Journal of Modern Physics
Vol.06 No.05(2015), Article ID:55726,4 pages
10.4236/jmp.2015.65067
Relationship between the Fundamental Constants of Physics Obtained from the Uncertainty Principle for Energy and Time
Stanisław Olszewski
Institute of Physical Chemistry, Polish Academy of Sciences Kasprzaka, Warsaw, Poland
Email: olsz@ichf.edu.pl
Copyright © 2015 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 27 January 2015; accepted 15 April 2015; published 16 April 2015
ABSTRACT
An attempt is done to calculate the value of the elementary electron charge from its relation to the Planck constant and the speed of light. This relation is obtained, in the first step, from the Pauli analysis of the strength of the electric field associated with an elementary emission process of energy. In the next step, the uncertainty principle is applied to both the emission time and energy. The theoretical result for e is roughly close to the experimental value of the electron charge.
Keywords:
Fundamental Constants of Physics, Uncertainty Principle for Energy and Time

1. Introduction
As soon as the atomic theory of matter occured to be a right idea, there arose a tendency to describe the physical properties of matter with the aid of a possibly low number of the elementary notions concerning the atoms and their structure. A further step in this direction has been provided by the quantum theory. In effect numerous properties of the atomic world could be represented in terms of the so-called fundamental constants of physics which are evidently few in their number. Perhaps the most widely used constants became e, m, h and c, which are respectively the electron charge and electron mass, the elementary action called the Planck constant and the speed of light.
Simultaneously a mutual reference between the constants mentioned above seemed to be not so much evident. The aim of the present paper is to demonstrate that, in fact, a reference between e, h and c can be supplied in effect of i) an elementary analysis of the forces entering the emission process of the electron energy, ii) an application of the uncertainty principle for energy and time which couples the parameters considered in i).
The energy-time aspect of the uncertainty principle has been presented originally by Heisenberg [1] (see also e.g. [2] ) in the formula
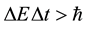 (1)
(1)
where 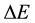 is the energy change in a quantum process the duration of which is
is the energy change in a quantum process the duration of which is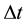 . But (1) has been objected on many occasions [3] - [5] and in fact numerous textbooks on quantum mechanics neglect (1) at all; see e.g. [6] . However, a modified approach to the coupling between
. But (1) has been objected on many occasions [3] - [5] and in fact numerous textbooks on quantum mechanics neglect (1) at all; see e.g. [6] . However, a modified approach to the coupling between  and
and 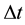 is also possible [7] - [9] . This gives instead of (1) a relation
is also possible [7] - [9] . This gives instead of (1) a relation
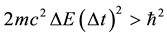 (2)
(2)
which on many occasions can be replaced by an approximate equation
 (2a)
(2a)
The formula (2a) allowed us to approach several problems of the elementary quantum theory, for example the spectrum of the Bohr hydrogen atom [10] and the spin mechanism [11] [12] . A check of (2) has been done in [13] . It gives the formula
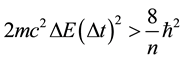 (2b)
(2b)
where 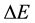 is the energy of transitions between the Bohr quantum levels n and
is the energy of transitions between the Bohr quantum levels n and 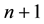 and
and 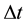 is the time of transition estimated in [13] . Evidently for n close to 10 the formula (2b) approaches (2a) and for
is the time of transition estimated in [13] . Evidently for n close to 10 the formula (2b) approaches (2a) and for  there is satisfied the relation (2). In the present paper the aim of (2a) is to put a bridge between e, h and c.
there is satisfied the relation (2). In the present paper the aim of (2a) is to put a bridge between e, h and c.
One of the notions useful to this purpose is a minimal distance between two particles having the same mass m. This is
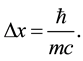 (3)
(3)
Equation (3) has been derived also on the basis of the formula (2a); see [10] . Before (2a) and (3) are applied, we refer to the Pauli analysis of the radiation emission process.
2. Pauli Analysis of the Electric Field Involved in the Radiation Emission Process [14]
Pauli’s idea was to consider the strength of the electric field  connected with an oscillator having a definite frequency
connected with an oscillator having a definite frequency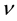 . The change of the number of quanta of the oscillator connected with the emission process is unknown. The average frequency
. The change of the number of quanta of the oscillator connected with the emission process is unknown. The average frequency 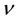 of the emitted light let be referred to the interval of time
of the emitted light let be referred to the interval of time 

In effect the intensity square of the electric field 


In order to derive (4a) two formulae for the momentum change 


where 

where 

or

On the other hand the change 



so

Therefore a final formula gives

According to Pauli the square root of 
However, for simplicity, let us assume that this excess is small and the change of the field intensity in the emission is

In result the force acting on the electron particle becomes

We assume that the force given in (11) is acting along an elementary (minimal) space interval 



3. Energy ΔE of (12) Applied in the Uncertainty Formula (2a) Gives an Equation for e
As a final step we substitute the energy change (12) to the uncertainty formula (2a). This gives an approximate equation

which can be simplified to the relation

or

coupling the constants h, c, and e. A substitution of

and

into (15) gives

The well-known value of the measured e is

The difference between (18) and (18a) is about 50 percent of (18). In some earlier papers (see [15] -[17] and also [18] instead of (3) the Compton length

is proposed which is larger than 



An approximate character of the formalism applied in the present paper is evident.
4. Summary
The electron charge e has been calculated from the Planck constant h and the speed of light c. This has been done on the basis of i) the Pauli expression for the emission strength of the electric field, ii) the uncertainty principle for energy and time. The emitted energy is obtained as a product of the force of the electric field and the elementary distance 
In case the factor of 1/2 introduced by Pauli as a multiplier of 

This number is different by less than 40 percent of its value from the experimental e represented in (18a).
The particle mass does not interfere in the equations of the paper. This implies that the absolute value of e can be the same for electrons and protons.
References
- Heisenberg, W. (1927) Zeitschrift fuer Physik, 43, 172. http://dx.doi.org/10.1007/BF01397280
- Schiff, L.I. (1968) Quantum Mechanics. 3rd Edition, McGraw-Hill, New York.
- Schommers, W. (1989) Space-Time and Quantum Phenomena. In: Schommers, W., Ed., Quantum Theory and Pictures of Reality, Springer, Berlin. http://dx.doi.org/10.1007/978-3-642-95570-9_5
- Allcock, G.R. (1969) Annals of Physics, 53, 253. http://dx.doi.org/10.1016/0003-4916(69)90251-6
- Bunge, M. (1970) Canadian Journal of Physics, 48, 1410. http://dx.doi.org/10.1139/p70-172
- Isaacs, A. (1990) Concise Dictionary of Physics. Oxford University Press, Oxford.
- Olszewski, S. (2011) Journal of Modern Physics, 2, 1305. http://dx.doi.org/10.4236/jmp.2011.211161
- Olszewski, S. (2012) Journal of Modern Physics, 3, 217. http://dx.doi.org/10.4236/jmp.2012.33030
- Olszewski, S. (2012) Quantum Matter, 1, 127. http://dx.doi.org/10.1166/qm.2012.1010
- Olszewski, S. (2014) Journal of Modern Physics, 5, 1264. http://dx.doi.org/10.4236/jmp.2014.514127
- Olszewski, S. (2014) Journal of Modern Physics, 5, 2022-2029. http://dx.doi.org/10.4236/jmp.2014.518198
- Olszewski, S. (2014) Journal of Modern Physics, 5, 2030-2040. http://dx.doi.org/10.4236/jmp.2014.518199
- Olszewski, S. (2012) Quantum Matter, 1, 59-62. http://dx.doi.org/10.1166/qm.2012.1006
- Pauli, W. (1933) Die allgemeine Prinzipien der Wellenmechanick. In: Geiger, H. and Scheel, K., Eds., Handbuch der Physik, Vol. 24, Part 1, Springer, Berlin.
- Ruark, A.E. (1928) Proceedings of the National Academy of Sciences of the United States of America, 14, 322-328. http://dx.doi.org/10.1073/pnas.14.4.322
- Flint, H.E. (1928) Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, 117, 630- 637. http://dx.doi.org/10.1098/rspa.1928.0025
- Flint, H.E. and Richardson, O.W. (1928) Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, 117, 637-649. http://dx.doi.org/10.1098/rspa.1928.0026
- Jammer, M. (1966) The Conceptual Development of Quantum Mechanics. McGraw-Hill, New York.

