Advances in Bioscience and Biotechnology
Vol.3 No.7(2012), Article ID:24857,9 pages DOI:10.4236/abb.2012.37112
Virulence properties of a peptide hemolysin produced by Enterococcus faecalis
![]()
1Department of Microbiology and Immunology, Biological Institute, Universidade Estadual de Campinas, Campinas, Brazil
2Research Group of Endemic and Parasitic Diseases, CEUMA University, São Luis, Brazil
3Medical Biology, Adolfo Lutz Institut, Campinas, Brazil
4Department Clinical, Toxicologicals and Bromatologicals Analisys, USP, Ribeirão Preto, Brazil
5Federal University of Maranhão, São Luís, Brazil
Email: #tyano@unicamp.br
Received 20 August 2012; revised 28 September 2012; accepted 30 October 2012
Keywords: Enterococcus faecalis; Cytotoxin, Heat-Stable Hemolysin; Human Epithelial Cells; Apoptosis
ABSTRACT
The characterization and prevalence of virulence factors associated with enterococcal invasiveness and severity of disease are important areas to be investigated. Recently, we described the production of a heatstable hemolysin by clinical isolates of Enterococcus faecalis cultived in BHI-GA (BHI with glucose and Larginine). Now, we purified the hemolysin from the culture supernatant by ultrafiltration (PM-10 membrane) and ethanol extraction followed by chromatography in a (Bondapak C18 and Superdex Peptide columns. The hemolytic activity was not affected by the proteolytic enzymes. Cholesterol, phospholipids, EDTA and also bivalent ions did not inhibit the hemolytic activity. Among the various carbohydrates, only dextran 4 protected the erythrocytes against lyse. Scanning electron microscopy showed that lyse of erythrocytes occured at once after the exposure to the hemolysin. The mitochondrial activity and the cell membrane integrity were significantly affected by the hemolysis, within 20 min of exposure and caused apoptosis after 12 h incubation, 51.92% in HeLa and 68% in HEp-2 cells, analyzed by flow cytometry. These results suggest that the heat-stable pore forming hemolysin might be a putative virulence factor in enterococci infections.
1. INTRODUCTION
Enterococcus faecalis is an important nosocomial pathogen that causes urinary tract infections, bacteremia and endocarditis [1].
Due to the importance of the Enterococcus species as nosocomial pathogens and the increasing prevalence of antimicrobial resistance among enterococci, the identification and characterization of virulence factors associated with enterococcal invasiveness and severity of disease are important areas to be investigated [2].
Enterococcal virulence factors such as hemolysin (Hly), aggregation substance (Agg or AS) and an enterococcal protease commonly referred to as gelatinase (Gel) have been suggested as possible contributors to the virulence of E. faecalis; however, the mechanisms involved remain unclear [2,3].
Hemolysin is commonly produced by Gram-positive and Gram-negative bacteria and, in most cases, is considered to be a virulence factor, although its relative contribution to disease is variable among microbes and different host species [4]. The cytolysin produced by E. faecalis is typically encoded by large, pheromone-responsive plasmids, the prototype of which is pAD1 [5] with a molecular weight of ~34,000 Da [6] and is able to lyse eukaryotic cells as well as kills bacterial cell. The cytolysin lyses mouse erythrocytes, macrophages and polymorphonuclear neutrophils [7], which may enable the infecting bacteria to obtain nutrients and to escape from immune clearance [8].
Recently, we observed among various virulence properties the expression of the heat-stable hemolytic activity in the culture supernatant of clinical isolates of E. faecalis [9] when grown in BHI (Brain Heart Infusion) medium supplemented with 1% glucose and 0.03% Larginine (BHI-GA) [5], but not in BHI medium alone.
In this work, we highly purified the heat-stable hemolysin produced by E. faecalis in order to investigate its physicochemical properties and biological effects on human epithelial cells. Biological characterization of this hemolysin can contribute to a better understanding of their role in the virulence of this specie.
2. MATERIALS AND METHODS
2.1. Bacterial Strain and Culture Conditions
Enterococcus faecalis isolate EF20 that produces heatstable hemolysin [9] was used in this study for hemolysin purification and characterization. The bacterium was cultured in BHI-GA (BHI supplemented with 1% glucose and 0.03% L-arginine) at 37˚C for 18 h with shaking [5]. The culture was then centrifuged at 12.000 × g for 20 min (Beckman, Model J2-20) to collect the supernatant.
2.2. Purification of Hemolysin (Hly)
The culture supernatant was ultrafiltrated through a PM- 10 membrane (Amicon, Millipore) and dissolved in a small volume of distilled water; then absolute ethanol was added to a final concentration of 90% [10]. The hemolysin ethanol-soluble fraction was dried by evaporation and applied on a mBondapak C18 column chromatography (Waters, Ireland) equilibrated with 0.1% trifluoroacetic acid (TFA) in distilled water [11] and eluted with a linear gradient of 30% acetonitrile in 0.1% TFA at a flow rate of 2 mL/min. The fraction with hemolytic activity was then concentrated and chromatographed in Superdex Peptide column (GE Healthcare) equilibrated with 0.1% trifluoroacetic acid (TFA) in distilled water at a flow rate of 0.5 mL/min. This material was considered highly purified and used to study some biological properties of this hemolysin.
2.3. Hemolytic Activity
The hemolytic activity was assayed in microplates using 50 mL of the culture supernatant or purified hemolysin, serially diluted with 5 mM phosphate buffer saline (PBS), pH 7.4, and mixed with an equal volume of a 1% sheep erythrocyte suspension. Then, it was incubated at 37˚C for 1 h, followed by a further incubation at 4˚C overnight. The hemolytic unit (HU) was defined as the highest diluted fraction that yet causes lyses in sheep erythrocytes.
2.4. Physicochemical Properties of Hemolysin
Purified hemolysin was tested for resistance by: 1) treatment with 100 mg/mL of trypsin and proteinase K (Calbiochem, CA, USA), as previously described [12], and 2) treatment with 180 mM 2-mercaptoethanol for 60 min. The residual hemolytic activity was assayed on sheep erythrocytes in triplicate.
2.5. Inhibition by Cholesterol and Phospholipids
Alcoholic solutions of cholesterol (Chol) (10 mg·mL–1) or phospholipid (EPC) (8.5 mg·mL–1) were diluted serially in 96-well microtiter plates so that the final Chol concentration ranged from 50 mg·mL–1 to 5 mg·mL–1 and EPC ranged from 40 mg·mL–1 to 4 mg·mL–1. Each microtiter plate either containing Chol or EPC was incubated with 100 mL aliquots of purified hemolysin for 60 min at 37˚C followed by 18h at 4˚C. The hemolytic activity was detected by incubating the above mixtures with 100 mL of 1% sheep suspension of erythrocytes for 60 min at 37˚C. The influence of Chol and EPC on hemolytic activity was tested as described [13].
2.6. Osmotic Protection of the Hemolytic Activity
The osmotic protection of the hemolytic activity was examined as described in the literature [14]. To hemolysin in 5 mM PBS, pH 7.4, 30 mM of sugars (raffinose, m-inositol, glucose, inositol, inulina, maltose, arabinose and dextran 4) were added. Moreover, the hemolysis inhibition was assayed with Ca2+ ions and EDTA (0.1 M).
2.7. Scanning Electron Microscopy (SEM)
The lysis of sheep erythrocytes was visualized by the scanning electron microscopy (SEM) technique using a slightly modification of the method described [15]. Sheep erythrocytes were incubated with 2 HU of hemolysin for different periods of time and the incubation was stopped with 4% formaldehyde. After treatment, one drop of erythrocytes was spread on cover glasses and postfixed with 2.5% glutaraldehyde in PBS for 30 min, followed by 1% osmium tetroxide for 2 h at 4˚C. The samples were dehydrated through a graded ethanol series and critical point dried. The specimens were then sputtercoated (Balzers SCD 050) and examined in a Jeol JSM 5800 LV scanning electron microscopy operated at 5 - 10 kV.
2.8. Cytotoxicity Assay
Human epithelial cells HeLa (human colon adenocarcinoma) and HEp-2 (human larynx epidermoid carcinoma) obtained from the Instituto Adolfo Lutz (São Paulo, SP, Brazil) were grown in Eagle’s minimal essential medium (EMEM, Nutricell) supplemented with 10% fetal calf serum (FBS) and 1% of an antibiotic solution (penicillin 100,000 U/L and 10 mg/L streptomycin-Sigma Chemical Co.) and incubated in a 5% CO2 atmosphere at 37˚C until a confluent monolayer was formed [16]. The purified hemolysin was filtered through 0.22 mm membranes (Millipore, USA) prior to testing in triplicate on HeLa and HEp-2. The morphological changes were assessed using an inverted microscope (Nikon Instruments, Japan) after 24 h of incubation.
2.9. Determination of Lactate Dehydrogenase (LDH)
Leakage of the cytosolic enzyme LDH was assessed [17] to investigate whether the hemolysin affected the integrity of the plasma membrane of HeLa and HEp-2 cells in triplicate. After incubation with 2 HU of hemolysin for different periods, the culture medium of HeLa and HEp- 2 cells was removed and kept in ice until measurement of LDH. An aliquot (0.1 mL) of each sample was added to a cuvette containing 0.2 mL of NADH solution (2.5 mg/ mL in 100 mM potassium phosphate buffer, pH 7.4) and 0.2 mL of sodium piruvate solution (1 mg/mL), and the rate of enzyme leakage/min was calculated and expressed as the percentage of the total LDH compared to untreated control cells.
2.10. Tetrazolium-Based Colorimetric Assay (MTT)
This assay involves the cellular reduction of MTT [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma) by the mitochondrial dehydrogenase of viable cells to produce a blue formazan product that can be spectrophotometrically measured. The HEp-2 cells were grown in 96-well plates for 24 h, treated with 2 HU of purified hemolysin in triplicate and the medium containing 100 mL of MTT solution (1 mg MTT/mL) was added to each well. After incubation for 4 h at 37˚C, the medium was removed and the formazan solubilized in absolute ethanol. The plate was shaken for 20 min and absorbance measured at 540 nm [18].
2.11. Apoptosis Detection by Annexin V Binding
The percentage of apoptotic cells was determined using Annexin V-FITC Apoptosis Detection Kit 1 (Pharmigen) and analyzed by flow cytometry in triplicate. After incubation with purified hemolysin, the cells were detached with trypsin (Nutricell) and suspended in EMEM-FBS. The suspension was centrifuged at 3000 × g for 12 min and the cell pellet was washed with PBS solution (pH 7.4) and stained with 5 mL of Annexin V-FITC and 10 mL of PI (propium Iodide, sock solution 0.05 mg·mL–1, final concentration of 0.7 mmol·L–1). After 15 min of incubation at room temperature, the cells were analyzed by flow cytometry.
3. RESULTS
3.1. Purification of Hemolysin
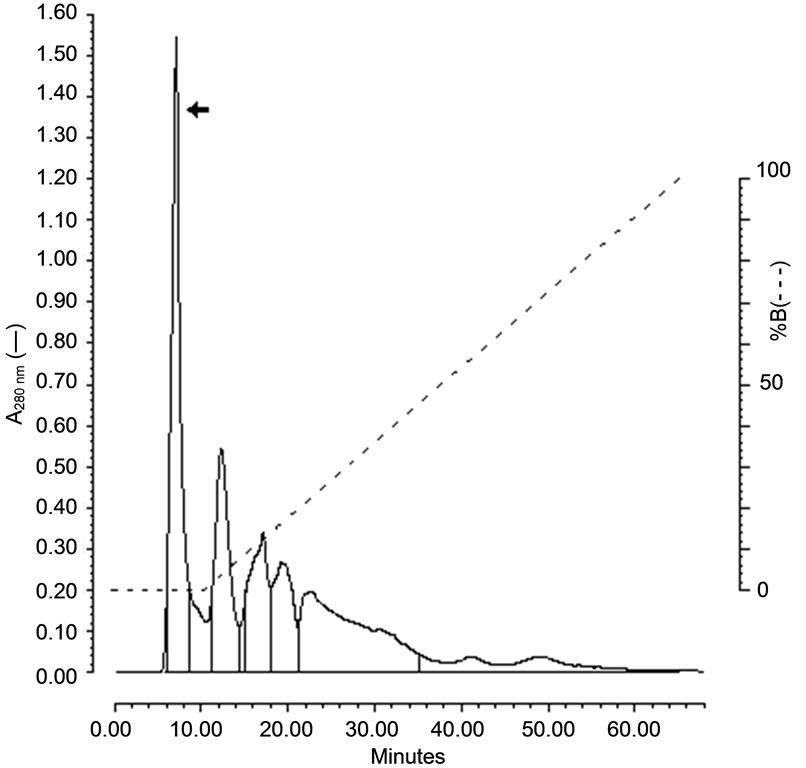
The hemolysin was purified after the ultrafiltration followed by precipitation with ethanol and was chromatographied on a mBondapak C18 column. The hemolytic activity was eluted as a sharp peak before the linear gradient of acetonitrile 66%, showing a hydrophilic character (Figure 1(a)). The hemolytic activity peak was concentrated by speed vac (Savant) and applied on a Superdex Peptide (GE Healthcare) column chromatography and the hemolytic activity was eluted at 47.95 min at a flow rate of 0.5 mL/min (Figure 1(b)).
3.2. Physic Chemical Properties of Hemolysin
The hemolytic activity was not affected by proteolytic enzymes such as protease K and trypsin (100 mg/mL).
3.3. Inhibition of Hemolytic Activity by Cholesterol and Phospholipids
The cholesterol and phospholipids (EPC) did not inhibit the hemolytic activity (Table 1).
3.4. Osmotic Protection
Only Dextran 4, among the others carbohydrates (raffinose, m-inositol, glucose, inositol, inuline, maltose, and arabinose) tested, protected the hemolysis caused by the hemolysin produced by E. faecalis. Moreover, the Ca2+ ions and ETDA (0.1 M) did not protect the hemolysis (Table 1).
3.5. Scanning Electron Microscopy (SEM)
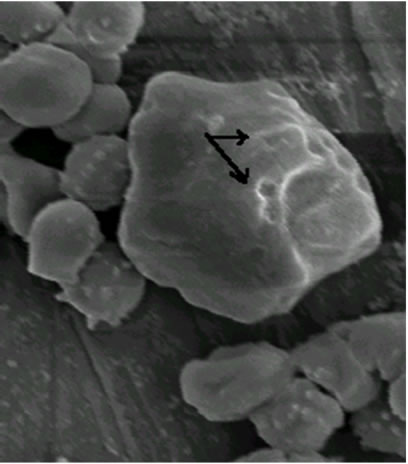
As shown in Figure 2, visualized by scanning electron microscopy (SEM) technique, the erythrocytes volume was unaltered after treatment for 1.5 min when compared with the erythrocytes control (a), although membrane lesions, including globular structures, were seen on the cell surface (b and c).
3.6. LDH Leakage
At 25 min, 30% of LDH leakage was observed as compared to the control and at 60 min, an increase to 80% above control LDH leakage has occurred (Figure 3).
3.7. MTT Assay
Mitochondrial activity started to rapidly decrease at 15 min of exposure to the hemolysin, indicating the onset of a toxic effect during this period and at 60 min, almost all of the mitochondrial activity was affected (Figure 4).
3.8. Apoptosis Detection by Annexin V Binding
The percentage of apoptotic cells, cell analysis and statistical analysis of the results mean are presented in Tables 2 and 3. The flow citometry assay showed that after 12 h of incubation, 51.92% of HeLa (Figure 5) and 68%
 (a)
(a)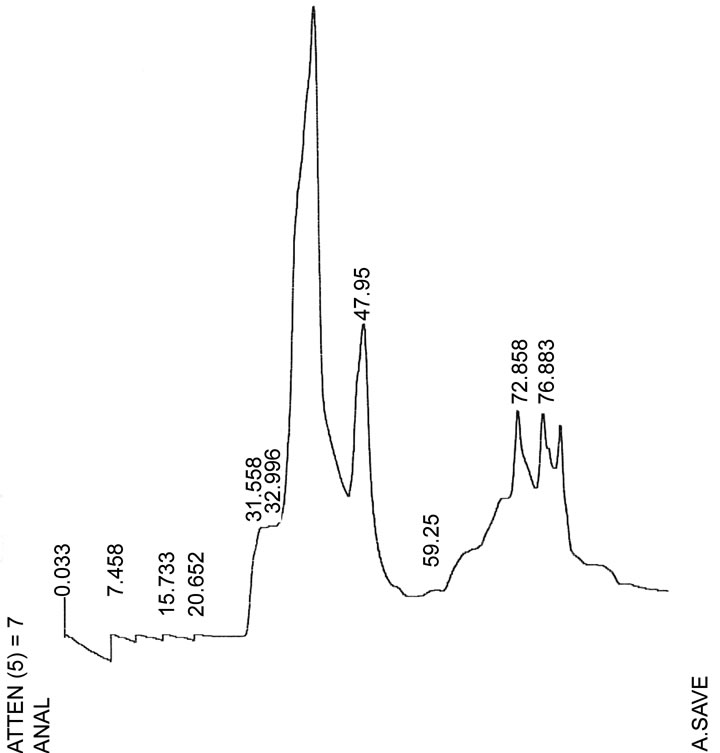 (b)
(b)
Figure 1. Chromatographic profiles of the purification of the enterococcal hemolysin. (a) Elution of hemolysin on mBondapak C18 reverse-phase HPLC. (b) Elution of hemolysin at 47.95 min (flow rate 0.5 mL/min) on reversephase HPLC. Arrows indicate the peak with the eluted hemolysin.

Table 1. Physicochemical characteristics of purified Encerococcus faecalis hemolysin.
 (a)
(a)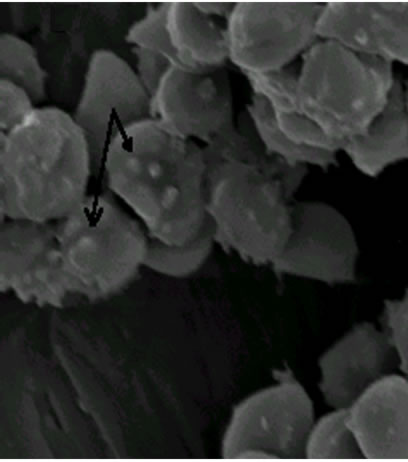 (b)
(b)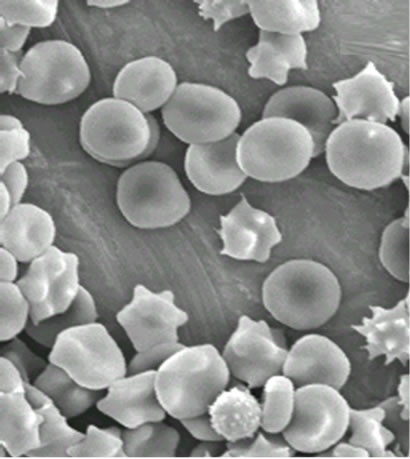 (c)
(c)
Figure 2. Electron microphotographs of sheep erythrocytes treated with hemolysin: (a) Controls (1800 × 10 kV); (b) Treated with 2 HU for 1.5 min (3000 × 10 kV); (c) for 2 min (2700 × 10 kV). Arrow indicates the lesions caused on the erythrocyte surface.
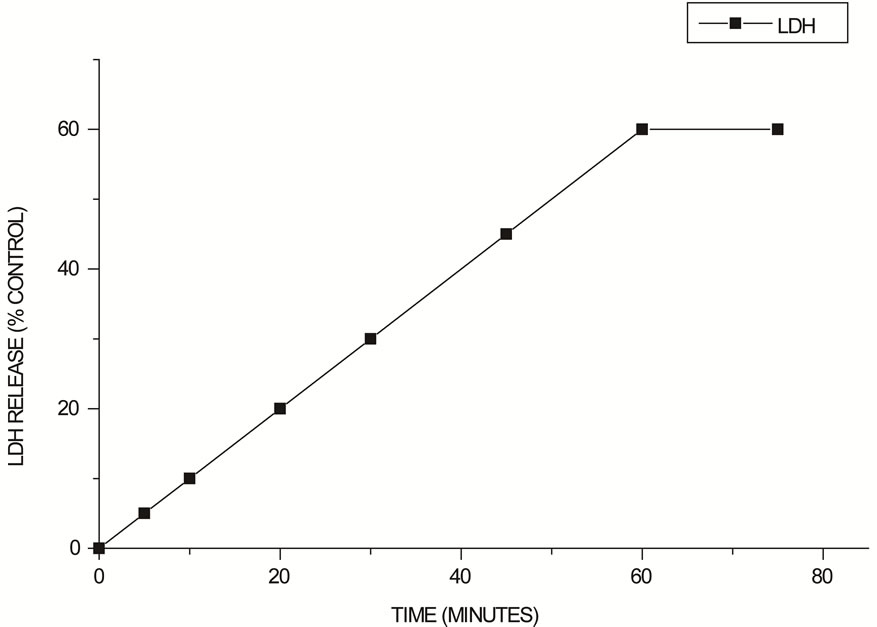
Figure 3. LDH Leakage in the HEp-2 cells after exposure to 2 HU E. faecalis hemolysin expressed as a percentage of control values.
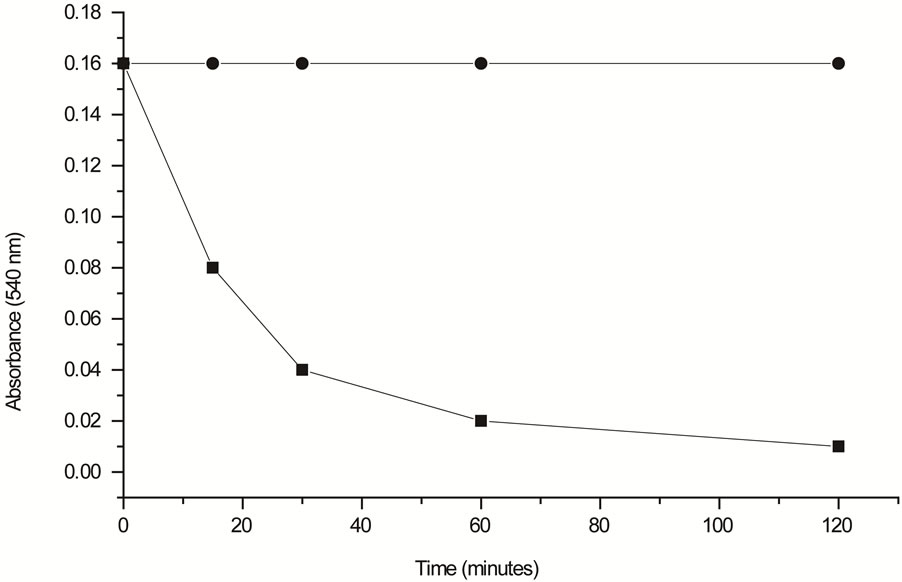
Figure 4. Mitochondrial activity rate estimated by MTT assay. Untreated HEp-2 cells (¨) used as a control and HEp-2 cells treated with 2 HU of hemolysin for different periods of exposure (■).
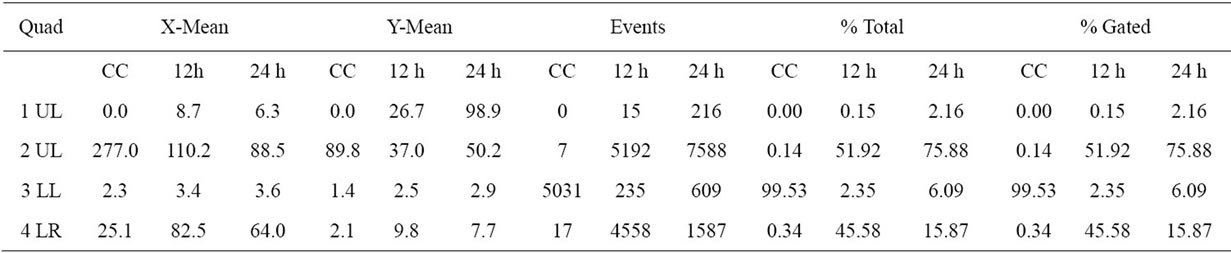
Table 2. The percentage of apoptotic cells, cell analysis and statistical analysis of HeLa cells results.
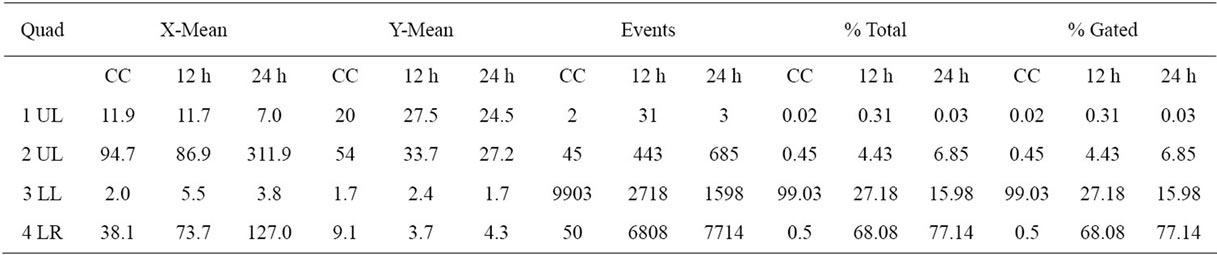
Table 3. The percentage of apoptotic cells, cell analysis and statistical analysis of HEp-2 cells results.
of HEp-2 (Figure 6) cells were dead by apoptosis.
4. DISCUSSION
Clinical isolates of E. faecalis produced a heat-stable hemolysin when cultivated in BHI-GA [3], but not in BHI [9]. In this work, we purified the hemolysin using ultrafiltration through a 10 kDa cutoff membrane (Amicon, Millipore) and mBondapak C18 column (Figure 1(a)) and the hemolysin was eluted at 47.95 min as a sharp peak on Superdex Peptide column (Figure 1(b)). This material was considered as the purified hemolysin and used here to study some biological characteristics.
The hemolytic activity was unaltered by treatment with proteolytic enzymes such as trypsin (100 mg/mL) and proteinase K (Table 1). Since trypsin cleaves only peptide bonds in which the carboxyl function is donated by either lysine or arginine [19], the hemolysin resistance to trypsin suggests that this molecule does not contain bonds involving the carboxy groups of these two amino acids. Moreover, proteinase K, an endopeptidase, did not digest the purified hemolysin. These results suggest that the heat-stable hemolysin produced by E. faecalis may
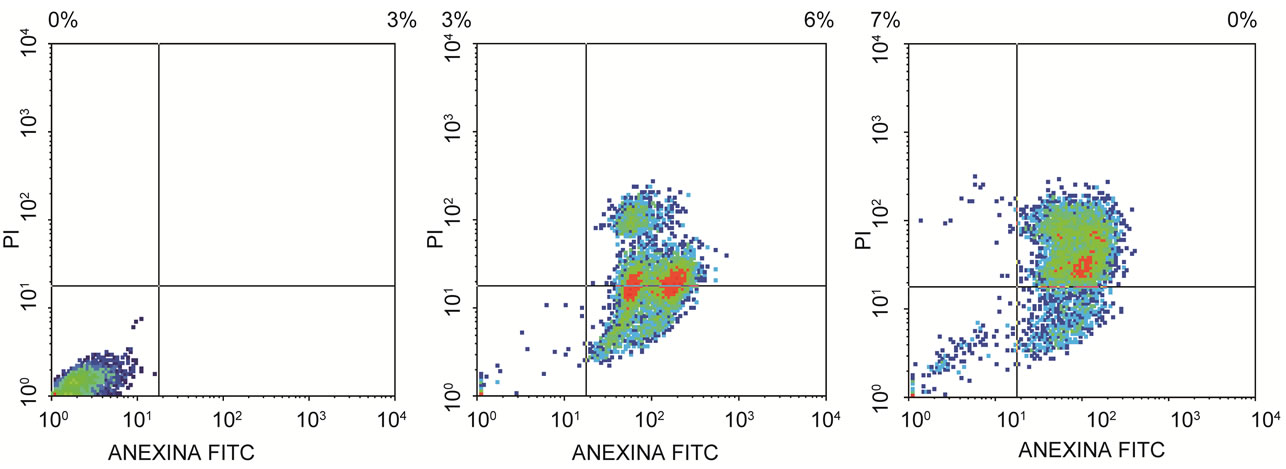 (a) (b) (c)
(a) (b) (c)
Figure 5. Flow citometry analysis. (a) HeLa control cell, (b) HeLa cell after 12 h and (c) 24 h of incubation with semi purified hemolysin.
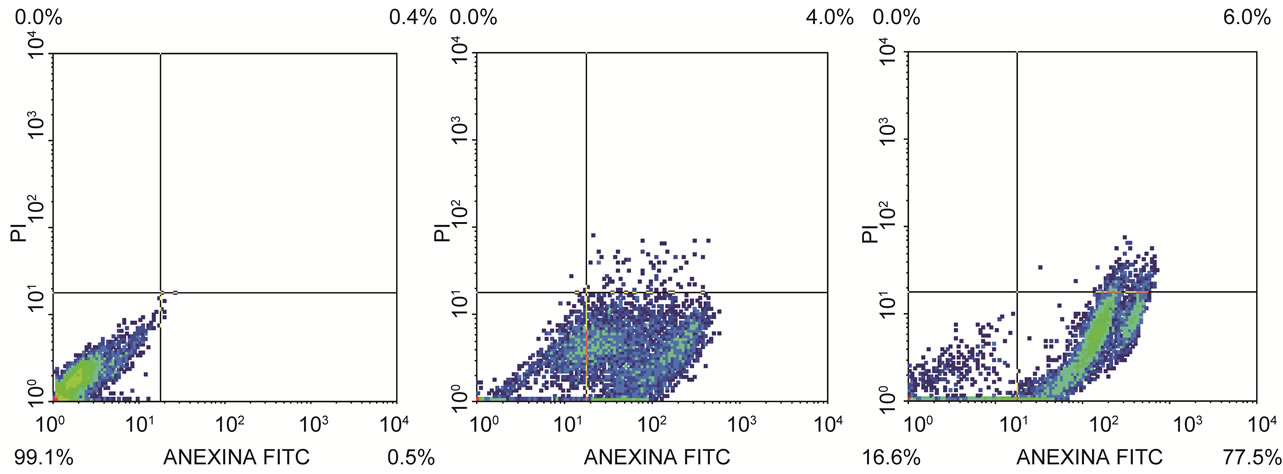 (a) (b) (c)
(a) (b) (c)
Figure 6. Flow citometry analysis. (a) HEp-2 control cell, (b) HEp-2 cell after 12 h and (c) 24 h of incubation with semi purified hemolysin.
be a peptide, smaller than 10 kDa as was determined by ultrafiltration on the PM-10 membrane (Amicon, Millipore).
Cytolytic toxins, such as hemolysins, cause damage to target membranes by three different mechanisms: Enzymatic degradation of membrane lipids, solubilization of the membrane through a detergent-like action (surfacetants), or pore formation [4].
E. faecalis hemolysin caused rapid damage on sheep erythrocytes at 2 min of exposure to hemolysin (Figure 2) and has shown osmotic protection by dextran 4. It probably involves the mechanisms of pore-forming on the target cell membrane to form a transmembrane pore with subsequent disruption of the membrane integrity [20] as a result of alterations in the passive ion permeability [21]. The cholesterol nor phospholipid did not protect from hemolysis suggesting that the hemolytic mechanism of the E. faecalis hemolysin is not due to the detergent-like action found in surfactants hemolysins nor to cytolisis by enzymatic degradation since the E. faecalis hemolysin did not require a period of chilling (0˚C - 4˚C) and incubation at 37˚C (“hot/cold”) as a hemolytic phenotype defined [4].
The hemolysins from the RTX family, such as HlyA of E. coli, are pore-forming toxins with the common trait of calcium requirement [22]. Furthermore, Aeromonas caviae and Plesiomonas shigelloides hemolysins are also stimulated by Ca2+ but inhibited by EDTA [23]. Our results indicate that the E. faecalis hemolysin differs from these hemolysins, since calcium ions did not affect their activity (Table 1).
Apoptosis is a morphologically distinct form of programmed cell death that plays major roles during development, homeostasis, and in many diseases including cancer, acquired immunodeficiency syndrome, and neurodegenerative disorders and is implicated in some types of bacterial pathogenesis [24] that involve a variety of virulence factors.
The strategies used by pathogens are not identical and are apparently designed to counteract host defense mechanisms or to allow tissue and organ invasion, thereby securing a niche for pathogen replication [25].
The results of the cytosolic enzyme LDH leakage (Figure 3) and the mitochondrial activity (Figure 4) of HEp- 2 treated with 2 HU (Figure 3) showed similar time of response and, at 60 min, almost all of the mitochondrial activity was affected. However, most of the cell death by apoptosis occurred at 12 h of incubation (Figures 5 and 6). Mitochondria may act as central executioner of apoptosis: apoptotic stimuli cause mitochondrial permeability transition, membrane despolarization and cytocrome C release, following caspase activation; the cell membrane lose its asymetry and phosphatidylserine is externalized to outer leaflet of the plasma membrane. Furthermore, condensation of chromatin occurs and endonucleases cause fragmentation of the cellular DNA [26].
In conclusion, clinical Enterococcus faecalis produced a low-molecular and heat-stable extracellular hemolysin with characteristics of pore-forming mechanism. In addition, the E. faecalis hemolysin induced apoptosis in human epithelial cells (HeLa and HEp 2) that may be a putative virulence factor in enterococci infections.
5. ACKNOWLEDGEMENTS
The authors thank Ana Stella Menegon Degrossoli for technical assistance. We thank the Microbiology Laboratory of the Hospital das Clínicas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil, for kindly providing the E. faecalis strains.
Financial support was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
![]()
![]()
REFERENCES
- Schaberg, D.R., Culver, D.H. and Gaynes, R.P. (1991) Major trends in microbial etiology of nosocomila infection. American Journal of Medicine, 91, 79S-82S. doi:10.1016/0002-9343(91)90346-Y
- Vergis, E.N., Shankar, N., Chow, J.W., Hayden, M.K., Snydman, D.R., Zervos, M.J., Linden, P.K., Wagener, M. M. and Muder, R.R. (2003) Association between the presence of enterococcal virulence factors gelatinase, hemolysin, and enterococcal surface protein and mortality among patients with bacteremia due to Enterococcus faecalis. Clinical Infectious Diseases, 35, 570-575. doi:10.1086/341977
- Dupont, H., Montravers, P., Mohler, J. and Carbon, C. (1998) Disparate findings on the role of virulence factors of Enterococcus faecalis in mouse and rat models of peritonitis. Infection and Immunity, 66, 2570-2575.
- Rowe, G.E. and Welch, R.A. (1994) Assay of hemolytic toxins. Methods in Enzymology, 235, 657-659. doi:10.1016/0076-6879(94)35179-1
- Booth, M.C., Bogie, C.P., Sahl, H.G., Siezen, R.J., Hatter, K.L. and Gilmore, M.S. (1996) Structural analysis and proteolytic activation of Enterococcus faecalis cytolysin, a novel lantibiotic. Molecular Microbiology, 21, 1175- 1184. doi:10.1046/j.1365-2958.1996.831449.x
- Gilmore, M.S., Segarra, R.A., Booth, M.C., Bogie, C.P., Hall, L.R. and Clewell, D.B. (1994) Genetic struture of Enterococcus faecalis plasmid pAD1-encode cytolytic toxin system and its relationship to lantibiotic determinants. Journal of Bacteriology, 176, 7335-7344.
- Miyazaki, S., Ohno, A., Kobayashi, I., Uji, T., Yamaguchi, K. and Goto, S. (1993) Cytotoxic effect of hemolytic culture supernatant from Enterococcus faecalis on mouse polymorphonuclear neutrophils and macrophages. Microbiology and Immunology, 37, 265-270.
- Jett, B.D., Huycke, M.M. and Gilmore, M.S. (1992) Virulence of enterococci. Clinical Microbiology Reviews, 7, 462-478.
- Furumura, M.T., Figueiredo, P.M.S., Carbonell, G.V., Darini, A.L.C. and Yano, T. (2006) Virulence-associated characteristics of Enterococcus faecalis strains isolated from clinical sources. Brazilian Journal of Microbiology, 37, 230-236.
- Takeda, Y., Takeda, T., Yano, T., Yamamoto, K. and Miwatani, T. (1979) Purification and partial characterization of heat-stable enterotoxin of enterotoxigenic Escherichia coli. Infection and Immunity, 25, 978-985.
- Arita, M., Takeda, T., Takeda, T., Honda, T. and Miwatani, T. (1986) Purification and characterization of Vibrio cholerae Non-01 heat-stable enterotoxin. Infection and Immunity, 52, 45-49.
- Leunk, R.D. (1991) Production of cytotoxin by Helicobacter pylori. Clinical Infectious Diseases, 13, 686-689. doi:10.1093/clinids/13.Supplement_8.S686
- Figueiredo, P.M.S., Catani, C.F. and Yano, T. (2003) Thiolindependent activity a cholesterol-binding enterohemolysin (EHly) produced by enteropathogenic Escherichia coli. Brazilian Journal of Medical and Biological Research, 36, 1495-1499. doi:10.1590/S0100-879X2003001100008
- Moayeri, M. and Welch, R. (1994) Effects of temperature, times, and toxin concentration on lesion formation by Escherichis coli hemolysin. Infection and Immunity, 62, 4124-4134.
- Gelderblom, H.R., Ozel, M., Hausmann, E.H.S., Winkel, T., Pauli, G. and Koch, M.A. (1988) Fine structure of human immunodeficiency virus (HIV), immunolocalization of structural proteins and virus-cell relation. Micron and Microscopica Acta, 19, 41-60. doi:10.1016/0739-6260(88)90039-1
- Rose, J.M., Houston, C.W., Coppenhaver, D.H., Dixon, J. D. and Kurosky, A. (1989) Purification and chemical characterization of cholera toxin-across-reactive cytolytic enterotoxin produced by human isolate of Aeromonas hydrophila. Infection and Immunity, 57, 1165-1169.
- Mitchell, L.R.M., Peiris, J.S.M., Cryz, S.J. and O’Brien, A.D. (1980) Evaluation of cytotoxicity in cultured cells by enzyme leakage. Journal of Tissue Culture Methods, 6, 113-116. doi:10.1007/BF02082861
- Souza-Pinto, N.C., Vercesi, A.E. and Hoffman, M.E. (1996) Mechanism of tetrahydroxy-1,4-quinone cytotoxicty: Involvement of Ca2+ and H2O2 in the impairment of DNA replication and mitochondrial function. Free Radical Biology & Medicine, 20, 657-666. doi:10.1016/0891-5849(95)02179-5
- Nelson, D.L. and Cox, M.M. (2000) Lehninger principles of biochemistry. 3rd Edition, Worth Publishers, New York.
- Füssle, R., Bhakdi, S., Sziegoleit, A., Tranum-Jensen, J., Kranz, T. and Wellensiek, H.J. (1981) On the mechanism of membrane damage by Staphylococcus aureus a-toxin. The Journal of Cell Biology, 91, 83-94. doi:10.1083/jcb.91.1.83
- Zavodnick, I.B., Lapshina, E.A., Zavodnick, L.B., Soszynski, G.B. and Bryszewska, M. (2002) Hypochlorous acidinduced oxidative damage of human red blood cells: Effects of tert-butyl hydroperoxide and nitrile on the HOCL reaction with erythrocytes. Bioelectrochemistry, 58, 127- 135. doi:10.1016/S1567-5394(01)00126-8
- Ludwing, A., Jarchau, T., Benz, R. and Goebel, W. (1988) The report domain Escherichia coli haemolysin (Hly A) is responsible for its Ca2+ dependent binding to erythrocytes. Molecular Genetics & Genomics, 214, 553-561. doi:10.1007/BF00330494
- Baratela, K.C., Saridaskis, H.O., Gaziri, L.C.J. and Pelayo, J.S. (2001) Effects of medium composition, calcium, iron and oxygen on haemolysin production by Plesiomonas shigelloides isolated from water. Journal of Applied Microbiology, 90, 482-487. doi:10.1046/j.1365-2672.2001.01270.x
- Thompson, C.B. (1995) Apoptosis in the pathogenesis and treatment of disease. Science, 267, 1456-1461. doi:10.1126/science.7878464
- Fernandez-Prada, C.M., Hoover, D.L., Tall, B.D. and Venkatesan, M.M. (1997) Human monocyte-derived macrophages infected with virulent Shigella flexneri in vitro undergo a rapid cytotoxic enterotoxin produced by Aeromonas hydrophila. Infection and Immunity, 65, 4299-4308.
- Tuschl, H. and Schwab, C.E. (2004) Flow cytometric methods used as screening tests for basal toxicity of chemicals. Toxicology in Vitro, 18, 483-491. doi:10.1016/j.tiv.2003.12.004
NOTES
*The two authors contribute equally to this article.
#Corresponding author.

