Journal of Modern Physics
Vol.06 No.09(2015), Article ID:58943,11 pages
10.4236/jmp.2015.69133
Non-Probabilistic Approach to the Time of Energy Emission in Small Quantum Systems
Stanisƚaw Olszewski
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka, Warsaw, Poland
Email: olsz@ichf.edu.pl
Copyright © 2015 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 21 July 2015; accepted 18 August 2015; published 21 August 2015
ABSTRACT
The energy emitted by an electron in course of its transition between two quantum levels can be considered as a dissipated energy. This energy is obtained within a definite interval of time. The problem of the size of the time interval necessary for transitions is examined both on the ground of the quantum approach as well as classical electrodynamics. It is found that in fact the emission time approaches the time interval connected with acceleration of a classical velocity of the electron particle from one of its quantum levels to a neighbouring one.
Keywords:
Dissipated Energy and Time Intervals of the Quantum Transitions, Electron Acceleration in Simple Quantum Systems Considered as a Test of the Theory

1. Introduction. Planck’s Approach to an Electron Transition and Its Difficulty
The phenomenon of the electron transitions between quantum levels is basic for the quantum theory. In fact the theory began by a fit of the transition energy of a set of oscillators to the intensity of the emission spectrum of the black body examined with respect to its dependence on the body temperature [1] [2] . A success of Planck was based on discovery that the energy of the oscillators is regularly a multiple
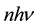 (1)
(1)
of the same expression
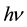 (2)
(2)
where 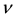 is the oscillator frequency and
is the oscillator frequency and  is the Planck constant.
is the Planck constant.
In a further development of the theory the result of (2) has been extended to any transition energy between two quantum levels, not necessarily those belonging to the oscillator. In effect the formula
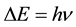 (3)
(3)
couples the energy interval
 (4)
(4)
with the frequency  of the electromagnetic wave characteristic for any transition between two different quantum levels.
of the electromagnetic wave characteristic for any transition between two different quantum levels.
However an important lack of the theory which remained in it was the problem of the time interval
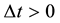 (5)
(5)
necessary for the process of the energy change 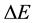 entering (3). In accordance with the statistical background of the energy spectrum of the black-body radiation the problem of
entering (3). In accordance with the statistical background of the energy spectrum of the black-body radiation the problem of  has not been examined for an individual transition between separate quantum levels, but approached on the basis of a probabilistic analysis of the population number of the quantum states forming the interval
has not been examined for an individual transition between separate quantum levels, but approached on the basis of a probabilistic analysis of the population number of the quantum states forming the interval . This kind of reasoning, characteristic already for the old quantum theory [3] [4] , has been consequently prolongated and extended in case of quantum mechanics; see e.g. [1] [5] -[7] .
. This kind of reasoning, characteristic already for the old quantum theory [3] [4] , has been consequently prolongated and extended in case of quantum mechanics; see e.g. [1] [5] -[7] .
The aim of the present paper is to examine the problem of the transition time
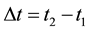 (6)
(6)
on the ground of a non-probabilistic (non-statistical) approach. Therefore formally the task becomes rather opposite to a treatment which has been usually applied. Only the emission rate of energy 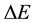 between two neighbouring quantum states is mainly considered. The obtained result is that
between two neighbouring quantum states is mainly considered. The obtained result is that 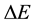 and
and  should satisfy the equation
should satisfy the equation
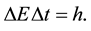 (7)
(7)
Formally (7) is much similar to the well-known Heisenberg principle of uncertainty for energy and time [8] which is

But the interval 




so 

Physical and philosophical implications of (9) have been discussed on many occasions, see e.g. [9] [10] . An important philosophical implication of (9) was to point out an indeterministic character of the relation between 

2. Quanta of the Dissipated Energy and Intervals of Time Necessary to Produce Them
In the first step, the aim of the formalism developed in the present paper is to demonstrate that 





Evidently 


The energy differences

between two neighbouring quantum states having the indices



for the hydrogen atom [15] ,

for a free particle having mass 


for the harmonic oscillator having the frequency
The time periods of the electron particle circulation on the orbits are defined by a physical character of each of the above systems. They are

for the case of electron in the hydrogen atom occupying the state 

for the particle of mass m being in state n in the potential box because of the relation between energy and velocity equal to
and

for all states 
A characteristic property of expressions (13)-(18) is that

holds for the atomic orbit 

for a free particle (free electron) in state 

for the harmonic oscillator [see (15) and (18)]. A common feature of (13a)-(15a) is that

If we note that the resistance 

where 



we obtain

In the last step in (22) the result of (19) is taken into account.
The 

Let the dissipation heat 

By putting

[see (10)] we obtain from (21)-(24) the following relation

which gives

But because of (19) the formula (26) can be transformed into

which implies that

In effect of (28) the relation (26) can be presented in a more familiar form:

cf. here (7).
A comparison of the time rate of energy emission calculated according to the method presented above with the quantum-mechanical method is done in [23] .
3. A Check of the Theory: Acceleration of Electrons Obtained in Effect of Their Quantum Transitions
The physics of the test is much similar to that entering the Tolman experiment [22] . The point is that the change of the electron energy in course of its transition between the quantum levels is accompanied by a change 




where the time of emission 




where 


Beginning with the hydrogen atom we have 




is given in (16), and



On the other side, a substitution of the absolute value of 

Evidently both expressions (33) and (34) are equal:
A similar operation can be repeated for the electron in the potential box. Here [see (17) and equation below of it] the velocity

so

The time period in state n is that given in (17) and

for any state n. Therefore the left-hand side of (30a) becomes

and the right-hand side is

In effect we obtain
which is a similar property to that calculated above in the case of the hydrogen atom.
The case of the harmonic oscillator is rather different than that of the electron in the hydrogen atom or the potential box because the velocity 

where k is the force constant. In consequence the formalism described in (30) and (30a) is applied solely to the velocity acceleration at a single point

The electron velocity 

where 

By considering solely the positive sign in (43) the increment of velocity due to the change of the quantum state becomes

The length 

where

so

In effect the left-hand side of Equation (30a) becomes

and the right-hand side of (30a) is

In consequence we obtain an approximate equality of both sides of (30a) represented by the relation
but not precisely the relation
4. Semiclassical Approach to the Dissipated Energy and Transition Time Deduced from the Ohm’s Law
An approach to the Joule-Lenz dissipation energy and its transition time can be done also on a semiclassical basis. First we note that the effective electric field 


where 

Here 


where 

can be provided by the energy difference 


The efficiency of the Joule-Lenz heat 


where 



and 

Since (see [22] )

we obtain because of (55) the following result

By assuming that
[see (10)] the formula (60) yields evidently the result

obtained in (28).
5. Energy Emission and Its Time in the Case of
In preceding sections the case of the neighbouring quantum states 

For example for the hydrogen atom the situation (62) implies

see (13) where 




Assuming that the end state of the energy emission has the index 

The length of the electron path covered within the time period 

see (32).
Our aim is to check the validity of the formula (30a) for the case of the emission from the state



We substitute on the left-hand side of (30a) the quantities 


On the right-hand side of (30a) a substitution of 


We find that the left-side of (30a) presented in (68) differs from the right-hand side presented in (69) solely by a factor of 2:
It is easy to demonstrate that a particle in the potential box and the harmonic oscillator submitted to the check given by the Equation (30a) do not satisfy this equation. For an electron in the potential box the energy 





It should be noted that for the transition 



This result is different from a similar product calculated in the case on 
6. Poynting’s Vector in the Hydrogen Atom and the Emission Time
We define [21] [22] [24]

as Poynting’s vector. The time rate of the loss of energy is [24]

A well-known formal asymmetry of the Bohr model of the hydrogen atom is the presence of the electric field strength

in the orbit plane for any quantum state n, but this presence is combined with the absence of a similar magnetic field strength




[

With the electron circulating with frequency (74a) is associated the field strength 

In effect

which is the size of a vector normal to the orbit plane.
A substitution of 


Since 




On the other side, the cross-section of the toroidal cylinder is dictated by the radius (see e.g. [22] )

of the electron particle which moves along the orbit. In effect the toroidal surface is approximated by the product of (78) and the circumference of the cross-section of the torus cylinder which is

The value of the Poynting vector for a thin electron orbit can be assumed as a constant number given in (77), therefore a non-vanishing term on the right of (72) becomes equal to

This is a product of (77), (78) and (80). In effect the Equation (72) has the form

Since the emitted energy in course of the electron transition between levels 
the emission time for that energy is

where the term taken in brackets is that calculated in (82).
The result of (83) should be compared with that given by the quantum-mechanical formula (29). This gives

which is a number larger by the factor of

than that of (83).
This is an expected situation because the emission rate described by the Poynting vector is not restricted to a single transition from level 
It seems of interest to demonstrate that 


The current 

where

is the volume occupied by the electron particle and

is the cross-section area of both of the volume 
Since the integral of 



which is a formula identical to that given in (76).
7. Summary and Comments
In his derivation of the formula (3) applied in the present paper, Einstein [4] has pointed out that statistical considerations supplemented by classical physics were necessary to obtain that formula. In effect no insight to an individual transition time of a particle between two quantum levels has been done. The aim of the paper was to bridge this gap.
The first step demonstrates that instead of (3) the Joule-Lenz dissipation energy can be applied [see (10) and (24)]. This yields an estimate of the emission time 
The 

It has been demonstrated that for 
A good fit of 


to the velocity in state n, viz.

A separate study of 
A similar agreement of emission time and acceleration time is present also for 
The only condition imposed on the applied formalism is that the electron states considered in a system are periodic in time. This property can be coupled rather easily with the idea of the electron orbit, for example that introduced by Bohr in the model of the hydrogen atom. In this case the electron remaining in a quantum stationary state is circulating incessibly along an orbit of a definite size within a definite constant period of time [15] .
In general the use of the orbit idea is well known in the whole domain of the atomic, molecular and solid-state physics [26] . For solids the idea became widely applied as soon as Bloch proposed to describe the electron wave functions with the aid of combinations of the atomic orbitals spread over all atoms building up regularly a given crystal sample [27] . Such combinations, classified according to the quantum parameter

where 


The Bloch model, especially of a one-dimensional crystal, is much similar to the model based on the standing-like wave functions characteristic for the electron particles enclosed in a potential box discussed in the present paper; see e.g. [28] .
Cite this paper
StanisƚawOlszewski, (2015) Non-Probabilistic Approach to the Time of Energy Emission in Small Quantum Systems. Journal of Modern Physics,06,1277-1288. doi: 10.4236/jmp.2015.69133
References
- 1. Rubinowicz, A. (1968) Quantum Mechanics. Elsevier, Amsterdam.
- 2. Loudon, R. (1991) The Quantum Theory of Light. 2nd Edition, Clarendon Press, Oxford.
- 3. Planck, M. (1910) Acht Vorlesungen ueber Theoretische Physik. S. Hirzel, Leipzig.
- 4. Einstein, A. (1917) Physikalische Zeitschrift, 18, 121.
- 5. Schiff, L.I. (1968) Quantum Mechanics. 3rd Edition, McGraw-Hill, New York.
- 6. Slater, J.C. (1968) Quantum Theory of the Atomic Structure. McGraw-Hill, New York.
- 7. Weinberg, S. (2013) Lectures on Quantum Mechanics. Cambridge University Press, Cambridge.
- 8. Heisenberg, W. (1927) Zeitschrift für Physik, 43, 172.
http://dx.doi.org/10.1007/BF01397280 - 9. Landau, L. and Peierls, R. (1931) Zeitschrift für Physik, 69, 56.
http://dx.doi.org/10.1007/BF01391513 - 10. Jammer, M. (1974) The Philosophy of Quantum Mechanics. Wiley, New York.
- 11. Schommers, W. (1989) Space-Time and Quantum Phenomena. In: Schommers, W., Ed., Quantum Theory and Pictures of Reality, Springer-Verlag, Berlin, 217-277.
http://dx.doi.org/10.1007/978-3-642-95570-9_5 - 12. Bunge, M. (1970) Canadian Journal of Physics, 48, 1410-1411.
http://dx.doi.org/10.1139/p70-172 - 13. Allcock, G.R. (1959) Annals of Physics, 53, 253-285.
http://dx.doi.org/10.1016/0003-4916(69)90251-6 - 14. Isaacs, A. (1990) Concise Dictionary of Physics. Oxford University Press, Oxford.
- 15. Sommerfeld, A. (1931) Atombau und Spektrallinien: Volume 1. 5th Edition, Vieweg, Braunschweig.
- 16. Eyring, H., Walter, J. and Kimball, G.E. (1957) Quantum Chemistry. Wiley, New York.
- 17. MacDonald, A.H., Ed. (1989) Quantum Hall Effect: A Perspective. Kluwer, Milano.
http://dx.doi.org/10.1007/978-94-010-9709-3 - 18. Olszewski, S. (2013) Quantum Matter, 2, 102-104.
http://dx.doi.org/10.1166/qm.2013.1030 - 19. Olszewski, S. (2013) Journal of Modern Physics, 4, 14-20.
http://dx.doi.org/10.4236/jmp.2013.411A1003 - 20. Olszewski, S. (2014) Quantum Matter, 3, 155-160.
http://dx.doi.org/10.1166/qm.2014.1108 - 21. Lass, H. (1950) Vector and Tensor Analysis. McGraw-Hill, New York.
- 22. Matveev, A.N. (1964) Electrodynamics and the Theory of Relativity. Izd. Wyzszaja Szkola, Moscow. (In Russian)
- 23. Olszewski, S. Quantum Matter, in Press.
- 24. Griffiths, D.J. (1999) Introduction to Electrodynamics. 3rd Edition, Prentice-Hall, Upper Saddle River.
- 25. Slater, J.C. (1967) Quantum Theory of Molecules and Solids. Volume 3, McGraw-Hill, New York.
- 26. Slater, J.C. (1963) and (1965) Quantum Theory of Molecules and Solids. Volume 1 and Volume 2, McGraw-Hill, New York.
- 27. Bloch, F. (1928) Zeitschrift für Physik, 52, 555-600.
http://dx.doi.org/10.1007/BF01339455 - 28. Mott, N.F. and Jones, H. (1958) Theory of the Properties of Metals and Alloys. Oxford University Press, Reprinted by Dover Publications, New York.










