Advances in Bioscience and Biotechnology
Vol.4 No.1(2013), Article ID:27239,8 pages DOI:10.4236/abb.2013.41012
L-ornithine hydrochloride ingestion increased carbohydrate oxidation but not lipid oxidation during submaximal endurance exercise following resistance exercise
![]()
1Graduate School of Natural Science & Technology, Kanazawa University, Kanazawa, Japan
2General Education Center, Fukui National College of Technology, Fukui, Japan
3Faculty of Medicine, Fukui University, Fukui, Japan
4Research and Education Center for Comprehensive Science, Akita Prefectural University, Akita, Japan
Email: demura@ed.kanazawa-u.ac.jp, *takay@fukui-nct.ac.jp, yamaji@u-fukui.ac.jp, uchiyama@akita-pu.ac.jp
Received 3 November 2012; revised 11 December 2012; accepted 9 January 2013
Keywords: L-Ornithine Hydrochloride; Endurance Exercise; Lipid Oxidation
ABSTRACT
This study aimed to examine the effect of L-ornithine hydrochloride ingestion on lipid oxidation during submaximal endurance exercise following resistance exercise. Ten healthy young male adults with no history of regular resistance exercise (age: 26.0 +/− 4.4) conducted resistance exercise after L-ornithine hydrochloride or placebo ingestion (0.1 g/kg). Subjects exercised for 60 min on an ergometer at 50% VO2peak 120 min after resistance exercise. Plasma ornithine concentrations measured immediately, 120 min and 180min after resistance exercise in the L-ornithine hydrochloride ingestion condition were significantly greater than those in the placebo condition. No significant difference was found in serum growth hor mone concentrations between both conditions (F = 4.4, p = 0.065). Serum free fatty acid concentrations were significantly greater immediately after sub-maximal ergometer bicycle exercise in both conditions than those before ingestion, immediately after resistance exercise and 120min after resistance exercise (F = 43.4, p < 0.001, 300% - 508%), but no significant difference was found between both conditions (F = 3.6, p = 0.090). A similar trend was observed in serum ketone bodies as well. Although total energy production during submaximal ergometer exercise did not significantly differ (t = 0.74, p = 0.238), a significant difference was found in energy production via carbohydrate and lipid oxidation; the former was greater in the L-ornithine hydrochloride ingestion condition (t = 1.89, p = 0.046, d = 0.44, 106%), and the latter was greater in the placebo condition (t = 1.89, p = 0.046, d = 0.78%, 145%). From the above, L-ornithine hydrochloride ingestion may not affect lipid metabolism during submaximal endurance exercise following resistance exercise. It may be involved in energy production via carbohydrate oxidation with glucogenic amino acid.
1. INTRODUCTION
A training program composed of resistance and endurance exercises has been widely recommended to control body mass and to maintain health [1-4]. Various combinations of these two exercise modes are generally conducted on the same day. With this type of program, Goto et al. [5] reported that the relative contribution of lipid oxidation to total energy production during submaximal endurance exercise following resistance exercise increased.
Resistance exercise, which preceded endurance exercise in this experiment, stimulates the endocrine response and markedly increases hormone secretion [6]. Among these hormones secreted after resistance exercise, catecholamine and growth hormone have strong lipolytic effects [7,8] and induce the gradual rise of blood concentrations of free fatty acids and glycerol after exercise [9] and their metabolism [10]. Meanwhile, growth hormone secretion is enhanced by not only resistance exercise and aerobic exercise, but also oral ingestion of free amino acids such as ornithine and arginine [11-14].
Bucci et al. [15] examined the effect of L-ornithine hydrochloride ingestion on enhancement of growth hormone secretion in body builders and reported that the blood concentration of growth hormone increased with L-ornithine hydrochloride ingestion. Moreover, Demura et al. [16] examined the effect of L-ornithine hydrochloride ingestion before resistance exercise on postexercise growth hormone secretion in ten healthy male adults and reported that blood concentration of growth hormone after resistance exercise was greater in the L-ornithine hydrochloride ingestion condition than the placebo ingestion condition. Namely, enhanced growth hormone secretion has been suggested by not only ingestion of free amino acids such as L-ornithine, but also subsequent resistance exercise after amino acid ingestion.
As stated above, blood concentrations of free fatty acid and glycerol increase with their release from triglycerides in adipose tissue by enhancing growth hormone secretion with oral ingestion of L-ornithine hydrochloride and resistance exercise. Due to energy consumption during exercise, an increase in lipid oxidation is expected when blood free fatty acid and glycerol concentrations increase. Although the lipolytic response accelerates with increased blood catecholamine and growth hormone concentrations induced by resistance exercise [7,8,17-20], the time course of the lipolytic response to these hormones may be different. In relation to the lipolytic response with growth hormone, blood free fatty acid and glycerol concentrations peak 120 - 160 min after resistance exercise [7,21]. Therefore, exercise for the above stated time is adequate.
This study aimed to examine the effect of L-ornithine hydrochloride ingestion on lipid oxidation during submaximal endurance exercise following resistance exercise.
2. SUBJECTS AND METHODS
2.1. Subjects
Ten healthy young trained male adults who majored in physical and health education participated in this study (age: 26.0 +/− 4.4 yr, height: 171.2 +/− 6.1 cm, body-mass: 70.7 +/− 8.9 kg). They habitually performed sports such as track and field, swimming, soccer and basketball over three times per week (3.1 +/− 0.9 times/ week), with moderate to high intensity over two hours per session (2.1 +/− 1.4 hour/time). Written informed consent was obtained from all subjects after a full explanation of the experimental purpose and protocol. Moreover, the experimental protocol was approved by the Kanazawa University Health & Sports Science Ethics Committee.
2.2. Experimental Design
The experimental design was a double-blinded crossover method. Namely, subjects participated in both conditions: L-ornithine hydrochloride supplementation and placebo (indigestible dextrin aqueous solution). Due to the cross-over design, all subjects participated in both conditions with a week wash-out period between conditions. Moreover, the test condition order was counter balanced to eliminate order effect. In addition, subjects were instructed to refrain from intensive exercise for two days prior to the experiment and fast overnight before the experiment to avoid a nutritional imbalance created by eating and drinking. Subjects were also instructed not to consume beverages or food containing caffeine during the experimental period.
2.3. Experimental Conditions
Subjects ingested L-ornithine hydrochloride or an indigestible dextrin aqueous solution with the same flavor (placebo) at the ratio of 0.1 g per kilogram body mass. Isomers exist in almost all amino acids, including ornithine, and are divided into levorotatory (L-) and dextrorotatory (D-) amino acids. L-ornithine corresponds to the former group of amino acids and is naturally occurring. The hydrochloride of the L-ornithine was used in this study.
The effect of L-ornithine hydrochloride ingestion was examined using the double-blinded cross-over method stated above. A placebo was required so that both subjects and testers are not biased to the effects of L-ornithine hydrochloride. A dextrin aqueous solution with the same flavor as L-ornithine hydrochloride solution is difficult to digest. Hence, the influence of the nutrients is considered to minimal.
2.4. Exercise Regimen
2.4.1. Determination of 10 Repetition Maximum (RM)
Ten repetition maximums of chest press, lat pull down, leg press, shoulder press, leg extension and leg curl were determined in each subject before participating in the experimental protocol in L-ornithine hydrochloride and placebo ingestion conditions. Ten repetitions using 50% - 70% of predicted 1RM were conducted as warm ups, and each muscle group was stretched before 10 RM measurement. Load was increased until subjects could complete 10 repetitions; this was determined as the 10 RM.
2.4.2. Determination of Peak Oxygen Consumption (VO2peak)
Peak oxygen consumption was measured at least a week after 10 RM measurement. Subjects conducted incrementally exhaustive ergometer bicycle exercise following non-loaded pedaling (0 watt) for 3 min. They were instructed to maintain 60 pedal revolutions per min during incremental exercise. We judged exhaustion when they could not continue pedaling at a rate of 40 revolutions per min. In addition, a graded rate of exercise load was calculated using the following formulas considering each subjects’ physical characteristics, and the exercise load was increased every minute during exercise.
Ÿ Incremental ratio of exercise load (Watts/min.) = (peak oxygen consumption (ml/min)a − oxygen consumption (ml/min)b)/100.
apeak oxygen consumption (ml/min.) = (height (cm)- age (yr)) × 20, boxygen consumption during unloaded pedaling (ml/min) = 150 + (6 × body-mass (kg)).
2.5. Experimental Protocol in Each Ingestion Condition
Subjects engaged in the experimental protocol (Figure 1) with L-ornithine hydrochloride and placebo ingestion conditions at least a week after VO2peak measurement. They conducted resistance exercise after L-ornithine hydrochloride or placebo ingestion following 60 min rest in a semi-recumbent position after entering the laboratory. Resistance exercise was designed according to previous studies [6,22]. The resistance exercise used in this study affects blood concentrations of anabolic hormones, such as catecholamine, growth hormone and insulin, and lactic acid [6,22,23]. Subjects conducted resistance exercises in the following order: chest presses, lat pull downs, leg presses, shoulder presses, leg extensions and leg curls. Three sets of each exercise were conducted and repeated 10 times using a 10 RM load determined in the first and second sets. Subjects repeated each exercise with 85% of 10 RM until exhaustion in the third set. In addition, a 90 second rest was performed with each set. Resistance exercises in both conditions were conducted with the same load and repetition number. Ergometer bicycle exercise at 50% of VO2peak for 60 min was conducted following a 120 min rest in a semirecumbent position after resistance exercise.
Subjects were instructed not to consume beverages or food containing caffeine during the experimental period. They were also instructed to refrain from intensive exercise for two days prior to the experiment. All experimental protocols started at 8:00 am and were completed by 1:00 pm, and they fasted overnight before the experiment to avoid a nutritional imbalance. Hydration (water) during the experiment was allowed as needed. In addition, laboratory temperature was maintained at 24˚C - 26˚C.
2.6. Blood and Expired Gas Analysis
The initial blood draw was conducted after 60 min rest in a semirecumbent position after entering the laboratory. The blood draw was conducted immediately after resistance exercise and before and after submaximal ergometer bicycle exercise (Figure 1). Each blood sample (12 ml) was collected from the antecubital vein and analyzed for growth hormone, free fatty acid, ketone bodies and ornithine. 7 ml blood was transferred into the blood sampling tube, which contains clotting and enhancing film and serum separating medium for growth hormone, free fatty acid and ketone bodies, and 5 ml of blood were transferred into 65IU heparin sodium for ornithine analysis. These processes were carried out within 30 s of the blood draw. The samples were immediately centrifuged, and the supernatants were placed in chilled containers and stored frozen at −80˚C until analysis. Serum growth hormone concentration was analyzed by radioimmunoassay using the kits from SRL Inc., Japan. Sensitivity, inter-assay and intra-assay coefficients of variation (CV) of this assay were 0.04 ng·m/L, 4.0% and 3.4%, respectively. Serum free fatty acid and ketone body concentrations were analyzed by the enzymatic method. These interassay and intraassay CV were 0.2% and 0.9% for free fatty acid and 0.6% and 0.7% for ketone bodies, respectively. The samples for ornithine determination were analyzed by high performance liquid chromatography using the an automatic amino acid analyzer (JOEL, JLC-500/V). Sensitivity, inter-assay and intra-assay coefficients of variation (CV) of this assay were 5.92 nmol/l, 4.94% and 0.00%, respectively.
Expired gases were continuously sampled in a breathby-breath method during submaximal ergometer bicycle
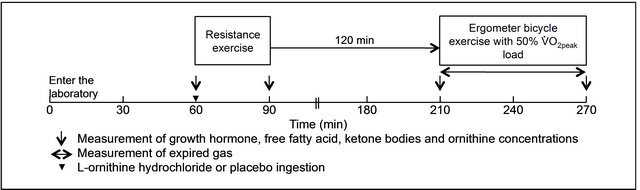
Figure 1. Protocol for exercise and blood sampling.
exercise for 60 min. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured with an automatic expired gas analytic system (AE-280S; Minato Medical Science, Japan). Respiratory exchange ratio (RER), which is calculated by VO2 and VCO2, was used to estimate the relative contribution of fat and carbohydrate oxidation to total energy production (% fat and carbohydrate oxidation) [24]. RER and % fat oxidation were determined without urinary nitrogen analysis because of the negligible contribution of protein to substrate oxidation during rest [25].
2.7. Statistical Analysis
Two-way repeated measures analysis of variance (ingestion condition × measurement time) was used to examine the effect of L-ornithine hydrochloride ingestion. When there was a significant main or interaction effect, Tukey’s honestly significant difference was used as post-hoc analysis to examine specific mean differences. Moreover, the paired t-test was used to examine the difference between both conditions for total energy production during submaximal endurance exercise. The effect sizes were calculated in the above stated analysis (ANOVA: partial η2, t-test: cohen’s d). An alpha concentration of 0.05 was used for all tests.
3. RESULTS
Figure 2 shows the changes of serum growth hormone, free fatty acid, ketone bodies (acetoacetate, 3-hydroxybutyrate) and plasma ornithine concentrations immediately, 120 min and 180 min after resistance exercise in Lornithine hydrochloride and placebo ingestion conditions. Although significant main time effects were found in all parameters (plasma ornithine: F = 156.0, p < 0.001, serum growth hormone: F = 16.7, p = 0.003, serum free fatty acid: F = 43.4, p < 0.001, acetoacetate: F = 24.7, p = 0.001, 3-hydroxybutyrate: F = 20.0, p = 0.002), main condition effect was not significant in all parameters (serum growth hormone: F = 4.4, p = 0.065, serum free fatty acid: F = 3.6, p = 0.090, acetoacetate: F = 0.2, p = 0.691, 3-hydroxybutyrate: F = 0.96, p = 0.352) except for plasma ornithine (F = 29.9, p < 0.001). In addition, plasma ornithine concentrations immediately after resistance exercise, as well as before and immediately after submaximal ergometer bicycle exercise, were significantly greater in the L-ornithine hydrochloride ingestion condition than in the placebo condition. Figure 3 shows changes of the relative contribution of fat and carbohydrate oxidation to total energy production (% fat and carbohydrate oxidation) and total energy production
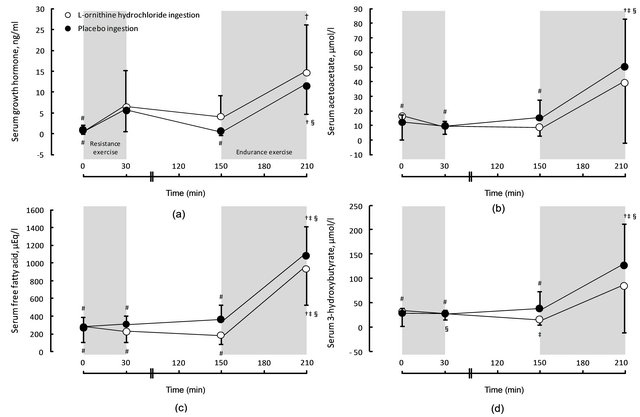
Figure 2. Serum growth hormone (a), free fatty acid (b), acetoacetate (c) and 3-hydroxy butyrate (d) concentrations in both conditioins. Values are means +/− SD. † significant difference was found with 0 min in each condition. ‡ significant difference was found with 30 min in each condition. § significant difference was found with 150 min in each condition. # significant difference was found with 210 min in each condition.
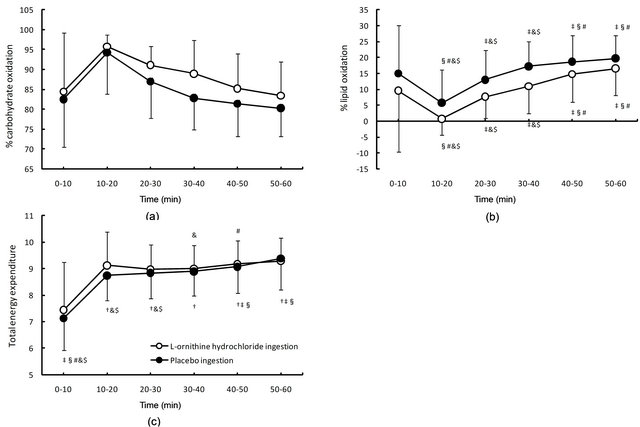
Figure 3. Relative contribution of carbohydrate (a) and lipid (b) oxidation to total energy expenditure and total energy expenditure (c) during submaximal endurance ergometer bicycle exercise for 60 min in both conditioins. Values are means +/− SD. † significant difference was found with 0 - 10 min during ergometer bicycle exercise in each condition. ‡ significant difference was found with 10 - 20 min during ergometer bicycle exercise in each condition. § significant difference was found with 20 - 30 min during ergometer bicycle exercise in each condition. # significant difference was found with 30 - 40 min during ergometer bicycle exercise in each condition. & significant difference was found with 40 - 50 during ergometer bicycle exercise min in each condition. $ significant difference was found with 50 - 60 min during ergometer bicycle exercise in each condition.
during submaximal endurance exercise in both conditions. Significant main effects of time were found in % lipid oxidation and total energy production (% carbohydrate oxidation: F = 3.6, p = 0.091, % lipid oxidation: F = 6.2, p = 0.035, Total energy production: F = 21.0, p = 0.001), but no significant main effect of ingestion condition was found (% carbohydrate oxidation: F = 1.1, p = 0.323, % lipid oxidation: F = 2.3, p = 0.167, Total energy production: F = 0.6, p = 0.468). Figure 4" target="_self"> Figure 4 shows energy production from carbohydrate and lipid oxidation and total energy production during submaximal endurance exercise in both conditions. A significant difference was found in energy production via carbohydrate and lipid oxidation. The former was higher in the L-ornithine hydrochloride ingestion condition, but the latter was higher in the placebo ingestion condition (t = 1.89, p = 0.046, d = 0.44; t = 1.89, p = 0.046, d = 0.78, respectively). No significant difference was found in total energy production (t = 0.74, p = 0.238).
4. DISCUSSION
Plasma ornithine concentration increased with L-ornithine hydrochloride ingestion and peaked 120 min after resistance exercise. However, serum growth hormone concentration did not increase (Figure 2). Bucci et al. [15] examined the effect of L-ornithine hydrochloride ingestion on enhancement of growth hormone secretion in 13 body builders. They reported that although significant increases were found in plasma ornithine concentrations 45 and 90 min after ingestion when subjects ingested 100 and 170 mg/kg of L-ornithine hydrochloride, serum growth hormone concentration increased significantly 90 min after ingestion of 170 ml/kg L-ornithine hydrochloride. The quantity of Lornithine hydrochloride ingestion in Bucci et al.’s report was more than that of the present study (Bucci et al.: 170 mg/kg vs present intake: 100 mg/kg), and the increase of plasma ornithine concentration with the above was also greater (Bucci et al.: approximately 650 μmol/l vs present result: approximately 370 μmol/l). Therefore, it is possible that there is no significant difference in serum growth hormone concentration between both conditions. However, Demura et al. [16] examined the effect of L-ornithine hydrochloride ingestion on enhancement of growth hormone secretion after resistance exercise using 10 male adults and reported that serum growth hormone concentration 30 min after resistance exercise in the
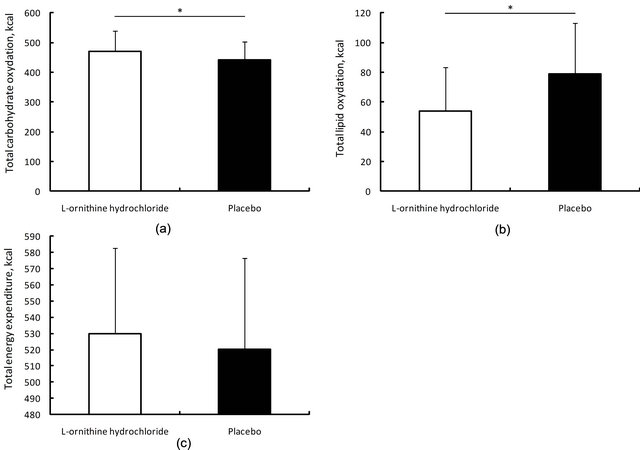
Figure 4. Total carbohydrate (a) and lipid (b) oxidation and total energy expenditure (c) for 60 min submaximal endurance ergometer bicycle exercise in both conditioins. Values are means +/− SD. * significant difference was found between both conditions.
L-ornithine hydrochloride ingestion condition was greater than that of the placebo ingestion condition. The inconsistency between the results of the present and previous studies may be attributed to measurement time of growth hormone rather than quantity of L-ornithine hydrochloride ingestion. Kraemer et al. [6] examined the responses of anabolic hormones and growth factors with resistance exercise and reported that blood growth hormone concentration with resistance exercise peaked 15 - 30 min after exercise. Moreover, Bucci et al. clarified that serum growth hormone concentration increased significantly 90 min after L-ornithine hydrochloride ingestion. Although growth hormone concentration in this study was measured immediately after resistance exercise (30 - 40 min after L-ornithine hydrochloride ingestion), it was not subsequently measured until 120 min after resistance exercise. Therefore, it is likely that a growth hormone increase with L-ornithine hydrochloride ingestion and resistance exercise could not be evaluated.
Free fatty acids are released from triglycerides in adipose tissue after an increase of blood growth hormone concentration. Namely, growth hormone has strong lipolytic effects and induces the gradual rise of blood concentrations of free fatty acids and glycerol [7-9]. Meanwhile, serum free fatty acid concentration after submaximal endurance exercise in both conditions increased significantly, but no significant difference was found between both conditions at any time (Figure 2(b)). Goto et al. [5] examined the effect of resistance exercise on subsequent lipid oxidation during submaximal endurance exercise by conducting 60 min bicycle ergometer exercise using loads corresponding to 50% of VO2peak with 0, 20 and 120 min rests after resistance exercise. They reported that serum free fatty acid concentration 120 min after resistance exercise was significantly greater than that of the other conditions, and it became about 230% of that during rest when conducting endurance exercise 120 min after rest. As stated above, the change in serum growth hormone concentration immediately and 120 min after resistance exercise is unclear. However, from the difference between the results of the present study and Goto et al.’s, it is inferred that the increase of growth hormone with resistance exercise in both conditions is not so great. Because a growth hormone increase with L-ornithine hydrochloride ingestion and subsequent increase of free fatty acid release in blood were not found, L-ornithine hydrochloride ingestion may not largely affect lipid metabolism. As stated in Figure 3, the time course of energy production during submaximal endurance exercise, which started 120 min after resistance exercise, was the same in both conditions (Figure 3(c)), and the relative contribution of fat and carbohydrate oxidation to total energy production (% fat and carbohydrate oxidation) was also similar (Figures 3(a) and (b)). If anything, it may be contradictory to our hypothesis. Energy production via carbohydrate and lipid oxidation during 60 min submaximal endurance exercise (Figures 4(a) and (b)) was significantly higher in the L-ornithine ingestion condition in the former and in the placebo ingestion condition in the latter. Lipolysis accentuated by an increase of blood catecholamine and growth hormone concentration with resistance exercise has been clarified in many studies [7,8,17-20]. However, the time course of the lipolytic response to these hormones may be different. Namely, the lipolytic response is induced by catecholamine immediately after exercise [8,26], whereas that induced by growth hormone causes a much-delayed response (about 120 - 160 min after exercise) [7,21]. We focused on the latter and examined the effects of Lornithine hydrochloride ingestion before resistance exercise on increase of lipid oxidation during subsequent submaximal endurance exercise. However, the accentuation of lipid oxidation with L-ornithine hydrochloride ingestion was not found, and the contribution of carbohydrate oxidation to total energy production was greater. Demura et al. [27] examined the effect of L-ornithine hydrochloride ingestion before incremental exhaustive ergometer bicycle exercise on metabolism of ammonia, which is produced during exercise. They reported that plasma ammonia concentration after exercise in the L-ornithine hydrochloride ingestion condition was significantly lower than that of the placebo ingestion condition. Moreover, the above result was reported to be induced by glutamic acid and glutamine synthesis with ammonia buffering in skeletal muscle. Glutamic acid, which is a representative glucogenic amino acid, is synthesized in skeletal muscle during intense exercise and transferred to the liver via blood. This is converted to glucose by gluconeogenesis in the liver and released to blood. Meanwhile, substantial ammonia is produced in skeletal muscle during resistance exercise in this study as well as in the study by Demura et al. [27]. Namely, it is inferred that glutamic acid, which is a glucogenic amino acid, is synthesized by ammonia buffering and more is synthesized in the L-ornithine hydrochloride ingestion condition. Greater energy production via carbohydrate oxidation during submaximal endurance exercise in the present L-ornithine hydrochloride ingestion condition may be suggested.
5. CONCLUSION
The L-ornithine hydrochloride ingestion may not affect lipid metabolism during submaximal endurance exercise following resistance exercise. It may be related to energy production via carbohydrate oxidation with glucogenic amino acid.
6. ACKNOWLEDGEMENTS
Authors received financial support from KYOWA HAKKO BIO CO., LTD. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- Poirier, P. and Després, J.P. (2001) Exercise in weight management of obesity. Cardiology Clinics, 19, 459-470. doi:10.1016/S0733-8651(05)70229-0
- Santa-Clara, H., Fernhall, B., Baptista, F., Mendes, M. and Sardinha, L.B. (2003) Effect of a one-year combined exercise training program on body composition in men with coronary artery disease. Metabolism, 52, 1413-1417. doi:10.1016/S0026-0495(03)00320-2
- Izquierdo, M., Ibañez, J., HAkkinen, K., Kraemer, W.J., Larrión, J.L. and Gorostiaga, E.M. (2004) Once weekly combined resistance and cardiovascular training in healthy older men. Medicine and Science in Sports and Exercise, 36, 435-443. doi:10.1249/01.MSS.0000117897.55226.9A
- Lambers, S., Van Laethem, C., Van Acker, K. and Calders, P. (2008) Influence of combined exercise training on indices of obesity, diabetes and cardiovascular risk in type 2 diabetes patients. Clinical Rehabilitation, 22, 483- 492. doi:10.1177/0269215508084582
- Goto, K., Higashiyama, M., Ishii, N. and Takamatsu, K. (2005) Prior endurance exercise attenuates growth hormone response to subsequent resistance exercise. European Journal of Applied Physiology, 94, 333-338. doi:10.1007/s00421-004-1296-x
- Kraemer, W.J., Marchitelli, L., Gordon, S.E., Harman, E., Dziados, J.E., Mello, R., Frykman, P., McCurry, D. and Fleck, S.J. (1990) Hormonal and growth factor responses to heavy resistance exercise protocols. Journal of Applied Physiology, 69, 1442-1150.
- Møller, N., Jørgensen, J.O., Alberti, K.G., Flyvbjerg, A. and Schmitz, O. (1990) Short-term effects of growth hormone on fuel oxidation and regional substrate metabolism in normal man. Journal of Clinical Endocrinology and Metabolism, 70, 1179-1186. doi:10.1210/jcem-70-4-1179
- Quisth, V., Enoksson, S., Blaak, E., Hagström-Toft, E., Arner, P. and Bolinder, J. (2005) Major differences in noradrenaline action on lipolysis and blood flow rates in skeletal muscle and adipose tissue in vivo. Diabetologia, 48, 946-953. doi:10.1007/s00125-005-1708-4
- Stich, V., de Glisezinski, I., Berlan, M., Bulow, J., Galitzky, J., Harant, I., Suljkovicova, H., Lafontan, M., Rivière, D. and Crampes, F. (2000) Adipose tissue lipolysis is increased during a repeated bout of aerobic exercise. Journal of Applied Physiology, 88, 1277-1283.
- Pritzlaff, C.J., Wideman, L., Blumer, J., Jensen, M., Abbott, R.D., Gaesser, G.A., Veldhuis, J.D. and Weltman, A. (2000) Catecholamine release, growth hormone secretion, and energy expenditure during exercise vs. recovery in men. Journal of Applied Physiology, 89, 937-946.
- Lambert, M.I., Hefer, J.A., Millar, R.P. and Macfarlane, P.W. (1993) Failure of commercial oral amino acid supplements to increase serum growth hormone concentrations in male body-builders. International Journal of Sport Nutrition, 3, 298-305.
- Fry, A.C., Kraemer, W.J., Stone, M.H., Warren, B.J., Kearney, J.T., Maresh, C.M., Weseman, C.A. and Fleck, S.J. (1993) Endocrine and performance responses to high volume training and amino acid supplementation in elite junior weightlifters. International Journal of Sport Nutrition, 3, 306-322.
- Cynober, L. (2007) Pharmacokinetics of arginine and related amino acids. Journal of Nutrition, 137, 1646S- 1649S.
- Cynober, L., Coudray-Lucas, C., de Bandt, J.P., Guéchot, J., Aussel, C., Salvucci, M. and Giboudeau, J. (1990) Action of ornithine alpha-ketoglutarate, ornithine hydrochloride, and calcium alpha-ketoglutarate on plasma amino acid and hormonal patterns in healthy subjects. Journal of the American College of Nutrition, 9, 2-12.
- Bucci, L., Hickson, J.F., Pivarnik, J.M., Wolinsky, I., Mc Mahon, J.C. and Turner, S.D. (1990) Ornithine ingestion and growth hormone release in bodybuilders. Nutrition Research, 10, 239-245.
- Demura, S., Yamada, T., Yamaji, S., Komatsu, M. and Morishita, K. (2010a) The effect of L-ornithine hydrochloride ingestion on human growth hormone secretion after strength training. Advances in Bioscience and Biotechnology, 1, 7-11. doi:10.4236/abb.2010.11002
- Binzen, C., Swan, P. and Manore, M. (2001) Postexercise oxygen consumption and substrate use after resistance exercise in women. Medicine and Science in Sports Exercise, 33, 932-938. doi:10.1097/00005768-200106000-00012
- Melby, C., Scholl, C., Edwards, G. and Bullough, R. (1993) Effect of acute resistance exercise on postexercise energy expenditure and resting metabolic rate. Journal of Applied Physiology, 75, 1847-1853.
- Ormsbee, M., Thyfault, J., Johnson, E., Kraus, R., Choi, M. and Hickner, R. (2007) Fat metabolism and acute resistance exercise in trained men. Journal of Applied Physiology, 102, 1767-1772. doi:10.1152/japplphysiol.00704.2006
- Melanson, E., Sharp, T., Seagle, H., Donahoo, W., Grunwald, G., Peters, J., Hamilton, J. and Hill, J. (2002) Resistance and aerobic exercise have similar effects on 24-h nutrient oxidation. Medicine and Science in Sports and Exercise, 34, 1793-1800. doi:10.1097/00005768-200211000-00016
- Gravhølt, C.H., Schmitz, O., Simonsen, L., Bülow, J., Christiansen, J.S. and Møller, N. (1990) Effects of a physiological GH pulse on interstitial glycerol in abdominal and femoral adipose tissue. American Journal of Physiology, 277, E848-E854.
- Thyfault, J.P., Carper, M.J., Richmond, S.R., Hulver, M.W. and Potteiger, J.A. (2004) Effects of liquid carbohydrate ingestion on markers of anabolism following high-intensity resistance exercise. Journal of Strength and Conditioning Research, 18, 174-179.
- Kraemer, W.J., Noble, B.J., Clark, M.J. and Culver, B.W. (1987) Physiologic responses to heavy-resistance exercise with very short rest periods. International Journal of Sports Medicine, 8, 247-252. doi:10.1055/s-2008-1025663
- Manetta, J., Brun, J.F., Perez-Martin, A., Callis, A., Prefaut, C. and Mercier, J. (2002) Fuel oxidation during exercise in middle-aged men: Role of training and glucose disposal. Medicine and Science in Sports and Exercise, 34, 423-429. doi:10.1097/00005768-200203000-00007
- Beidleman, B.A., Rock, P.B., Muza, S.R., Fulco, C.S., Gibson, L.L., Kamimori, G.H. and Cymerman, A. (2002) Substrate oxidation is altered in women during exercise upon acute altitude exposure. Medicine and Science in Sports Exercise, 34, 430-437. doi:10.1097/00005768-200203000-00008
- Marion-Latard, F., De Glisezinski, I., Crampes, F., Berlan, M., Galitzky, J., Suljkovicova, H., Riviere, D. and Stich, V. (2001) A single bout of exercise induces beta-adrenergic desensitization in human adipose tissue. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, 280, R166-R173.
- Demura, S., Yamada, T., Yamaji, S., Komatsu, M. and Morishita, K. (2010b) The effect of L-ornithine hydrochloride ingestion on performance during incremental exhaustive ergometer bicycle exercise and ammonia metabolism during and after exercise. European Journal of Clinical Nutrition, 64, 1166-1171. doi:10.1038/ejcn.2010.149
NOTES
*Corresponding author.

