Advances in Bioscience and Biotechnology
Vol.4 No.4A(2013), Article ID:30644,6 pages DOI:10.4236/abb.2013.44A012
VACCINATION of Atlantic salmon leads to long-lasting higher levels of serum immunoglobulin and possible skewed ratios of two distinct IgM isotypes
![]()
1Department of Biology, University of Bergen, Bergen, Norway
2Matre Research Station, Institute of Marine Research, Bergen, Norway
3Department of Molecular Biology, University of Bergen, Bergen, Norway
4The Norwegian School of Veterinary Science, Oslo, Norway
Email: *ivar.hordvik@bio.uib.no
Copyright © 2013 Atif Kamil et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 19th, 2013; revised March 15th, 2013; accepted April 18th, 2013
Keywords: Teleost; Atlantic Salmon; Vaccine; IgM; Tetraploidy
ABSTRACT
Primary and secondary antibody responses in blood of teleost fish are mainly IgM mediated, as they lack an IgG homolog and a class switch mechanism. Thus, the major serum immunoglobulin is tetrameric IgM. A unique antibody class in teleosts; named IgT, appears to be specialized for mucosal immunity and is present in low concentrations in serum. A third class; IgD was recently detected in serum of rainbow trout, but is less abundant than IgT. In the present study, relative quantification of total IgM showed that Atlantic salmon (Salmo salar) which had received an oiladjuvanted commercial vaccine maintained about 2-fold higher levels of IgM; 14 and 17 months after intraperitoneal injection, in comparison to unvaccinated fish kept in the same cage. Similar results were obtained by ELISA (serum IgM) and reverse transcription real time PCR (spleen mRNA). Analyses conducted in our lab have shown that several salmonid species possess two slightly different IgM isotypes as a result of ancestral tetraploidy. In Atlantic salmon, two distinct IgM subpopulations (A and B) can be separated by anion exchange chromatography. The IgM-B tetramer exhibits a higher degree of disulfide cross-linking than IgM-A, presumably due to an extra cysteine near the C-terminus of the heavy chain. The typical A/B ratio is approximately 40/60. Anion exchange elution profiles of serum IgM from vaccinated fish indicate that prolonged triggering of the immune system can lead to a skewed ratio of IgM-A/IgM-B. In the context of recent results from rainbow trout (Onchorhynchusmykiss), showing that high affinity antibodies are more highly polymerized and have a longer half life time, Atlantic salmon is an interesting model to elucidate these aspects further since tools are available to distinguish IgM-A and IgMB in this species.
1. INTRODUCTION
The major form of serum immunoglobulin in teleost fish is IgM, whereas two other classes of antibodies; IgT and IgD, are present in relatively low quantities [1]. The IgT isotype, which is unique for teleosts, appears to be specialized to mucosal immune responses. The plasma concentration of IgT was estimated to be 1000 fold lower than IgM in naïve fish [2].Transcripts encoding membrane bound IgD seem to be dominant in most teleosts, but recently it was shown that alternative mRNA usage generates a secreted form of IgD in rainbow trout [3]. In catfish, a separate gene encodes secretory IgD, but this is not typical for teleost fish [4]. Teleosts constitute a heterogenous group of vertebrates with several examples of unique Ig structures, gene duplications and splicing variants [1,5-8]. In contrast to higher vertebrates, the expression of Ig isotypes in teleost fish is not regulated by a class switch mechanism.
The IgM concentration in fish varies to a large extent: from species to species, between males and females, by age, and from one individual to another [9,10]. Furthermore, there are reproduction related immunoglobulin changes, and seasonal changes presumably related to water temperature. The IgM serum concentration in a series of examined fish species listed in [9] ranged from 0.63 to 17.2 mg/ml, and the proportion of Ig protein in the sera varied from 2% to 47%. Atlantic salmon (Salmo salar) are among teleost fish with a relatively low serum IgM concentration (~0.8 - 1.3 mg/ml serum), constituting about 2% of the total serum protein [11,12]. In comparison, Atlantic cod has a high IgM concentration (~5.6 - 11.5 mg/ml), constituting up to 50% of the total serum protein [12].
Typical serum concentrations in human are, for IgM: 1.5 mg/ml, IgG: 13.5 mg/ml, and IgA: 3.5 mg/ml. Serum immunoglobulin levels (of IgM, IgG and IgA) are higher in adults than in children, and IgM levels are higher in females than males. IgG and IgA continue to increase throughout adult life [13,14]. In general, variations among humans are small compared to those in fish.
It has been suggested that post translational diversity of IgM in teleosts might compensate to some degree for the absence of for example IgG during secondary immune responses. In rainbow trout (Onchorhynchus mykiss) high affinity IgM antibodies (i.e. tetramers) have a higher degree of disulfide polymerization and a longer half life time [15,16].
As a result of a tetraploid event in the ancestor of the salmonid fish family there are two paralogous Ig heavy chain gene complexes (or remnants) in the genome of several species belonging to Salmonidae [17-23]. IgM heavy chain sub-variants have been characterized in Atlantic salmon, brown trout (Salmo trutta) and arctic char (Salvelinus alpinus). In members of the genus Salmo (i.e. Atlantic salmon and brown trout) an additional cysteine residue has been introduced in the C-terminus of the IgM-B heavy chain [20,23]. In agreement with this, the IgM-B tetramers hold a higher degree of disulfide bonding than the accompanying sub-variant IgM-A [23]. Thus, Atlantic salmon and brown trout may be promising models to study functional aspects related to IgM heterogeneity since IgM-A and IgM-B have distinct features and can be distinguished by use of the monoclonal antibody MAb4C10 [24]. The objective of the present study was 1) to quantify the relative abundance of transcripts encoding the heavy chains of IgM (m), IgT (t) and IgD (d) in spleen; and 2) to reveal possible effects of vaccination on serum IgM, i.e. changes in total IgM concentration and the proportion of IgM-A and IgM-B antibodies. To estimate the relative abundance of the highly similar mA and mB mRNAs different approaches were attempted and evaluated. Both diploid and triploid fish were included in the study to reveal possible ploidy effects on Ig expression.
2. MATERIAL AND METHODS
2.1. Fish
Atlantic salmon were provided by The Industrial and Aquatic Laboratory (ILAB) at the High Technology Center in Bergen for initial analyses of Ig gene expression. Subsequently, sampling of unvaccinated and vaccinated Atlantic salmon (diploid and triploid fish) was performed at Matre research station (Institute of Marine Research). Hatching of these fish started on 05.02.2010 and was completed by 18.02.2010. Intra-peritoneal vaccination was performed 25.11.2010. In total the experiment included 3120 fish and represents the source for several studies. More detailed information on the fish can be found in [25,26]. Our samplings were performed on 19.01.2012 and 30.04.2012, respectively. During sampling the fish were treated in accordance with the regulations for euthanasia of fish in aquaculture issued by the Norwegian Directorate of Fisheries. The fish were pittagged and the status of each individual (diploid versus triploid, and vaccinated versus unvaccinated) were recorded before killing. During the first sampling (n = 20) 5 diploids and 5 triploids were selected from unvaccinated and vaccinated fish, respectively. The weight of the fish was from 1945 g to 4850 g and the length was from 53 cm to 71 cm. Gender was not recorded during the first sampling. During the second sampling (n = 24) 6 diploids and 6 triploids were selected from unvaccinated and vaccinated fish, respectively. The weight of the fish varied from 2830 g to 6535 g and the length from 60 cm to 76 cm. Among the unvaccinated fish there were 5 females and 7 males. Among the vaccinated fish there were 7 females and 5 males.
2.2. Isolation of RNA and Synthesis of cDNA
RNA was isolated by use of Trizol Reagent (Life Technologies, USA). First strand cDNA was synthesized by use of MMLV reverse transcriptase (Promega, Madison, USA) and an oligo-dT primer.
2.3. Conventional and Real Time PCR
Reverse transcription quantitative PCR (RT-qPCR) was performed as described in [1]. Real time assays utilized in the present study are listed in Table 1. Conventional PCR followed by restriction enzyme analysis was performed as described in [24]. Primers J.s (TTTGACTACTGGGGGAAAGG) and m3.anti (CCCATTGCTCCAGTCCTCAT) were used for amplification of m mRNA.
2.4. ELISA
Salmon sera in several different dilutions (1/10, 1/20, 1/100, 1/200, 1/300 and 1/500) in coating buffer (carbonate-bicarbonate buffer from Sigma Aldrich, Product No: C3041) were used for coating flat bottom 96-well plates overnight at 4˚C. The plate was washed three
Table 1. Real time PCR assays.

times with PBST after each step. The plate was blocked with 5% dry milk in PBS for 2 hours at room temperature. Thereafter the plate was incubated with polyclonal rabbit anti-salmon IgM (1:3000) or monoclonal mouse antitrout IgM (MAb4C10) antibodies (1:500) overnight at 4˚C. Horseradish peroxidase (HRP)-conjugated anti-rabbit (1:10,000) or anti-mouse (1:5000) respectively was applied for 2 hours at room temperature. For colorimetric measurement liquid substrate for HRP (Sigma-Aldrich Product No: T0440) 3,3’,5,5’-tetramethylbenzidine (TMB) was applied to all the wells for 30 min in dark at room temperature. Then equal volume of TMB stop reagent (Sigma-Aldrich Catalog No: S5814) was added to all wells and the absorbance of the resulting yellow color was measured at 450 nm.
2.5. Purification of IgM
Blood was kept for 2 - 15 hours at 4˚C before centrifugation. 1 - 3 ml of serum (fresh or stored at −80˚C) was purified by gel filtration followed by anion-exchange chromatography as described previously [11,24].
3. RESULTS
3.1. Immunoglobulin Gene Expression in Unvaccinated and Vaccinated Fish
Atlantic salmon (unvaccinated and vaccinated, diploid and triploid fish) were sampled at two time points; 14 and 17 months after vaccination. At both samplings, the abundance of m transcripts was higher (>2 fold) in spleen of vaccinated fish compared to unvaccinated fish (Table 2). The abundance of t transcripts was low (>5 CT values higher than m), and the d expression was too low to give reliable results (data not shown). The m gene expression was somewhat higher in males than in females (Table 3), but confirmation of this variation requires a more comprehensive study. Prior to the examination of samples from the vaccination experiment, a series of tissues from healthy fish were subjected to reverse transcription real time PCR to evaluate the relative abundance of mA and mB transcripts. The main conclusion of this analysis was that both sub-types are uniformly expressed in healthy fish, but minor deviations
Table 2. Fold increase in m gene expression (RT-qPCR) and IgM concentration (ELISA) in vaccinated (V) versus unvaccinated (UV) fish.
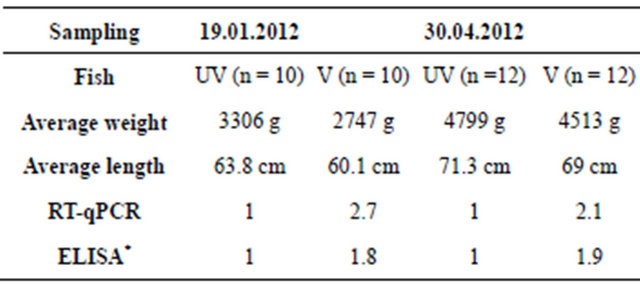
*Measured by use of MAb4C10.
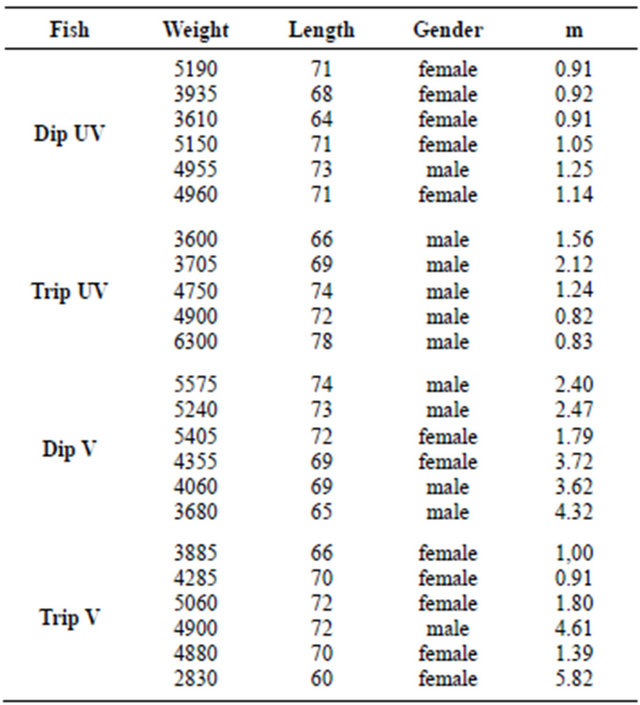
Table 3. Relative abundance (fold) of m transcripts in spleen of individual diploid (Dip) and triploid (Trip) fish sampled 30. 04.2012.
might have been ignored due to methodological limitations. Accordingly, differences in the A/B ratio of m transcripts in spleen of vaccinated versus unvaccinated fish could not detected by PCR/restriction analysis (see discussion).
3.2. Serum Immunoglobulin in Vaccinated Versus Unvaccinated Atlantic Salmon
In accordance with the reverse transcription real time PCR analysis, ELISA showed that total IgM in serum was higher (1.8 - 1.9 fold) in vaccinated fish versus unvaccinated fish, utilizing the monoclonal antibody MAb4C10 (Table 2). Fold increase of IgM was estimated to be somewhat lower when a polyclonal antiserum was employed instead of Mab4C10. However, it is plausible to assume that this discrepancy was attributed to polyclonal antibody cross-reaction with other proteins in serum. Previous studies including samples from fish farms have shown that serum IgM concentration can vary considerably between groups [27-29], but average fold increase is in agreement with the present measurements. Since MAb4C10 only detects IgM-A in Atlantic salmon the increase of total IgM might have been underestimated to some degree if IgM-B is generally more abundant in vaccinated fish.
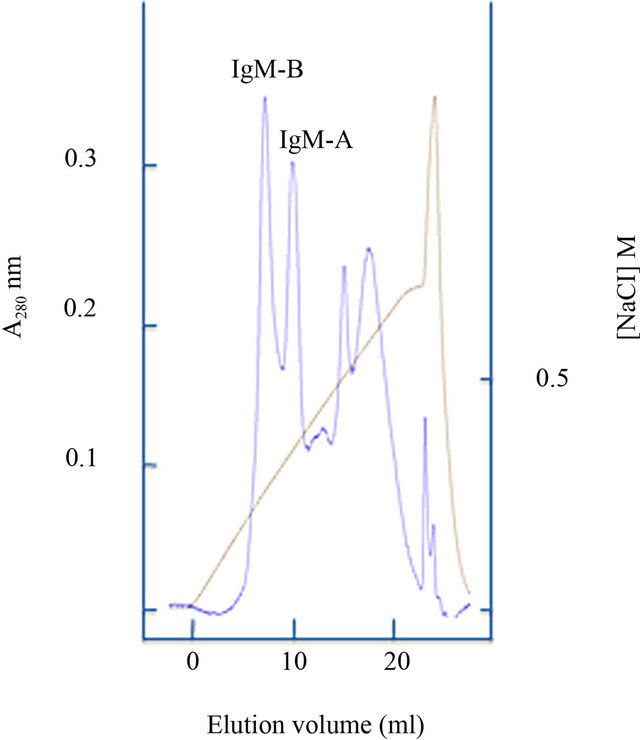
Correlation to ploidy status was not found in the present study. However, males and females were not uniformly distributed in diploid and triploid groups (Table 3), and males appeared to have a somewhat higher abundance of IgM transcripts than females. Thus, ploidy effects could have been overshadowed by other variables. Interestingly, gradient anion exchange chromatography of salmon IgM indicated that vaccination can lead to a skewed ratio of IgM-A/IgM-B (Figure 1).
4. DISCUSSION
The present work supports previous studies showing that oil-adjuvanted commercial vaccines trigger a prolonged increase of total IgM in blood [27-29]. In our study, real time PCR showed that the abundance of m transcripts in spleen was higher in vaccinated fish versus unvaccinated, supporting the ELISA results. Gradient anion exchange chromatography profiles obtained from fish used in this study (and IgM purifications conducted in our laboratory during many years) indicate that the IgM-A/IgM-B ratio can be skewed in vaccinated fish. In accordance with typical serum IgM profiles, the mA/mB mRNA ratio was previously estimated to be approximately 40/60 in Atlantic salmon [20]. In agreement with this, a recent study based on immunostaining and flow cytometry reported 40/60, 36/64 and 29/71 ratios of IgM-A/IgM-B expressing cells in peripheral blood, spleen and head kidney leukocytes of Atlantic salmon[30].
Exact ratios of mA and mB transcripts could not be obtained by the present PCR approaches. Since mA and mB mRNAs are highly similar it is difficult to design real time PCR assays that distinguish 100% between the variants. The choice of amplicon becomes more restricted because nucleotides that are different in mA and mB are
 (a)
(a)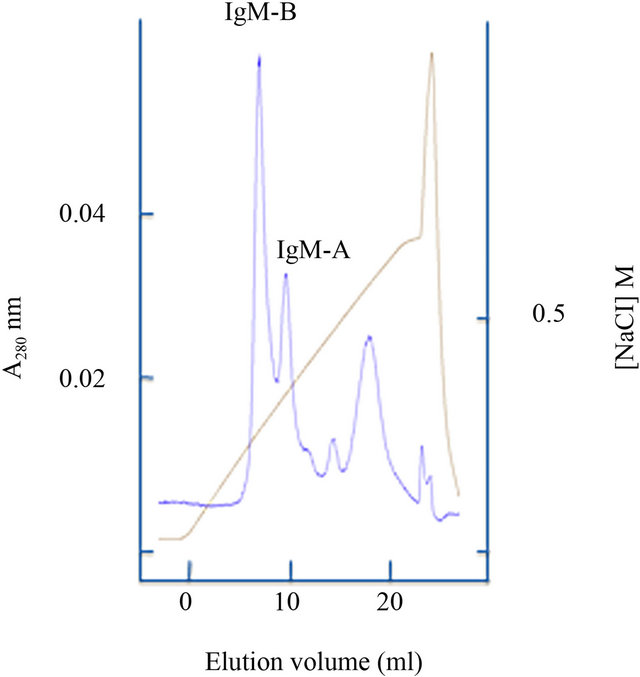 (b)
(b)
Figure 1. Anion exchange chromatography elution profiles of IgM from Atlantic salmon. (a) typical profile; (b) profile from a vaccinated triploid individual.
not uniformly distributed. Reducing the region from where primers/probes can be selected may result in less optimal PCR, and in the next hand to difficulties in the interpretation of the results. The m3 assays in this study were designed from the most diverged (coding) region of mA and mB, covering 5 different nucleotides. By use of plasmid clones it was shown that both assays cross-react with the accompanying sub-variant to some degree although standard conditions and recommended melting temperatures for probes and primers were utilized. It may be possible to develop more precise qPCR tools to distinguish between sub-variants, but the precision and appropriateness of real time PCR can be questioned. We decided to switch to a RT-PCR/restriction enzyme based approach to reveal possible differences in the proportion of mA and mB mRNA [20,24], but could not obtain the required resolution with this method either (results not shown). Application of high throughput sequencing will allow more detailed quantification of the different Ig transcripts in future studies, as recently reported from rainbow trout [31].
Based on the present knowledge it is plausible to assume that expression of the paralogous Ig heavy chain loci in Atlantic salmon are subjected to a random selection process equivalent to allotypic exclusion in mammals. Presumably, this process occurs in the head kidney and results in a mixed population of IgM-A and IgM-B expressing lymphocytes with a broad spectrum of specificities contributed from both gene complexes [18-19,22]. The typical ratio of mA/mB transcripts might reflect the number of successfully recombined variable genes from each locus and/or distinct features associated with the constant region. A skewed IgM-A/IgM-B ratio in serum of vaccinated fish, as illustrated in Figure 1 could indicate that there has been a clonal expansion of cells expressing a favorable fraction of the IgM-B subpopulation and/or that the expanded IgM-B population is more robust with a longer half life time, resulting in a dominance of these antibodies. Notably, the IgM-B tetramer in Atlantic salmon exhibits a higher degree of inter-chain disulfide bonding than IgM-A [23]. In rainbow trout it has been shown that the fraction of fully cross-linked IgM tetramers increases during the antibody response against the pathogen Streptococcus iniae, as well as other defined antigens [15,16].
As described in Materials and Methods, fish analyzed in the present study were part of a large trial setup, including both diploid and triploid fish. Triploid salmon are commercially produced in several countries, but very few studies have been done on the immune system of these fish [32]. Triploid fish are expected to have fewer and larger immune cells compared to diploids. Thus, at the outset of this study, we thought that including fish with an extra set of chromosomes could possibly reveal relevant information regarding the ratio of IgM-A/IgM-B, e.g. that triploid fish could show a more pronounced skewed IgM-A/IgM-B ratio after prolonged triggering of the immune system. However, as mentioned in the Results section a more comprehensive study is needed to study possible impact of ploidy on Ig expression in Atlantic salmon.
5. ACKNOWLEDGEMENTS
AtifKamil was supported by a grant from Higher Education Commission (HEC) of Pakistan. We thank Ole Horvli at Department of Molecular Biology for excellent technical assistance and Dr. Knut Falk for providing the monoclonal antibody MAb4C10.
REFERENCES
- Tadiso, T.M., Lie, K.K. and Hordvik, I. (2011) Molecular cloning of IgT from Atlantic salmon, and analysis of the relative expression of tau, mu and delta in different tissues. Veterinary Immunology and Immunopathology, 139, 17-26. doi:10.1016/j.vetimm.2010.07.024
- Zhang, Y.-A., Salinas, I., Li, J., Parra, D., Bjork, S., Xu, Z., LaPata, S.E., Bartholomew, J. and Sunyer, J.O. (2010) IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nature Immunology, 11, 827-835. doi:10.1038/ni.1913
- Ramirez-Gomez, F., Greene, W., Rego, K., Hansen, J.D., Costa, G., Kataria, P. and Bromage, E.S. (2012) Discovery and characterization of secretory IgD in rainbow trout: Secretory IgD is produced through a novel splicing mechanism. Journal of Immunology, 188, 1341-1349. doi:10.4049/jimmunol.1101938
- Bengten, E., Quiniou, S.M.-A., Stuge, T., Katagiri, T., Miller, N.W., Clem, L.W., Warr, G.W. and Wilson, M. (2002) The IgH locus of the channel catfish, Ictaluruspunctatus, contains multiple constant region gene sequences: Different genes encode heavy chains of membrane and secreted IgD. Journal of Immunology, 169, 2488- 2497.
- Quiniou, S.M.A., Wilson, M. and Boudinot, P. (2011) Processing of fish Ig heavy chain transcripts: Diverse splicing patterns and unusual nonsense mediated decay. Developmental and Comparative Immunology, 35, 949-958. doi:10.1016/j.dci.2010.12.007
- Coscia, M.R., Varriale, S., Giacomelli, S. and Oreste, U. (2011) Antarctic teleost immunoglobulins: More extreme, more interesting. Fish & Shellfish Immunology, 31, 688- 696. doi:10.1016/j.fsi.2010.10.018
- Savan, R., Aman, A., Nakao, M., Watanuki, H. and Sakai, M. (2005) Discovery of a novel immunoglobulin heavy chain gene chimera from common carp (Cyprinuscarpio L.). Immunogenetics, 57, 458-463. doi:10.1007/s00251-005-0015-z
- Stenvik, J. and Jørgensen, T.O. (2000) Immunoglobulin D (IgD) of Atlantic cod has a unique structure. Immunogenetics, 51, 452-461. doi:10.1007/s002510050644
- Israelsson, O., Petersson, A., Bengten, E., Wiersma, E.J., Andersson, J., Gezelius, G. and Pilström, L. (1991) Immunoglobulin concentration in Atlantic cod, Gadusmorhua L., serum and cross-reactivity between anti-cod-antibodies and immunoglobulins from other species. Journal of Fish Biology, 39, 265-278. doi:10.1111/j.1095-8649.1991.tb04361.x
- Bromage, E.S., Ye, J. and Kaattari, S.L. (2006) Antibody structural variation in rainbow trout fluids. Comparative Biochemistry and Physiology, 143, 61-69. doi:10.1016/j.cbpb.2005.10.003
- Haavarstein, L.S., Aasjord, P.M., Ness, S. and Endresen, C. (1988) Purification and partial characterization of an IgM-like serum immunoglobulin from Atlantic salmon (Salmosalar). Developmental and Comparative Immunology, 12, 773-785. doi:10.1016/0145-305X(88)90052-3
- Magnadottir, B. (1998) Comparison of immunoglobulin (IgM) from four fish species. Icelandic Agricultural Sciences, 4, 49-54.
- Stoop, J.W., Zegers, J.M., Sander, P.C. and Ballieux, R.E. (1969) Serum immunoglobulin levels in healthy children and adults. Clinical and Experimental Immunology, 4, 101- 112.
- Kalff, M.W. (1970) A population study on serum immunoglobulin levels. Clinica Chimica Acta, 28, 277-289. doi:10.1016/0009-8981(70)90092-6
- Ye, J., Bromage, E.S. and Kaattari, S.L. (2010) The strength of B cell interaction with antigen determines the degree of IgM polymerization. Journal of Immunology, 184, 844-850. doi:10.4049/jimmunol.0902364
- Costa, G., Danz, H., Kataria, P. and Bromage, E. (2012) A holistic view of the dynamisms of teleost IgM: A case study of Streptococcus iniae vaccinated rainbow trout (Oncorhynchusmykiss). Developmental and Comparative Immunology, 36, 298-305. doi:10.1016/j.dci.2011.04.011
- Hordvik, I., Voie, A.M., Glette, J., Male, R. and Endresen, C. (1992) Cloning and sequence analysis of two isotypic IgM heavy chain genes from Atlantic salmon, Salmosalar L. European Journal of Immunology, 22, 2957-2962. doi:10.1002/eji.1830221130
- Hordvik, I. (1998) The impact of ancestral tetraploidy on antibody heterogeneity in salmonid fishes. Immunological Reviews, 166, 153-157. doi:10.1111/j.1600-065X.1998.tb01260.x
- Solem, S.T., Hordvik, I., Killie, J.E.A., Warr, G.W. and Jørgensen, T.Ø. (2001) Diversity of the immunoglobulin heavy chain in the Atlantic salmon (Salmosalar L.) is contributed by genes from two parallel IgHisoloci. Developmental and Comparative Immunology, 25, 403-417. doi:10.1016/S0145-305X(01)00008-8
- Hordvik, I., Berven, F.S., Solem, S.T., Hatten, F. and Endresen C. (2002) Analysis of two IgM isotypes in Atlantic salmon and brown trout. Molecular Immunology, 39, 313-321. doi:10.1016/S0161-5890(02)00114-1
- Hansen, J.D., Landis, E.D. and Phillips, R.B. (2005) Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proceedings of the National Academy of Sciences of the United States of America, 102, 6919-6924. doi:10.1073/pnas.0500027102
- Yasuike, M., de Boer, J., von Schalburg, K.R., Cooper, G.A., McKinnel, L., Messmer, A., So, S., Davidson, W.S. and Koop, B.F. (2010) Evolution of duplicated IgH loci in Atlantic salmon, Salmosalar. BMC Genomics, 11, 486. doi:10.1186/1471-2164-11-486
- Kamil, A., Raae, A., Fjelldal, P.G., Koppang, E.O., Kari, F.E. and Hordvik, I. (2013) Comparative analysis of IgM sub-variants in salmonid fish and identification of a residue in µ3 which is essential for MAb4C10 reactivity. Fish & Shellfish Immunology, 34, 667-672. doi:10.1016/j.fsi.2012.12.006
- Kamil, A., Falk, K., Sharma, A., Raae, A., Berven, F., Koppang, E.O. and Hordvik, I. (2011) A monoclonal antibody distinguishes between two IgM heavy chain isotypes in Atlantic salmon and brown trout: protein characterization, 3D modeling and epitope mapping. Molecular Immunology, 48, 1859-1867. doi:10.1016/j.molimm.2011.05.005
- Cantas, L., Fraser, T.W.K., Fjelldal, P.G., Mayer, I. and Sørum, H. (2011) The culturable intestinal microbiota of triploid and diploid juvenile Atlantic salmon (Salmosalar)—A comparison of composition and drug resistance. BMC Veterinary Research, 7, 71. doi:10.1186/1746-6148-7-71
- Fraser, T.W.K., Rønneseth, A., Haugland, G.T., Fjelldal, P.G. and Mayer, I. (2012) The effect of triploidy and vaccination on neutrophils and B-cells in the peripheral blood and head kidney of 0+ and 1+ Atlantic salmon (Salmosalar L.) post-smolts. Fish & Shellfish Immunology, 33, 60-66. doi:10.1016/j.fsi.2012.04.001
- Koppang, E.O., Bjerkås, I., Haugarvoll, E., Chan, E.K.L., Szabo, N.J., Ono, N., Akikusa, B., Jirillo, E., Poppe, T.T., Sveier, H., Tørud, B. and Satoh, M. (2008) Vaccinationinduced systemic autoimmunity in farmed Atlantic salmon. Journal of Immunology, 181, 4807-4814.
- Haugarvoll, E., Bjerkås, I., Szabo, N.J., Satoh, M. and Koppang, E.O. (2010) Manifestations of systemic autoimmunity in vaccinated salmon. Vaccine, 28, 4961-4969. doi:10.1016/j.vaccine.2010.05.032
- Satoh, M., Bjerkås, I., Haugarvoll, E., Chan, E.K., Szabo, N.J., Jirillo, E., Poppe, T.T., Sveier, H., Tørud, B. and Koppang, E.O. (2011) Polyclonal hypergammaglobulinemia and autoantibody production induced by vaccination in farmed Atlantic salmon. Fish & Shellfish Immunology, 30, 1080-1086. doi:10.1016/j.fsi.2011.02.006
- Hedfors, I.A., Bakke, H., Skjødt, K. and Grimholt, U. (2012) Antibodies recognizing both IgM isotypes in Atlantic salmon. Fish & Shellfish Immunology, 33, 1199- 1206. doi:10.1016/j.fsi.2012.09.009
- Castro, R., Jouneau, L., Pham, H.P., Bouchez, O., Giudicelli, V., Lefranc, M.P., Quillet, E., Benmansour, A., Cazals, F., Six, A., Fillatreau, S., Sunyer, O. and Boudinot, P. (2013) Teleost fish mount complex clonal IgM and IgT responses in spleen upon systemic viral Infection. PLoS One, 8, Article ID: 55302. doi:10.1371/journal.pone.0055302
- Piferrer, F., Beaumont, A., Falguiere, J.C., Flajshans, M., Haffray, P. and Colombo, L. (2009) Polyploid fish and shellfish: production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture, 293, 125-156. doi:10.1016/j.aquaculture.2009.04.036
NOTES
*Corresponding author.

