Journal of Cancer Therapy
Vol.06 No.02(2015), Article ID:54033,8 pages
10.4236/jct.2015.62023
How Safe Are Reduced Doses per Fraction in Target Volumes of 2nd to 4th Order in the Simultaneous Integrated Boost Irradiation Technique in Head and Neck Carcinoma Patients?
A. Buchali1*, C. Schroeder1,2, C. Boerrnert1, I. Maekelburg1,
1Department of Radiooncology, Ruppiner Kliniken GmbH, Neuruppin, Germany
2Justus Liebig University, Giessen, Germany
3Department of Otolaryngology, Ruppiner Kliniken GmbH, Neuruppin, Germany
Email: *a.buchali@ruppiner-kliniken.de
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 19 January 2015; accepted 11 February 2015; published 12 February 2015
ABSTRACT
Aim: The simultaneous irradiation of target volumes of different total dose levels using intensity modulated radiotherapy leads to reduced doses per fraction and longer treatment times in target volumes of 2nd to 4th order. Does the thereby caused reduced biological effectiveness induce an increased recurrence risk? The current work deals with the problem of recurrences of patients with head and neck carcinomas treated either with an intensitiy (IMRT) or with a volumetric modulated (VMAT) irradiation technique. Methods: From October 2002 to September 2014, 699 patients with carcinomas of the head and neck were irradiated using IMRT or VMAT. The median follow up of the patients was 21.9 months (2 to 145 months). Primary tumor regions (1st order target volume) of 565 patients were treated with doses per fraction of 2 Gy. Accordingly, further 133 target volumes of the primary tumor received reduced doses per fraction. In 1 patient, the lymphatic drainage was treated solely without irradiation of the primary region. For the lympatic drainage, 854 1st order target volumes were treated with a dose per fraction of 2 Gy. Reduced doses per fraction were applied to further 1780 target volumes. Results: 54 of 699 patients developed a recurrence in the primary tumor region after radio-(chemo) therapy, 4 patients developed a recurrence of the primary tumor and a unilateral recurrence of the lymphatic drainage, 2 patients a recurrence of the primary tumor and a bilateral lymph node recurrence. 18 patients showed an isolated unilateral recurrence and additionally 2 patients a bilateral recurrence of the lymphatic drainage. 619 patients stayed recurrence free. In primary tumor regions, receiving a dose per fraction of 2 Gy 55 patients (9.7%) developed a recurrence, whereas in target volumes receiving a reduced dose per fraction 5 patients (3.8%) developed a recurrence (p < 0.001). In lympatic drainage target volumes receiving a dose per fraction of 2 Gy, 25 target volumes (2.9%) developed a recurrence, whereas in target volumes receiving a reduced dose per fraction, 5 patients (0.3%) developed a recurrence (p = 0.001). Conclusion: The recurrence risk in target volumes of 2nd to 4th order was not increased due to reduced doses per fraction deposited by means of a simultaneous integrated boost technique. Therefore, the simultaneous irradiation of target volumes with different dose levels is safely applicable within one treatment plan.
Keywords:
Head and Neck Carcinoma, Simultaneous Integrated Boost Technique, Dose Painting, Dose per Fraction, Recurrence Risk

1. Introduction
Irradiating patients with head and neck carcinomas, it is necessary to treat target volumes with different total doses, depending on the presence of macroscopic or microscopic tumor manifestations. Using a conventional 3D-dimensional radiation technique, usually, a new treatment plan with a reduced target volume is calculated after reaching a certain dose level. Thereby, all target volumes are irradiated with the same single dose.
The intensity modulated (IMRT) as well as the volumetric modulated arc radiotherapy (VMAT) allows to treat several dose levels in one treatment plan. As a consequence, different target volumes are irradiated with different doses per fraction. Therefore, the biological efficacy varies compared to a conventional fractionated radiotherapy due to reduced doses per fraction and increased treatment times. This is discussed controversially, especially in fast growing tumors, like squamous cell carcinomas.
With the introduction of the IMRT in October 2002, all patients with head and neck carcinomas got simultaneous integrated boost irradiation techniques. Since June 2011, the VMAT was additionally introduced as an alter- native treatment technique for patients with head and neck carcinomas, but without changing the treatment strategy. Preventing late side effects, the dose per fraction was limited to 2 Gy for the target volume with the highest total dose (1st order target volume). Target volumes of 2nd to 4th order received lower doses per fraction, respectively.
The frequency of recurrences has been analysed to survey the impact of the reduced biological efficacy following the radiation schedule of target volumes of 2nd or 4th order due to lower doses per fraction and increased treatment times. Finally, it is used as an indicator to estimate the harmlessness of a simultaneous irradiation of different target volumes with varying dose levels.
2. Material and Methods
From October 2002 to September 2014, 699 patients (130 female, 569 male) with head-neck squamous cell carcinomas were treated using IMRT or VMAT. The mean age at the start of the treatment was 58.2 (30.5 - 84.2) years. The median follow-up of the patients was 21.9 months (2 to 145 months). The patient characteristics are shown in Table 1.
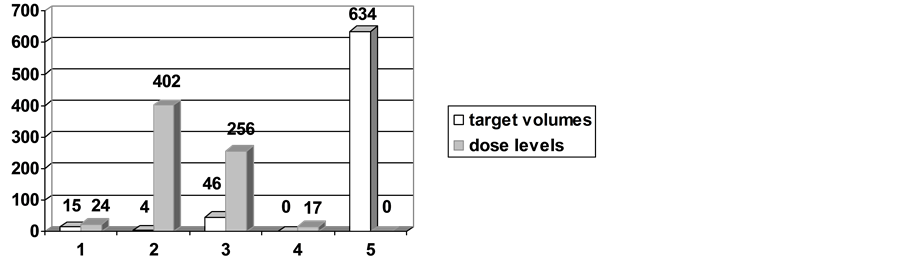
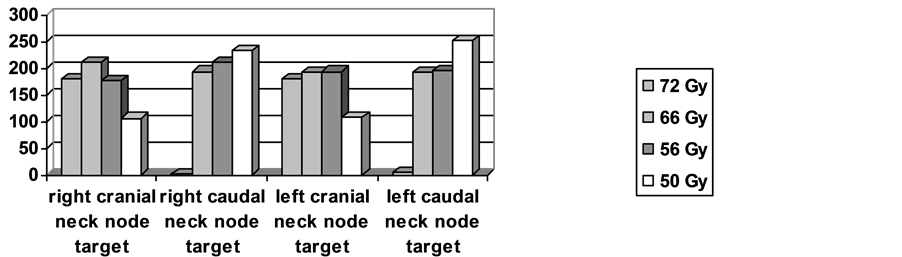
The treatment plan of each patient contains from one up to five target volumes, one target volume for the primary tumor and a maximum of 4 target volumes for the lymphatic drainage. The irradiation of the lymph node area was divided in a caudal and a cranial part. The caudal part included Level IIB, Level III, the cranial Levels V and VI, as well as Level I or IIA, if indicated. The caudal part consisted of the caudal Levels V and VI in addition of the supraclavicular node area. A typical example of a treatment plan is demonstrated in Figure 1. 20 patients received a unilateral treatment of the lymphatic drainage and in 15 patients, the primary tumor region was treated without and lyphatic dranage. Eventually, an exclusive irradiation of the cranial part of the neck node area was delivered in additionally 29 patients. The number of target volumes per patient is shown in Figure 2 and the number of lymphatic drainage volumes is shown in Figure 3.
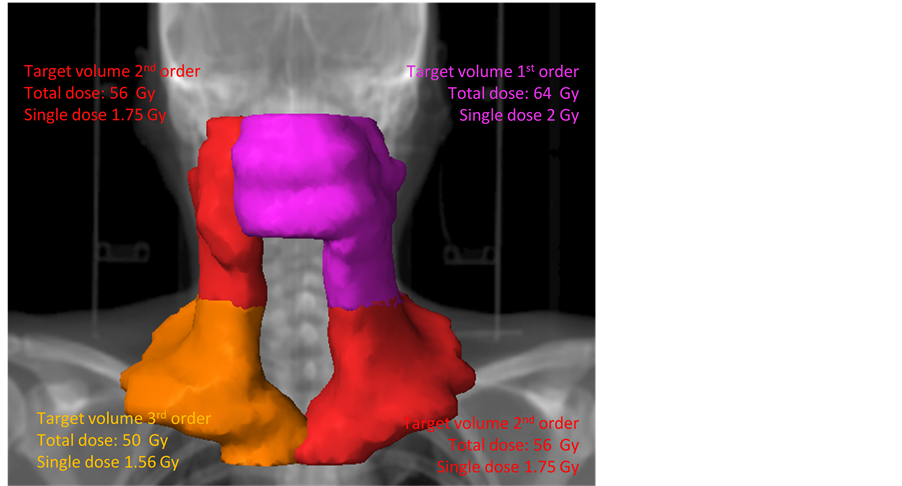
Figure 1. Treatment plan of a patient. Diagnosis: Carcinoma of the oropharynx pT4. pN2c: right: 3/15 no extracapsular spread; left: 7/17 extracapsular spread. Resection: Resection of the primary: R1, bilateral neck dissection. Ra- diochemotherapy: Target volume 1: 64 Gy: primary tumor extension and, cra- nial left neck node area, PTV: purple. Target volume 2: 56 Gy: cranial right neck node area, and caudal left neck node area, PTV: red. Target volume 3: 50 Gy: caudal right neck node area, PTV: orange.
Figure 2. Number of targets volumes and dose levels per patient (n = 699).
Figure 3. Number of the volumes of the neck node areas per dose level. The cranial part included Level IIB, Level III, the cranial Levels V and VI, as well as Level I or IIA if indicated. The caudal part included caudal Levels V and VI and the supraclavicular node area.
Table 1. Characteristics of patients (n = 699).
The treatment was accomplished by means of four groups of total dose levels. Within the first group a dose of 72 Gy was disposed to macroscopic tumor volumes, either for a definitive treatment of the primary tumor, the tumor bed after R2-resection, or for an identifiable tumor mass within the treatment planning CT (n = 230 target volumes). In case of macroscopic lymph node metastases, lymph nodes received total doses of 72 Gy as well (n = 365 target volumes). A total dose of 66 Gy was applied for patients of the second group characterized by target volumes of microscopic tumor sites, i.e. to treat the primary tumor bed after R1-resection (n = 218 target volumes), the postoperative lymphatic drainage in case of extracapsular infiltration, or 1st level of clinical non involved lymph node areas within a definitive treatment course (n = 786 target volumes). Accordingly reduced total doses were exhibited to the primary tumor bed after R0-resection. In those cases (n = 208 target volumes) as well as for a postoperative treatment of the neck node area (if there was no extracapsular spread, or if the second lymphatic drainage was not involved, n = 784), a total dose of 56 Gy was irradiated. Finally, total doses of 50 Gy were applied to the naso-, oro- and hypopharynx in patients with a CUP syndrome (n = 42 target volumes) or to the lymph node level in a pN0 situation (n = 699 target volumes). The number of dose levels per patient is also shown in Figure 2.
Clinical target volumes (CTV) and planning target volumens (PTV) were obtained by surrounding gross tumor volumens (GTV) and CTV’s, respectively, each with a margin of 6 mm in all directions. Treatment plans were calculated to a single dose of 2 Gy for the target volume with the highest total dose according to the criteria of the ICRU. The target volumes of 2nd to 4th order were also calculated according the homogeneity criteria of the ICRU, excluding a margin of 5 mm to the PTV of higher order, to achieve the dose criteria in the PTV’s of higher order.
Descriptive statistics were performed using MS Excel (Microsoft Corp. Redmond WA). For further analysis using Chi Square and Man & Whitney U-test, SPSS for Windows (SPSS Inc., Chicago IL) was used.
3. Results
After radio-(chemo-) therapy 54 of 699 patients developed a recurrence in the primary tumor region, 4 patients developed a recurrence of the primary tumor and a unilateral recurrence of the lymphatic drainage, 2 patients a recurrence of the primary tumor and a bilateral lymph node recurrence. 18 patients showed an isolated unilateral recurrence and additionally 2 patients a bilateral recurrence of the lymphatic drainage without a recurrence of the primary tumor region. 619 patients stayed recurrence free.
In primary tumor regions receiving a dose per fraction of 2 Gy, 55 patients (9.7%) developed a recurrence. Within this patient group, 10 of 134 patients developed a recurrence after treatment with a total dose 56 Gy (R0 resection), 17 of 201 after 66 Gy (R1 resection), and 28 of 203 after 72 Gy (definitive treatment). In the group of patients with target volumes receiving a reduced dose per fraction, 5 patients (3.8%) developed a recurrence (p < 0.001). Three of them were treated within the primary tumor region with a total dose of 56 Gy after R0 resection, whereat according treatment plans contained higher total doses of 66 Gy addressed to the lymphatic drainage. Thereby, a dose per fraction of 1.7 Gy resulted for the primary tumor region. The remaining two recurrences occurred after 66 Gy (R1 resection) for treatment plans with total doses of 72 Gy aimed to macroscopic lymph node metastases. A single dose of 1.83 Gy followed for the primary tumor bed.
Within 698 primary tumor regions, 565 PTV’s were treated with a dose per fraction of 2 Gy (1st order PTV’s). Accordingly, reduced doses per fraction were delivered to the remaining 133 PTV’s. In primary tumor PTV’s receiving a dose per fraction of 2 Gy, 55 patients (9.7%) developed a recurrence, whereas in PTV’s receiving reduced doses per fraction, 5 patients (3.8%) developed a recurrence (p < 0.001) (Table 2).
Irradiating the lympatic drainage, all together 854 PTV’s were treated with a dose per fraction of 2 Gy (1st order PTV’s) and 1780 PTV’s received reduced doses per fraction. For both groups the recurrence rate was low (Table 3). First order PTV’s developed a recurrence in 25 cases (2.9 %). The number of recurrences was 17 of 365 after 72 Gy, 4 of 291 after 66 Gy, and 4 of 198 after 56 Gy, respectively. Target volumes treated with a reduced dose per fraction developed recurrences only in 5 cases (p = 0.001). Four of them occurred after a dose of 56 Gy in treatment plans with total doses of 66 Gy leading single doses of 1.7 Gy. The remaining recurrence appeared after 50 Gy in a treatment plan with a total dose of 66 Gy, which resulted in a dose per fraction of 1.52 Gy.
The recurrence risk was not increased in 2nd to 4th order target volumes, neither for the primary tumor region nor for the lymphatic drainage.
4. Discussion
The total rates of locoregional recurrences inside the treated PTV’s amounts to 11.4%. These results are in good agreement with other studies of varying follow up periods, showing recurrence rates from 5.6% to 31% as a result of a definitive radiation therapy [1] -[7] and between 7% and 10% after a postoperative radiation therapy [1] - [3] .
Table 2. Recurrence rate of primary tumor bed related to the single dose (n = 699).
*1The total dose of the whole treatment plan is calculated for a single dose of 2 Gy for the target volume, needing the highest total dose; *2The indications leading to the total dose of the primary are: 50 Gy: CuP: Naso-, Oro-, Hypopharynx; 56 Gy: R0 resection; 66 Gy: R1 resection; 72 Gy: R2 resection or definitive treatment; *3In 1 patient only lymph nodes without primary tumor have been treated.
Table 3. Recurrence rate of lymph node levels related to the single dose.
*1The total dose of the whole treatment plan is calculated for a single dose of 2 Gy for the target volume, needing the highest total dose; *2The indications leading to the total dose of the lymph node level are: 50 Gy: pN0; 56 Gy: pN+ and no extracapsular spread or 2nd noninvolved level; 64 Gy: pN+ and extracapsular spread or 1st noninvolved level; 72 Gy: cN+; *3In 15 patients the primary was treated without lymphatic drainage, in 20 patients only unilateral lymphatic drainage and in 29 patients only the cranial part of lymphatic drainage has been treated.
The distribution of stages reveals that some departments show a more favourable risk profile of patients [2] . Our data again match previous studies according to locoregional control rates after definitive therapy of 56% to 95% after 2 to 5 years [1] [3] [6] [8] - [15] and after postoperative therapy of 85% after 3 years [3] [16] .
However, no data could be found concerning the problem addressed in the current work: the question, whe- ther a lower biological efficacy, which is caused by lower doses per fraction and increased corresponding treatment times, leads to a higher rate of recurrences for simultaneous irradiated PTV’s of 2nd to 4th order? The current study shows, that among all in-field recurrences only 3 recurrences emerged within the primary tumor region and further 3 within the lymphatic drainage after a treatment by means of reduced single doses. They count for 8.3% of the in-field recurrences of the primary tumor region and for 15.8% of the in-field recurrences of the lymphatic drainage, respectively. Most in-field recurrences have been developed in our investigation after irradiation of macroscopic tumor mass with doses per fraction of 2 Gy. In agreement with these results, Collan et al. also located all of the observed recurrences inside target volumes, which were irradiated receiving high total doses and consecutively high doses per fraction [1] .
Several groups emphasize risk factors for locoregional recurrence. They include the volume of the tumor and lymph node metastases, the perinodal infiltration, the number of lymph nodes involved and the resection rim, forming different risk groups [17] - [19] . Patients with these risk factors were treated more rigidly in our department concerning total radiation dose and/or simultaneous chemotherapy. Appropriate PTV’s received highest total doses with doses per fraction of 2 Gy, respectively.
PTV’s of lower risk for recurrencies (e.g. pN+ without perinodal infiltration, pN0) were irradiated with lower doses per fraction and total doses, since, especially for the low dose elective target volumes (pN0 situation) a very low recurrence rate is expected. Nevertheless, there are known cases, for which the indication of a radiation therapy of a pN0 situation seems to be questionable [20] - [24] .
Further factors are known to influence the recurrence rate. Among tumors of the same kind there is a broad variability of intrinsic radiation sensitivity [25] . Further, the quality of target volume determination within the radiation planning process is crucial [26] - [30] . The limitation of total radiation treatment time of macroscopic tumor regions seems to have a high relevance to the recurrence rate, too [31] .
5. Conclusion
According to the shown results, recurrence rates of primary tumors as well as of lymphatic drainages are not increased after treating them in a risk adapted concept as target volumes of 2nd to 4th order with decreased doses per fraction and consequently increased treatment time.
Conflict of Interest
The authors state that there are no conflicts of interest.
Statement
The accompanying manuscript does not include studies on humans or animals.
References
- Collan, J., Kapanen, M., Mäkitie, A., et al. (2012) Submandibular Gland-Sparing Intensity Modulated Radiotherapy in the Treatment of Head and Neck Cancer: Sites of Locoregional Relapse and Survival. Acta Oncologica, 51, 735-742. http://dx.doi.org/10.3109/0284186X.2011.640348
- Garden, A.S., Dong, L., Morrison, W.H., et al. (2013) Patterns of Disease Recurrence Following Treatment of Oropharyn- geal Cancer with Intensity Modulated Radiation Therapy. International Journal of Radiation Oncology, 85, 941-947. http://dx.doi.org/10.1016/j.ijrobp.2012.08.004
- Van Gestel, D., Van Den Weyngaert, D., Schrijvers, D., et al. (2011) Intensity-Modulated Radiotherapy in Patients with Head and Neck Cancer: A European Single-Centre Experience. British Journal of Radiology, 84, 367-374. http://dx.doi.org/10.1259/bjr/67058055
- Hoebers, F., Rios, E., Troost, E., et al. (2013) Definitive Radiation Therapy for Treatment of Laryngeal Carcinoma. Impact of Local Relapse on Outcome and Implicaions for Treatment Strategies. Strahlentherapie und Onkologie, 189, 834-841. http://dx.doi.org/10.1007/s00066-013-0414-2
- Liauw, S.L., Amdur, R.J., Morris, C.G., et al. (2007) Isolated Neck Recurrence after Definitive Radiotherapy for Node-Posi- tive Head and Neck Cancer: Salvage in the Dissected or Undissected Neck. Head Neck, 29, 715-719. http://dx.doi.org/10.1002/hed.20580
- Ng, W.T., Lee, M.C.H., Hung, W.M., et al. (2011) Clinical Outcomes and Patterns of Failure after Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma. International Journal of Radiation Oncology*Biology*Physics, 79, 420- 428. http://dx.doi.org/10.1016/j.ijrobp.2009.11.024
- Oksuz, D.C., Prestwich, R.J., Carey, B., et al. (2011) Recurrence Patterns of Locally Advanced Head and Neck Squa- mous Cell Carcinoma after 3D Conformal (Chemo)-Radiotherapy. Radiation Oncology, 24, 54. http://dx.doi.org/10.1186/1748-717X-6-54
- Buchali, A., Schröder, C., Sidow, D., et al. (2013) Influence of the Radiation Dose to Slaivary Glands on Xerostomia in Patients with Head and Neck Carcinomas. JCT, 4, 188-194. http://dx.doi.org/10.4236/jct.2013.41028
- Budach, V., Stuschke, M., Budach, W., et al. (2005) Hyperfractionated Accelerated Chemoradiation with Concurrent Fluorouracil-Mitomycin Is More Effective than Dose-Escalated Hyperfractionated Accelerated Radiation Therapy Alone in Locally Advanced Head and Neck Cancer: Final Results of the Radiotherapy Cooperative Clinical Trials Group of the German Cancer Society 95-06 Prospective Randomized Trial. Journal of Clinical Oncology, 23, 1125- 1135. http://dx.doi.org/10.1200/JCO.2005.07.010
- Chen, J.L.Y., Huang, Y.S., Kuo, S.H., et al. (2013) Intensitiy Modulated Radiation Therapy for T4 Nasopharyngeal Carcinoma. Treatment Results and Locoregional Recurrence. Strahlentherapie und Onkologie, 189, 1001-1008. http://dx.doi.org/10.1007/s00066-013-0429-8
- Kuhnt, T., Mueller, A.C., Pelz, T., Haensgen, G., Bloching, M., Koesling, S., et al. (2005) Impact of Tumor Control and Presence of Visible Necrosis in Head and Neck Cancer Patients Treated with Radiotherapy or Radiochemotherapy. Journal of Cancer Research and Clinical Oncology, 131, 758-764. http://dx.doi.org/10.1007/s00432-005-0018-z
- Lambrecht, M., Dirix, P., Van den Bogaert, W. and Nuyts, S. (2009) Incidence of Isolated Regional Recurrence after De- finitive (Chemo-) Radiotherapy for Head and Neck Squamous Cell Carcinoma. Radiotherapy and Oncology, 93, 498- 502. http://dx.doi.org/10.1016/j.radonc.2009.08.038
- Lambrecht, M., Nevens, D. and Nuyts, S. (2013) Intensity-Modulated Radiotherapy vs. Parotid-Sparing 3D Conformal Radiotherapy. Effect on Outcome and Toxicity in Locally Advanced Head and Neck Cancer. Strahlentherapie und Onkologie, 189, 223-229. http://dx.doi.org/10.1007/s00066-012-0289-7
- Lau, H., Brar, S., Hao, D., MacKinnon, J., Yee, D. and Gluck, S. (2006) Concomitant Low-Dose Cisplatin and Three- Dimensional Conformal Radiotherapy for Locally Advanced Squamous Cell Carcinoma of the Head and Neck: Analysis of Survival and Toxicity. Head and Neck, 28, 189-196. http://dx.doi.org/10.1002/hed.20324
- Schoenfeld, G.O., Amdur, R.J., Morris, C.G., Li, J.G., Hinerman, R.W. and Mendenhall, W.M. (2008) Patterns of Failure and Toxicity after Intensity-Modulated Radiotherapy for Head and Neck Cancer. International Journal of Radiation Oncology, Biology, Physic, 71, 377-385. http://dx.doi.org/10.1016/j.ijrobp.2007.10.010
- Moon, S.H., Jung, Y.S., Ryu, J.S., Choi, S.W., Park, J.Y., Yun, T., et al. (2011) Outcomes of Postoperative Simultaneous Modulated Accelerated Radiotherapy for Head-and-Neck Squamous Cell Carcinoma. International Journal of Radiation Oncology, Biology, Physic, 81, 140-149. http://dx.doi.org/10.1016/j.ijrobp.2010.04.068
- Bockel, L.W., Monninkhof, E.M., Pameijer, F.A. and Terhaard, C.H.J. (2013) Importance of Tumor Volume in Supraglottic, and Glottic Laryngeal Carcinoma. Strahlentherapie und Onkologie, 189, 1011-1014.
- Feng, M., Jabbari, S., Lin, A., Bradford, C.R., Chepeha, D.B., Teknos, T.N., et al. (2005) Predictive Factors of Local- Regional Recurrences Following Parotid Sparing Intensity Modulated or 3D Conformal Radiotherapy for Head and Neck Cancer. Radiotherapy & Oncology, 77, 32-38. http://dx.doi.org/10.1016/j.radonc.2005.07.008
- Langendijk, J.A., Slotman, B.J., van der Waal, I., Doornaert, P., Berkof, J. and Leemans, C.R. (2005) Risk-Group Definition by Recursive Partitioning Analysis of Patients with Squamous Cell Head and Neck Carcinoma Treated with Surgery and Postoperative Radiotherapy. Cancer, 104, 1408-1417. http://dx.doi.org/10.1002/cncr.21340
- Chen, J.Z., Le, Q.T., Han, F., Lu, L.Y., Huang, S.M., Lin, C.G., et al. (2013) Results of a Phase 2 Study Examining the Effects of Omitting Elective Neck Irradiation to Nodal Levels IV and Vb in Patients with N0-1 Nasopharyngeal Carcinoma. International Journal of Radiation Oncology, Biology, Physic, 85, 929-934. http://dx.doi.org/10.1016/j.ijrobp.2012.07.2356
- Gao, Y., Zhu, G., Lu, J., Ying, H., Kong, L., Wu, Y., et al. (2010) Is Elective Irradiation to the Lower Neck Necessary for No Nasopharyngeal Carcinoma? International Journal of Radiation Oncology, Biology, Physic, 77, 1397-1402. http://dx.doi.org/10.1016/j.ijrobp.2009.06.062
- Ho, F.C., Tham, I.M., Earnest, A., Lee, K.M. and Lu, J.J. (2012) Patterns of Regional Lymph Node Metastasis of Nasopharyngeal Carcinoma: A Meta-Analysis of Clinical Evidence. BMC Cancer, 12, 98. http://dx.doi.org/10.1186/1471-2407-12-98
- Ng, S.H., Chang, J.T., Chan, S.C., Ko, S-F., Wang, H.M., Liao, C.T., et al. (2004) Nodal Metastasis of Nasopharyngeal Carcinoma: Patterns of Disease on MRI and FDG-PET. European Journal of Nuclear Medicine and Molecular Imaging, 31, 1073-1080. http://dx.doi.org/10.1007/s00259-004-1498-9
- Tang, L., Mao, Y., Liu, L., Liang, S., Chen, Y., Sun, Y., et al. (2009) The Volume to Be Irradiated during Selective Neck Irradiation in Nasopharyngeal Carcinoma: Analysis of the Spread Patterns in Lymph Nodes by Magnetic Resonance Imaging. Cancer, 115, 680-688. http://dx.doi.org/10.1002/cncr.24049
- Begg, A.C. (2012) Predicting Recurrence after Radiotherapy in Head and Neck Cancer. Seminars in Radiation Oncology, 22, 108-118. http://dx.doi.org/10.1016/j.semradonc.2011.12.002
- Cannon, D.M. and Lee, N.Y. (2008) Recurrence in Region of Spared Parotid Gland after Definitive Intensity-Modu- lated Radiotherapy for Head and Neck Cancer. International Journal of Radiation Oncology, Biology, Physic, 70, 660- 665. http://dx.doi.org/10.1016/j.ijrobp.2007.09.018
- Lee, A.W.M., Sze, H. and Tong Ng, W. (2013) Is Selective Neck Irradiation Safe for Node-Negative Nasopharyngeal Cancer. International Journal of Radiation Oncology, Biology, Physic, 85, 902-903. http://dx.doi.org/10.1016/j.ijrobp.2012.09.010
- Mendenhall, W.M. and Mancuso, A.A. (2009) Radiotherapy for Head and Neck Cancer―Is the “Next Level” Down? International Journal of Radiation Oncology, Biology, Physic, 73, 645-646. http://dx.doi.org/10.1016/j.ijrobp.2008.08.061
- Peters, L.J., O’Sullivan, B., Giralt, J., Fitzgerald, T.J., Trotti, A., Bernier, J., et al. (2010) Critical Impact of Radiotherapy Protocol Compliance and Quality in the Treatment of Advanced Head and Neck Cancer: Results from TROG 02.02. Journal of Clinical Oncology, 28, 2996-3001. http://dx.doi.org/10.1200/JCO.2009.27.4498
- Buchali, A., Schroeder, C., Sidow, D. and Blank, E. (2013) Influence of the Radiation Dose to Salivary Glands on Xerostomia in Patients with Head and Neck Carcinomas. Journal of Cancer Therapy, 4, 188-194. http://dx.doi.org/10.4236/jct.2013.41028
- Bourhis, J., Overgaard, J., Audry, H., Ang, K.K., Saunders, M., Bernier, J., et al. (2006) Hyperfractionated or Accelerated Radiotherapy in Head and Neck Cancer: A Meta-Analysis. Lancet, 368, 843-854. http://dx.doi.org/10.1016/S0140-6736(06)69121-6
NOTES

*Corresponding author.