Chinese Medicine
Vol. 3 No. 1 (2012) , Article ID: 18126 , 7 pages DOI:10.4236/cm.2012.31012
A Hypothetical Anti-Aging Mechanism of “Yang-Invigorating” Chinese Tonic Herbs*
Division of Life Science, The Hong Kong University of Science & Technology, Hong Kong, China
Email: #bcrko@ust.hk
Received September 29, 2011; revised October 19, 2011; accepted November 6, 2011
Keywords: Chinese Medicine; Yang; Mitochondrial Decay; Age-Related Diseases
ABSTRACT
Chinese tonic herbs are generally classified into Yin and Yang categories based on their health-promoting action. Emerging evidence has suggested that in addition to up-regulating mitochondrial functional status, Yang tonic herbs also enhance cellul-ar/mitochondrial antioxidant capacity, and may thus prevent age-related diseases and prolong the healthy part of lifespan (i.e. healthspan). The proposed biochemical mechanism underlying the antioxidant action of Yang tonic herbs involves a sustained and low level of mitochondrial reactive oxygen species production, which is secondary to the increased activity of the electron transport chain, with the possible involvement of mitochondrial uncoupling. “Yang invigoration” improves antioxidant defense in the body in the long term and thereby offers a promising prospect for preventing or possibly delaying age-related diseases and the detrimental effects of aging.
1. Mitochondrial Decay and Age-Related Diseases
Aging is a process characterized by a general decline in physiological functions, and it is also considered as a major risk factor for many age-related diseases, including, but not limited to, neurodegenerative diseases, cardiovascular disorders, and metabolic diseases [1-3]. The “Mitochondrial Free Radical Theory of Aging” (MFRTA) attempts to explain the role of reactive oxygen species (ROS) arising from mitochondrial electron transport in the aging process [4]. At odds with the MFRTA is the observation that the significant loss-of-function mitochondrial DNA (mtDNA) mutations accumulated only to low levels in most tissues, even by very old age. To address this anomaly, Aubrey de Grey proposed the “Reductive Hotspot Hypothesis of Mammalian Aging” as a supplement of the MFRTA. This theory attempts to explain how the relatively few cells that have lost oxidative phosphorylation capacity due to mtDNA mutations may be toxic to the rest of the body and result in the development of age-related diseases [5,6]. Since then, mitochondrial decay in aging has been implicated in a broad spectrum of degenerative and metabolic diseases [1,7-9]. The “Double-Agent Theory”, which endeavors to provide a unifying view of aging and diseases, postulated that mitochondrial ROS generation produces a genetic response mimicking that triggered by infection associated increase in intracellular oxidative stress [10]. However, the continuous mitochondrial ROS production would lead to a persistent shift in gene expression, with resultant chronic inflammation which is commonly involved in age-related diseases. In this connection, in order to decrease the vulnerability to age-related diseases, we must find ways to attenuating the mitochondrial ROS production [10]. Despite conflicting views concerning the primary role of mitochondrial ROS as a cause of aging [11], the generation of ROS within mitochondria remains the most viable theory to explain the process of aging. Increased levels of ROS within mitochondria are the principal trigger not only for mitochondrial dysfunction, but also for diseases associated with aging in general [12]. Several studies have revealed a complex network of signaling pathways modulated by nutrients, such as IGF-1, TOR, sirtuins, AMP kinase and PGC-1α that are connected and then converge to inhibit oxidative stress within the mitochondrion [13-17].
Under normal physiological conditions, mitochondria not only serve as the powerhouse of cells by generating ATP through oxidative phosphorylation, but also regulate a wide range of cellular processes, including cellular signaling, differentiation and proliferation, as well as cell survival and death [18]. Experimental studies showed that mitochondria from liver tissue of elderly rats generated a larger amount of ROS than liver mitochondria of young rats, indicative of increasing oxidative stress as a function of aging [19]. The age-related increase in mitochondrial oxidative stress can disrupt mitochondrial structural and functional integrity, thereby triggering a vicious cycle of ROS generation. Experimental findings indicate that the age-related decrease in mitochondrial respiratory efficiency was associated with the significant decline in respiratory complex (I-V) activities, presumably mediated by self-inflicted oxidative damage [20-22]. In addition, the extent of oxidative damage on key metabolic enzymes increases with age, with consequent decreases in substrate binding affinity and mitochondrial ATP generation capacity [23,24]. The oxidation of DNA, RNA, protein and lipid molecules in mitochondria and other cellular components can culminate in functional impairment in cells, tissues, and ultimately in vital organs such as brain, heart and liver [19,25-27]. Taken together, the capacity to produce ATP and respond to cellular stress decrease as a function of age during the age-associated deterioration of mitochondrial structure and function. The mitochondrial dysfunction results in increased ROS generation, which tilts the cellular environment towards an oxidative state (i.e., impairment of cellular redox balance) and increases the susceptibility to diseases associated with aging [18,28,29].
2. Pharmacological Basis of “Yang-Invigoration” in Chinese Medicine
According to traditional Chinese medicine (TCM) theory, tonic herbs are classified into four categories, based on their health-promoting actions: Yang-invigorating; Yinnourishing; Qi-invigorating and Blood-enriching. The Qiinvigorating and Blood-enriching herbs are further grouped under the Yang and Yin categories, respectively. Holistically, it is believed that the Yang-invigorating herbs enhance physiological cellular activities, which is in turn critically dependent on mitochondrial ATP generation through the oxidative phosphorylation process at the cellular level. A previous study in our laboratory has shown that short-term oral treatment with the methanol extract of Yang-invigorating herbs, including Cortex Eucommiae, Herba Cistanches, Herba Cynomorii, Rhizoma Curculiginis, Herba Epimedii, Radix Dipsaci, Rhizoma Drynariae, Fructus Psoraleae, Semen Cuscutae, Radix Morindae, and Semen Alliion, enhanced myocardial ATP generation and produced significant stimulatory action on pyruvate-supported mitochondrial electron transport in mice [30]. This finding is corroborated by a recent study involving Yang and Yin tonic herbs using a cell-based assay of ATPgenerating capacity, which showed that Yang but not Yin tonic herbs enhanced mitochondrial ATP generation capacity in H9c2 cardiomyocytes [31]. Activity-directed fractionation of three Yang tonic herbs (namely, Herba Cistanches, Herba Cynomorii, and Semen Cuscutae) indicated that the active ingredient(s) appeared to reside in the butanol and/or ethylacetate fraction [31]. Moreover, long-term treatment with a Yang-invigorating Chinese herbal formula (VI-28; composed of Radix Ginseng, Cornu Cervi, Cordyceps, Radix Salviae, Semen Allii, Fructus Cnidii, Fructus Evodiae and Rhizoma Kaempferiae) was found to enhance mitochondrial ATP generation in brain, heart, liver and skeletal muscle tissues of male and female rats [32]. Although studies on isolated mitochondria may not directly reflect the in vivo mitochondrial function, it provided important information to support the rationale that Yang-invigorating herbs may enhance the functional capacity of mitochondria. Interestingly, in addition to stimulating mitochondrial ATP generation, VI-28, a proprietary Chinese herbal formula, also increased the levels/activities of mitochondrial antioxidant components such as reduced glutathione (GSH), α-tocopherol (α-TOC) and manganese-superoxide dismutase (Mn-SOD), indicative of upregulation of mitochondrial redox status by “Yang-invigoration” [32]. In connection with this, several Yang herbs have been shown to possess antioxidant activity both in vitro and in vivo [33]. Studies from various laboratories showed that Yang tonic herbs produced antioxidant actions by free radical-scavenging [34], inhibition of oxidant production [35], inhibition of NADPH-dependent lipid peroxidation [36] and increase of antioxidant enzyme activities [37], with a resultant protection against oxidative tissue damage. These findings were consistent with our earlier study which showed that Yang tonic herbs possessed stronger free radical scavenging activity than that of tonic herbs of other functional categories [38]. As mentioned earlier, ROS are unavoidably generated during the oxidative phosphorylation process, particularly under conditions of increased electron transport activity. Given that increased formation of ROS within mitochondria can cause an adaptive response that leads to the enhancement of mitochondrial antioxidant capacity [39], the induction of mitochondrial antioxidant components by the Yang-invigorating VI-28 may, at least in part, be mediated by an increase in ROS production from complex I and/or complex III resulting from the stimulation of mitochondrial electron transport (see Figure 1).
3. Protection against Oxidant-Induced Tissue Injury by Yang-Invigorating Tonic Herbs/Formulae
“Yang-invigoration”, which can up-regulate cellular/mitochondrial antioxidant components, may protect against oxidant-induced injury. In a study using rodent models of cerebral/myocardial ischemia/reperfusion injury, carbon tetrachloride hepatotoxicity, and gentamicin nephrotoxicity, long-term treatment with VI-28 significantly ameliorated oxidative stress-induced tissue damage in various organs, including brain, heart, liver and kidney. The tissue protection afforded by VI-28 pretreatment was associated with increases in the levels/activities of mitochondrial antioxidant components (GSH, α-TOC and
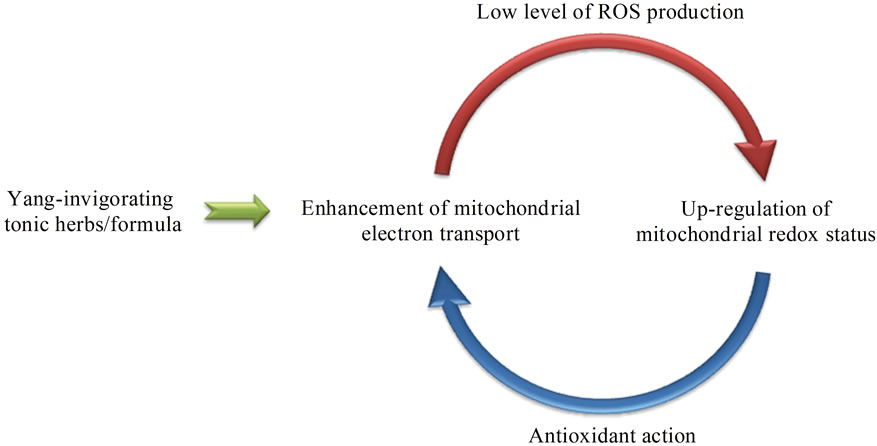
Figure 1. Pharmacological basis of “Yang-invigoration” in Chinese medicine. Yang-invigorating herbs/formulae enhance mitochondrial functional capacity with a concomitant production of ROS which triggers an adaptive antioxidant response that enhances mitochondrial antioxidant defense and safeguards mitochondrial function.
Mn-SOD), as well as the preservation of mitochondrial structural integrity [40]. In addition, Wu-Zi-Yan-ZhongWan (WZ), a Chinese herbal formula containing five herbs, namely, Fructus Lycii, Semen Cuscutae, Fructus Rubi, Semen Plantaginis and Fructus Schisandrae, is prescribed for treating “Yang deficiency” in TCM. Studies showed that WZ decreased the extent of ethanol-induced ROS production and lipid peroxidation as well as prevented ethanol-induced decreases in cellular/mitochondrial GSH levels and mitochondrial membrane potential in CYP2E1 cDNA-transfected human HepG2 (E47) cells [41] and chronic ethanol-intoxicated rats [42]. Taken together, both cell-based and animal studies provide convincing evidence to support the role of Yang tonic herbs/formulae in protecting the body against oxidantinduced injury, presumably by up-regulating cellular/mitochondrial antioxidant defense components in a tissue non-specific manner.
4. “Yang-Invigoration” and Prolongation of Healthspan
According to the United Nations organization, the world’s elderly population (60 years of age or older) is currently 650 million, and it is forecasted to reach 2 billion by the year 2050 [43]. The rapid increase in the elderly population will entail major challenges in societies, particularly in the area of healthcare expenditures for age-related diseases. Preventive measures for achieving a healthy lifespan are therefore instrumental in relieving the financial burden in aging societies. A growing body of evidence has revealed the crucial involvement of mitochondrial dysfunction and impaired antioxidant status in the pathogenesis of various age-related diseases and the aging process in general [2,44,45]. However, outcomes of experiments which attempt to increase tissue antioxidants levels through dietary supplementation, pharmacological induction or transgenic techniques were unsatisfactory in terms of extending longevity [46-48]. Numerous randomized, double-blinded clinical trials have also shown that antioxidant supplements cause no beneficial effect and occasionally harmful to human [49]. Yang tonic herbs/ formulae, which can induce endogenous mitochondrial antioxidant status and functional capacity enhancement [30,40], may therefore offer a promising prospect for preventing or possibly delaying age-related diseases and the detrimental effects of aging. With respect to Chinese medicine, more than 50% of the elderly people in China were found to show a deficiency of Yang (or Qi) in body function [50], and Yang tonic herbs/formulae are therefore commonly used for retarding the adverse consequences of aging in the practice of Chinese medicine. According to TCM theory, a deficiency of Yang is believed to be one of the causative factors for the development Parkinson’s disease (PD), a common neurodegenerative disease that severely compromises the quality of life in many elderly individuals [51]. This notion is paralleled by a clinical study which revealed the gradual development of “Yang-deficiency” symptoms prior to the clinical manifestations of PD [52]. In this connection, a pilot study investigating whether a Yang-invigorating Chinese herbal suppository preparation (ViNeuro; a VI-28-related product) could produce any symptom-relieving effect in patients suffering from PD was conducted [53]. The results indicated that all PD patients (40 - 69 years of age; 6 males and 3 females) showed improvement in various Parkinsonian symptoms, particularly relieving reduction in rigidity and tremor, after taking ViNeuro for 6 months. The relief of “Yang-deficiency” symptoms such as aversion to cold, poor appetite, frequent urination, and constipation were also observed [53]. Based on the finding that ViNeuro can enhance the mitochondrial ATP generation capacity (a “Yang-invigoration” property), it is plausible that the relief of Parkinsonian symptoms involves an improvement of cellular energy status that eventually leads to an enhancement of neuronal function. A recent study showed that long-term dietary supplementation with VI-28, the aforementioned Yang-invigorating herbal formula, can extend the median lifespan of both male and female C57BL/6J mice [54]. The enhanced survival was associated with the mitigation of age-dependent progressive impairments in mitochondrial antioxidant status and functional capacity [54]. Conceivably, the retardation of mitochondrial decay in structure and function by “Yang invigoration” delays the onset of age-related diseases, thereby enhancing longevity and thus increasing the healthy part of lifespan (i.e. healthspan) of animals. In this regard, the finding of myocardial protection with various interventions on mitochondrial oxidative stress suggested the prominent role of mitochondrial decay in cardiac aging [12].
5. “Yang-Invigoration” Enhances Mitochondrial Functional Capacity and Antioxidant Status
Preliminary studies in our laboratory using animal and cell models showed that a semi-purified fraction of Herba Cistanches not only stimulated state-3 respiration, but also caused an increase in state-4 respiration in rat mitochondria and H9c2 cardiomyocytes, with the latter being reversed by GDP, an uncoupling protein inhibitor (unpublished data). The experimental findings suggested the involvement of mitochondrial uncoupling in the “Yanginvigoration” produced by Herba Cistanches. Taken together with our previous observations, we hypothesize that “Yang-invigoration” can produce both short-term and long-term beneficial effects in the body (see Figure 2). In the short term, “Yang-invigoration” stimulates mitochondrial electron transport, presumably by increasing both complex I and III activities, with a resultant increase in ATP generation activity (i.e., functional capacity). The stimulation of electron transport is accompanied by an increase in ROS generation and a rise in membrane potential, with the latter also inhibiting further electron transport but favouring ROS production [51]. On the one hand, the increased mitochondrial ROS generation triggers a retrograde response to up-regulate cellular/mitochondrial antioxidant defense components [52]. On the other hand, mitochondrial ROS also stimulate the uncoupling protein activity and thus lower the membrane potential through dissipation of the proton gradient [53,54]. This restoration of membrane potential allows the re-activation of electron transport and the associated ROS production at low level. As such, with a recurring “Yanginvigoration”, a sustained and low level of mitochondrial ROS production can result in both the up-regulation of antioxidant defense components characteristic of mitohormesis [52,55,56] and the prolonged activation of mitochondrial uncoupling. The reduced level of mitochondrial ROS generation associated with increased electron transport due to uncoupling may be an effect secondary to the up-regulation of antioxidant defense or the decrease in membrane potential [55,56]. A low level of mitochondrial ROS generation has also been shown to be a potential key factor that contributes to the low rate of aging in almost all long-lived species [57]. While the role of mitochondrial uncoupling in influencing lifespan remains to be established, experimental investigations in transgenic mice have ascribed a role for uncoupling protein-1 dependent uncoupling in increasing healthspan [57]. Therefore, in addition to the enhancement of mitochondrial function capacity, “Yang invigoration” improves antioxidant defense in the body in the long term and thereby offers a promising prospect for preventing or possibly delaying age-related diseases and the detrimental effects of aging.
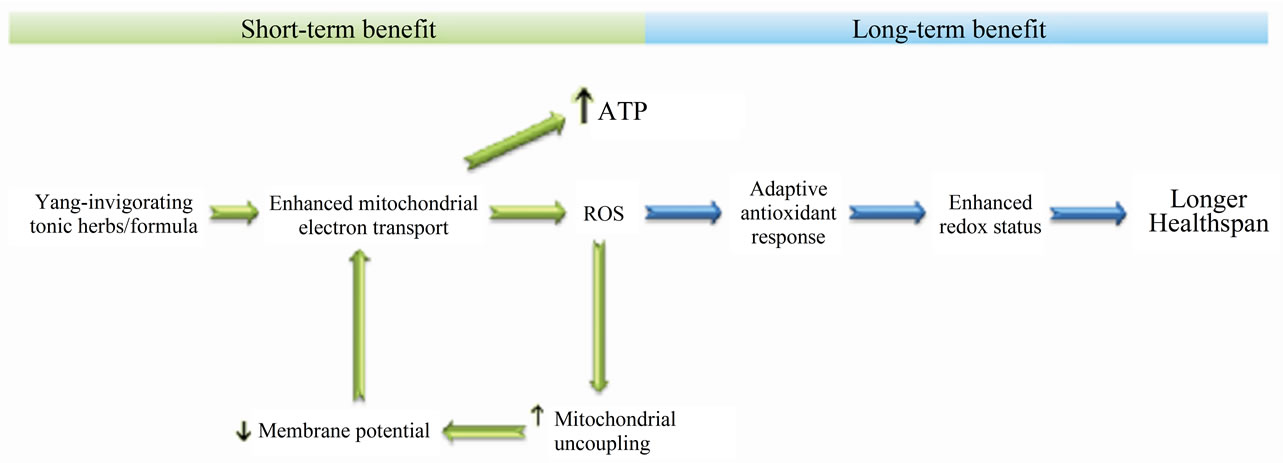
Figure 2. Up-regulation of cellular/mitochondrial antioxidant defense mechanism by “Yang-invigoration”. “Yang-invigoration” may produce both short-term and long-term beneficial effects (please refer to the text for details).
6. Acknowledgements
Some research works described in this paper were fully supported by a research contract from Vigconic (International) Ltd., Hong Kong. Vigconic (International) Ltd., Hong Kong does not involve in the interpretation or publication of any work mentioned in the current article.
REFERENCES
- M. K. Shigenaga, T. M. Hagen and B. N. Ames, “Oxidative Damage and Mitochondrial Decay in Aging,” Proceedings of the National Academy of Sciences USA, Vol. 91, No. 23, 1994, pp. 10771-10778. doi:10.1073/pnas.91.23.10771
- B. N. Ames, M. K. Shigenaga and T. M. Hagen, “Oxidants, Antioxidants, and the Degenerative Diseases of Aging,” Proceedings of the National Academy of Sciences USA, Vol. 90, No. 17, 1993, pp. 7915-7922. doi:10.1073/pnas.90.17.7915
- D. Harman, “Aging: A Theory Based on Free Radical and Radiation Chemistry,” Journal of Gerontology, Vol. 11, No. 3, 1956, pp. 298-300.
- D. Harman, “The Biologic Clock: The Mitochondria?” Journal of the American Geriatrics Society, Vol. 20, No. 4, 1972, pp. 145-147.
- A. D. de Grey, “The Reductive Hotspot Hypothesis of Mammalian Aging: Membrane Metabolism Magnifies Mutant Mitochondrial Mischief,” European Journal of Biochemistry, Vol. 269, No. 8, 2002, pp. 2003-2009. doi:10.1046/j.1432-1033.2002.02868.x
- A. D. de Grey, “The Reductive Hotspot Hypothesis: An Update,” Archives of Biochemistry and Biophysics, Vol. 373, No. 1, 2000, pp. 295-301. doi:10.1006/abbi.1999.1509
- K. B. Beckman and B. N. Ames, “Mitochondrial Aging: Open Questions,” Annals of the New York Academy of Sciences, Vol. 854, 1998, pp. 118-127.
- K. B. Beckman and B. N. Ames, “The Free Radical Theory of Aging Matures,” Physiological Reviews, Vol. 78, No. 2, 1998, pp. 547-581.
- T. M. Hagen, D. L. Yowe, J. C. Bartholomew, C. M. Wehr, K. L. Do, J. Y. Park and B. N. Ames, “Mitochondrial Decay in Hepatocytes from Old Rats: Membrane Potential Declines, Heterogeneity and Oxidants Increase,” Proceedings of the National Academy of Sciences USA, Vol. 94, No. 7, 1997, pp. 3064-3069. doi:10.1073/pnas.94.7.3064
- N. Lane, “A Unifying View of Ageing and Disease: The Double-Agent Theory,” Journal of Theoretical Biology, Vol. 225, No. 4, 2003, pp. 531-540. doi:10.1016/S0022-5193(03)00304-7
- J. Lapointe and S. Hekimi, “When a Theory of Aging Ages Badly,” Cellular and Molecular Life Sciences, Vol. 67, No. 1, 2010, pp. 1-8. doi:10.1007/s00018-009-0138-8
- J. Wanagat, D. F. Dai and P. Rabinovitch, “Mitochondrial Oxidative Stress and Mammalian Healthspan,” Mechanisms of Ageing and Development, Vol. 131, No. 7-8, 2010, pp. 527-535. doi:10.1016/j.mad.2010.06.002
- M. Holzenberger, J. Dupont, B. Ducos, P. Leneuve, A. Geloen, P. C. Even, P. Cervera and Y. Le Bouc, “IGF-1 Receptor Regulates Lifespan and Resistance to Oxidative Stress in Mice,” Nature, Vol. 421, No. 6919, 2003, pp. 182-187. doi:10.1038/nature01298
- S. J. Lin, P. A. Defossez and L. Guarente, “Requirement of NAD and SIR2 for Life-Span Extension by Calorie Restriction in Saccharomyces Cerevisiae,” Science, Vol. 289, No. 5487, 2000, pp. 2126-2128. doi:10.1126/science.289.5487.2126
- T. J. Schulz, K. Zarse, A. Voigt, N. Urban, M. Birringer and M. Ristow, “Glucose Restriction Extends Caenorhabditis Elegans Life Span by Inducing Mitochondrial Respiration and Increasing Oxidative Stress,” Cell Metabolism, Vol. 6, No. 4, 2007, pp. 280-293. doi:10.1016/j.cmet.2007.08.011
- S. Wullschleger, R. Loewith and M. N. Hall, “TOR Signaling in Growth and Metabolism,” Cell, Vol. 124, No. 3, 2006, pp. 471-484. doi:10.1016/j.cell.2006.01.016
- R. M. Anderson, J. L. Barger, M. G. Edwards, K. H. Braun, C. E. O’Connor, T. A. Prolla and R. Weindruch, “Dynamic Regulation of PGC-1α Localization and Turnover Implicates Mitochondrial Adaptation in Calorie Restriction and the Stress Response,” Aging Cell, Vol. 7, No. 1, 2008, pp. 101-111. doi:10.1111/j.1474-9726.2007.00357.x
- L. A. Loeb, D. C. Wallace and G. M. Martin, “The Mitochondrial Theory of Aging and Its Relationship to Reactive Oxygen Species Damage and Somatic mtDNA Mutations,” Proceedings of the National Academy of Sciences USA, Vol. 102, No. 52, 2005, pp. 18769-18770. doi:10.1073/pnas.0509776102
- T. M. Hagen, J. Liu, J. Lykkesfeldt, C. M. Wehr, R. T. Ingersoll, V. Vinarsky, J. C. Bartholomew and B. N. Ames, “Feeding Acetyl-L-Carnitine and Lipoic Acid to Old Rats Significantly Improves Metabolic Function While Decreasing Oxidative Stress,” Proceedings of the National Academy of Sciences USA, Vol. 99, No. 4, 2002, pp. 1870- 1875. doi:10.1073/pnas.261708898
- G. Paradies, F. M. Ruggiero, G. Petrosillo, M. N. Gadaleta and E. Quagliariello, “Effect of Aging and Acetyl-L-Carnitine on the Activity of Cytochrome Oxidase and Adenine Nucleotide Translocase in Rat Heart Mitochondria,” FEBS Letters, Vol. 350, No. 2-3, 1994, pp. 213-215. doi:10.1016/0014-5793(94)00763-2
- V. G. Desai, R. Weindruch, R. W. Hart and R. J. Feuers, “Influences of Age and Dietary Restriction on Gastrocnemius Electron Transport System Activities in Mice,” Archives of Biochemistry and Biophysics, Vol. 333, No. 1, 1996, pp. 145-151. doi:10.1006/abbi.1996.0375
- A. Navarro and A. Boveris, “Rat Brain and Liver Mitochondria Develop Oxidative Stress and Lose Enzymatic Activities on Aging,” American Journal of Physiology— Regulatory, Integrative and Comparative Physiology, Vol. 287, No. 5, 2004, pp. R1244-R1249. doi:10.1152/ajpregu.00226.2004
- R. J. Feuers, “The Effects of Dietary Restriction on Mitochondrial Dysfunction in Aging,” Annals of the New York Academy of Sciences, Vol. 854, 1998, pp. 192-201.
- S. Moghaddas, C. L. Hoppel and E. J. Lesnefsky, “Aging Defect at the QO Site of Complex III Augments Oxyradical Production in Rat Heart Interfibrillar Mitochondria,” Archives of Biochemistry and Biophysics, Vol. 414, No. 1, 2003, pp. 59-66. doi:10.1016/S0003-9861(03)00166-8
- J. Liu, E. Head, A. M. Gharib, W. Yuan, R. T. Ingersoll, T. M. Hagen, C. W. Cotman and B. N. Ames, “Memory Loss in Old Rats Is Associated with Brain Mitochondrial Decay and RNA/DNA Oxidation: Partial Reversal by Feeding Acetyl-L-Carnitine and/or R-α-Lipoic Acid,” Proceedings of the National Academy of Sciences USA, Vol. 99, No. 4, 2002, pp. 2356-2361. doi:10.1073/pnas.261709299
- J. Liu, D. W. Killilea and B. N. Ames, “Age-Associated Mitochondrial Oxidative Decay: Improvement of Carnitine Acetyltransferase Substrate-Binding Affinity and Activity in Brain by Feeding Old Rats Acetyl-L-Carnitine and/or R-α-Lipoic Acid,” Proceedings of the National Academy of Sciences USA, Vol. 99, No. 4, 2002, pp. 1876-1881. doi:10.1073/pnas.261709098
- J. Liu, H. Atamna, H. Kuratsune and B. N. Ames, “Delaying Brain Mitochondrial Decay and Aging with Mitochondrial Antioxidants and Metabolites,” Annals of the New York Academy of Sciences, Vol. 959, 2002, pp. 133- 166.
- A. Ugidos, T. Nystrom and A. Caballero, “Perspectives on the Mitochondrial Etiology of Replicative Aging in Yeast,” Experimental Gerontology, Vol. 45, No. 7-8, 2010, pp. 512-515. doi:10.1016/j.exger.2010.02.002
- B. Halliwell, “Antioxidants in Human Health and Disease,” Annual Review of Nutrition, Vol. 16, 1996, pp. 33- 50.
- K. M. Ko, T. Y. Leon, D. H. Mak, P. Y. Chiu, Y. Du and M. K. Poon, “A Characteristic Pharmacological Action of ‘Yang-Invigorating’ Chinese Tonifying Herbs: Enhancement of Myocardial ATP-Generation Capacity,” Phytomedicine, Vol. 13, No. 9-10, 2006, pp. 636-642. doi:10.1016/j.phymed.2006.02.007
- H. S. Wong, H. Y. Leung and K. M. Ko, “Yang-Invigorating Chinese Tonic Herbs Enhance Mitochondrial ATP Generation in H9c2 Cardiomyocytes,” Chinese Medicine, Vol. 2, No. 1, 2011, pp. 1-5.
- H. Y. Leung, P. Y. Chiu, M. K. Poon and K. M. Ko, “A Yang-Invigorating Chinese Herbal Formula Enhances Mitochondrial Functional Ability and Antioxidant Capacity in Various Tissues of Male and Female Rats,” Rejuvenation Research, Vol. 8, No. 4, 2005, pp. 238-247. doi:10.1089/rej.2005.8.238
- K. M. Ko and H. Y. Leung, “Enhancement of ATP Generation Capacity, Antioxidant Activity and Immunomodulatory Activities by Chinese Yang and Yin Tonifying Herbs,” Chinese Medicine, Vol. 2, 2007, p. 3.
- W. Liu, T. Ogata, S. Sato, K. Unoura and J. Onodera, “Superoxide Scavenging Activities of Sixty Chinese Medicines Determined by an ESR Spin-Trapping Method Using Electrogenerated Superoxide,” Yakugaku Zasshi, Vol. 121, No. 4, 2001, pp. 265-270. doi:10.1248/yakushi.121.265
- H. C. Liu, R. M. Chen, W. C. Jian and Y. L. Lin, “Cytotoxic and Antioxidant Effects of the Water Extract of the Traditional Chinese Herb Gusuibu (Drynaria fortunei) on Rat Osteoblasts,” Journal of the Formosan Medical Association, Vol. 100, No. 6, 2001, pp. 383-388.
- H. Haraguchi, J. Inoue, Y. Tamura and K. Mizutani, “Inhibition of Mitochondrial Lipid Peroxidation by Bakuchiol, a Meroterpene from Psoralea Corylifolia,” Planta Medica, Vol. 66, No. 6, 2000, pp. 569-571. doi:10.1055/s-2000-8605
- Y. T. Wong, K. K. Wong, K. Yeung and G. Mo, “Pharmacology and Clinical Research on Dipsacus Asperoides,” Pharmacology and Clinics of Chinese Materia Medica, Vol. 3, 1996, pp. 20-24.
- T. K. Yim and K. M. Ko, “Antioxidant and Immunodulatory Activities of Chinese Tonifying Herbs,” Pharmaceutical Biology, Vol. 40, No. 5, 2002, pp. 329-335. doi:10.1076/phbi.40.5.329.8457
- N. Chen and M. Ko, “Schisandrin B-Induced Glutathione Antioxidant Response and Cardioprotection Are Mediated by Reactive Oxidant Species Production in Rat Hearts,” Biological and Pharmaceutical Bulletin, Vol. 33, No. 5, 2010, pp. 825-829. doi:10.1248/bpb.33.825
- P. Y. Chiu, H. Y. Leung, A. H. Siu, N. Chen, M. K. Poon and K. M. Ko, “Long-Term Treatment with a Yang-Invigorating Chinese Herbal Formula Produces Generalized Tissue Protection against Oxidative Damage in Rats,” Rejuvenation Research, Vol. 11, No. 1, 2008, pp. 43-62. doi:10.1089/rej.2007.0577
- M. L. Chen, S. P. Ip, S. H. Tsai, K. M. Ko and C. T. Che, “Biochemical Mechanism of Wu-Zi-Yan-Zong-Wan, a Traditional Chinese Herbal Formula, against AlcoholInduced Oxidative Damage in CYP2E1 cDNA-Transfected HepG2 (E47) Cells,” Journal of Ethnopharmacology, Vol. 128, No. 1, 2010, pp. 116-122. doi:10.1016/j.jep.2009.12.036
- M. L. Chen, S. H. Tsai, S. P. Ip, K. M. Ko and C. T. Che, “Long-Term Treatment with a ‘Yang-Invigorating’ Chinese Herbal Formula, Wu-Zi-Yan-Zong-Wan, Reduces Mortality and Liver Oxidative Damage in Chronic Alcohol-Intoxicated Rats,” Rejuvenation Research, Vol. 13, No. 4, 2010, pp. 459-467. doi:10.1089/rej.2009.0985
- Population Division of the Department of Economic and Social Affairs of the United Nation Secretariat, “World Population Ageing: 1950-2050,” New York, 2001.
- M. F. Beal, “Mitochondria Take Center Stage in Aging and Neurodegeneration,” Annals of Neurology, Vol. 58, No. 4, 2005, pp. 495-505. doi:10.1002/ana.20624
- D. C. Chan, “Mitochondria: Dynamic Organelles in Disease, Aging, and Development,” Cell, Vol. 125, No. 7, 2006, pp. 1241-1252. doi:10.1016/j.cell.2006.06.010
- T. T. Huang, E. J. Carlson, A. M. Gillespie, Y. Shi and C. J. Epstein, “Ubiquitous Overexpression of CuZn Superoxide Dismutase Does Not Extend Life Span in Mice,” Journal of Gerontology Series A: Biological Sciences and Medical Sciences, Vol. 55, No. 1, 2000, pp. B5-B9. doi:10.1093/gerona/55.1.B5
- R. J. Mockett, A. C. Bayne, L. K. Kwong, W. C. Orr and R. S. Sohal, “Ectopic Expression of Catalase in Drosophila Mitochondria Increases Stress Resistance But Not Longevity,” Free Radical Biology and Medicine, Vol. 34, No. 2, 2003, pp. 207-217. doi:10.1016/S0891-5849(02)01190-5
- W. C. Orr, R. J. Mockett, J. J. Benes and R. S. Sohal, “Effects of Overexpression of Copper-Zinc and Manganese Superoxide Dismutases, Catalase, and Thioredoxin Reductase Genes on Longevity in Drosophila Melanogaster,” Journal of Biological Chemistry, Vol. 278, No. 29, 2003, pp. 26418-26422. doi:10.1074/jbc.M303095200
- S. R. Steinhubl, “Why Have Antioxidants Failed in Clinical Trials?” American Journal of Cardiology, Vol. 101, No. 10A, 2008, pp. 14D-19D. doi:10.1016/j.amjcard.2008.02.003
- Y. Huang, “Gerontology,” Shanghai Science and Technology Press, Shanghai, 1989.
- G. H. Li, “The Differentiation and Treatment of Parkinson’s Disease According to Traditional Chinese Medicine,” Journal of Chinese Medicine, Vol. 30, 1989, pp. 1- 4.
- X. Yu, Q. Meng, G. Zhao and B. Chen, “Clinical Management of Parkinson’s Disease by Chinese Medicine,” Proceedings of 4th National Conference on Integrated Chinese and Western Medicine on Health Promotion and Rehabililation Medicine, 2004, pp. 355-359.
- K. M. Ko, K. Chan, L. Shek, J. Yeung and S. H. Chui, “A ‘Yang-Invigorating’ Chinese Herbal Suppository Preparation Relieves Symptoms in Patients with Parkinson’s Disease,” New Trends in Alzheimer and Parkinson Related Disorders ADPD, 2005, pp. 243-246.
- K. M. Ko, P. Y. Chiu, H. Y. Leung, A. H. Siu, N. Chen, E. P. Leong and M. K. Poon, “Long-Term Dietary Supplementation with a Yang-Invigorating Chinese Herbal Formula Increases Lifespan and Mitigates Age-Associated Declines in Mitochondrial Antioxidant Status and Functional Ability of Various Tissues in Male and Female C57BL/6J Mice,” Rejuvenation Research, Vol. 13, No. 2-3, 2010, pp. 168-171. doi:10.1089/rej.2009.0893
- E. J. Anderson, H. Yamazaki and P. D. Neufer, “Induction of Endogenous Uncoupling Protein 3 Suppresses Mitochondrial Oxidant Emission during Fatty Acid-Supported Respiration,” Journal of Biological Chemistry, Vol. 282, No. 43, 2007, pp. 31257-31266. doi:10.1074/jbc.M706129200
- L. J. Toime and M. D. Brand, “Uncoupling Protein-3 Lowers Reactive Oxygen Species Production in Isolated Mitochondria,” Free Radical Biology and Medicine, Vol. 49, No. 4, 2010, pp. 606-611. doi:10.1016/j.freeradbiomed.2010.05.010
- G. Barja, “Aging in Vertebrates, and the Effect of Caloric Restriction: A Mitochondrial Free Radical ProductionDNA Damage Mechanism?” Biological Reviews of the Cambridge Philosophical Society, Vol. 79, No. 2, 2004, pp. 235-251. doi:10.1017/S1464793103006213
NOTES
*Conflict of Interest: The authors have not received any funding or benefits from industry or elsewhere to compose this article.
#Corresponding author.

