved thermometry and thus help improve the hyperthermia therapy. 5. CONCLUSION Nanosize Mn-Zn ferrite and Gd substituted Mn-Zn ferrite particles have been synthesized by chemical co- precipitation method. The samples are observed to be soft-magnetic. Addition of Gd3+ ions up to proportions of x=0.5 results in an increase in the net moment. Further addition of the Gd3+ ions results in a decrease in the net moment. The saturation magnetization increases then decreases with increasing proportion of Gadolinium. The Curie temperature increases with addition of Gadolinium. But addition of Gd in proportions more than x=1.0 re- sults in a decrease in Curie temperature. Mn0.5.Zn0.5.Ferrite ferromagnetic nanomagneticparticles showed sharp ferromagnetic to paramagnetic transition behavior at specific temperature. ACKNOWLEDGEMENTS The project was partially funded by a grant from the Florida State Uni- versity Research Foundation to the Center of Nanomagnetics and Bio- technology. Tallahassee, FL. Authors are grateful for assistance of Virendra Mohite and Dr Saleh Hayek for their graduate work in this grant. The data was partly presented by Dr Chen CJ at ASME IMEC Seattle meeting 2008 IMECE2007-43617NSTI meeting 2007. REFERENCES [1] W. Busch, (1966), Rheinisch-Westfael. Akad. Nat. Ing. Wirtschaftswiss Vortr. 23, 28. [2] W. B. Coley, (1993) Am. J. Med. Sci. 105 488. [3] G. Crile, (1963) Cancer Res. 23, 372. [4] R. Cavaliere, E. C. Ciocatta, B. C Giovanella, C. Heidelberger, (1967) R. D. et al. Cancer 20, 1351. [5] J. W. Hand and G. ter Haar, (1981) Br. J. Radiol. 54, 443. [6] D. A. Christensen and C. H. Durney, J. Microwave Power 16 (1981) 89). [7] J. W. Strohbehn and E. B. Douple, (1984) IEEE Trans. Biomed. Eng. BME-31, 779. [8] C. C. Vernon, J. W. Hand, S. B. Field, et al. (1996) Int. J. Rad. Oncol. Biol. Phys. 35, 731-744. [9] J. Van der Zee, (2002) Annals Oncology 13, 1173-1184. [10] G. Multhoff, C. Botzler, M. Wiesnet, E. Muller, T. Meier, (1995) W. Wilmanns and R. D. Issle , Int. J.Cancer 61, 272-279. [11] B. Park, B. S. Koo, Y. K. Kim, M. K. Kim, (2002) Korean J Radiol, 3, 98-104. [12] S. Hayek. Chapter 5. In:Ph.D thesis submitted to Florida State University, Tallahassee. [13] R. Sharma. Chapter 4. In: Ph.D thesis submitted to Indian Institute of Technology, New Delhi. [14] S. Deger, D. Boehmer, I. Turk, J. Roigas, V. Budach, S. Loening, (2002) European Urology, 42, 147-153. [15] A. Jordan, R. Scholz, P. Wust, H. Fahling. R. Felix, (1999) J Mag Mag Matr, 201, 413-419. [16] N. Brusentsov, L. Nikitin, T. Brusentsova, A. Kuznetsov, F. Bayburtskiy, L.Schumakov, N.Jurchenko, (2002) J. Mag. Mag. Matr., 252, 378-380. [17] E. Auzans, D. Zins, M. M. Maiorov, E. Blums, R. Massart, (1999) Magn. Gidrodinamika 38, 78-86. [18] E. Auzans, D. Zins, E. Blums, R. Massart, (1999) J. Mater. Sci. 34, 1253 – 1260. [19] E. Auzans, (1999) Mn-Zn ferrite nanoparticles for water- and hydrocarbone-based ferrofluids:preparation and properties, The- sis. [20] C. Kittel, Intro. To Solid State Physics, John Wiley & Sons, NY, 4th Ed. [21] G. Burns, (1985) Solid State Physics, Academic Press Inc. [22] K. Schroder, (1978) Elec., Mag. And Thermal Properties of Solid Mat., Marcel Dekker Inc. NY. [23] R. Kubo, T. Nagamiya, (1969) Solid State Physics, McGraw Hill. [24] J. Smit And H. P. J. Wijn, Ferrites, John Wiley & Sons, NY, 1959, p 139-142. [25] R. V. Upadhyay, R.V. Mehta, K. Parekh, D. Shrinivas, R.P. Pant, (1999) J. Magn. Magn. Mater. 201,129 – 132. [26] F. Settecase, M.S. Sussman, (2007) TPL Roberts, Contrast Media Mol Imaging, 2,50-54. [27] R Hergt,S Dutz,R Muller,M Zeisberger.(2006) J Phys Cond Mat.18:S2919-S2934. [28] T. Matsui, T. Kakeda, (2008.)J Neurotrauma.25(6):709-15. [29] H. W. Chen, H. T. Kuo, S. J. Wang, T.S. Lu, R.C. Yang, (2005) Shock. 24(3):232-8. |

