Journal of Environmental Protection
Vol. 3 No. 1 (2012) , Article ID: 16862 , 10 pages DOI:10.4236/jep.2012.31012
Degradation and Detoxification of Persistent Organic Pollutants in Soils by Plant Alkaloid Anabasine
![]()
1Institute of the Chemistry of Plant Substances, Academy of Sciences Republic of Uzbekistan, Tashkent, Uzbekistan; 2Institute of Ecology and Evolution of Rusian, Academy of Sciences, Moscow, Russia.
Email: hamid_khodjaniyazov@yahoo.com
Received September 7th, 2011; revised October 8th, 2011; accepted November 13th, 2011
Keywords: chlorinated pesticides; HCH; TeCB; DDT; DDE; trichlorobenzene; plant extracts; sum of alkaloids; Anabasis Aphylla; anabasine; detoxification
ABSTRACT
The development of a technology employing materials containing specific alkaloids from biomasses of agricultural production would be extremely beneficial and relatively inexpensive for the remediation of polluted soils and may represent a possible new industrial and commercial opportunity. We have studied detoxification and degradation of persistent chloroorganic pesticides by using plant extracts, which contained alkaloids. Reactions DDT with alkaloid Anabasine and extractive sum of alkaloids, isolated from ground up part plant Anabasis аphylla proceed more easy without solvent. It is established, that at interaction alkaloids of Anabasis aphylla with pesticide DDT occurs degradation to formation of less toxic DDE. Results the carried out researches on studying interaction sum of alkaloids, isolated from ground up part plant Anabasis Ahylla, with DDT in various ratio (1:1, 2:1, 3:1) have shown, that thus there are detoxification pesticide DDT on 35% - 45%, 75% - 80% and 80% - 85% according to formation dichlorodiphenyldichloroethylene (DDE), and in presence humic acids the degree degradation achieves to 95% - 97%.
1. Introduction
Polychlorinated organic pesticides (POP) are well-known recalcitrant environmental pollutants. Although the metabolism of the POPs has been intensively studied, very little is known about their mechanism of toxicity in living organisms or how they are degraded. We have examined interaction between POPs with some plant alkaloids (Anabasine) and its extractive sum with other analogical compounds.
The full-scale and widespread use of agricultural pesticides in Uzbekistan in the 1960-1980 decades resulted in a serious pollution of the environment (soil, water and atmosphere). During this period, persistent halogenated pesticides such as DDT, lindane, heptachlor, pentachlorophenol, aldrin, keltan and their several combined derivatives were used in large scale due to their efficiency and non-specificity. Moreover, DDT and hexachlorocyclohexane were used as effective pesticides for treatment of plant seeds, soil, and crops, and in livestock production, as well as disinfectants against bugs, mosquitoes, flies and other harmful insects for household use.
Uzbekistan was a major producer of cotton and pesticides were applied to the cotton cultivation in huge quantities. For example, based on the data of the State Stock Holding Company “Uzagricultural-chemistry”, in the year 1971-1988, the cited pesticides were used on 1, 7, 2, 1 billion hectares of Uzbekistan cropping areas for cotton and in the treatment of cotton seeds in the amount of 11 - 12 kg per ton annually. For this purposes 1800 - 2000 tons of fentiuram, which consists of 40% TDTM, 15% hexachlorocyclohexane and 10% 2,3,5-trichlorophenolate of copper, were employed [1]. It is noteworthy that technical trichlorophenolate of copper is reported to contain an amount of the highly toxic and environmentally persistent 2,3,7,8-tetrachlorodibenzo-p-dioxin [2].
Therefore, during 17 years only one of these persistent organic pollutants, pentachloronitrobenzene was applied in more than 30,000 tons to struggle against the cotton wilt disease. Pentachlorophenol was also largely used as antiseptic, desiccant and herbicide. This persistent substance is also very dangerous because photolytic reactions can change it into dioxin isomers.
Besides the past heavy contamination of soils and extended environment, the prohibition of further application of halogenated pesticides following new regulation after the years 1989-1990 have left huge quantities of these pesticides unused with their accumulation in uncontrolled dumps directly in soils, thereby representing a serious risk to further the environmental pollution. This situation is no longer acceptable and measures have to be taken to reduce the existing environmental contamination and preserve future generations from health hazards.
2. Interaction DDT with Alkaloid Anabasine
We have studied interaction of DDT with anabasine isolated from a plant of Anabasis Aphylla. The lead researches have shown that in this case of interactions of DDT with anabazine goes easier without solvents in formating a new compound. For a finding of an optimum ratio anabasine and DDT, reaction spent in various ratio alkaloid: pesticide (table 1).
From the resulted data it is visible that optimum conditions are: a ratio of 3:1, heating at 50˚C - 55˚C, thus up to 85% - 90% yield of DDE is achieved.
Search interaction sum of alkaloids, isolated from a plant Anabasis Aphylla with DDT have shown, that in this case effective there was also a ratio 3:1 (conversion 80% - 85%).
Further search interaction sum of alkaloids isolated from a plant Anabasis Aphylla with DDT in presence of humic acid in ratio 2:1:4. Thus also there are 95% - 97% degradation DDT by forming less toxic product. With the help of column chromatography pure product of transformations were isolated and identified by GC-MS (Figure 1). Results of analysis are given in table 2.
From the data of the table it is visible, that thus less toxic product of transformation DDE is formed.
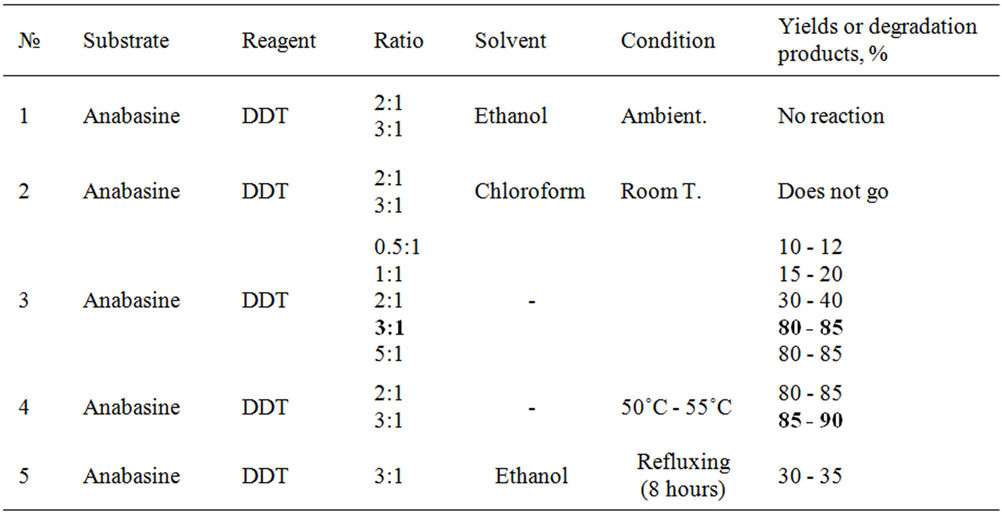
Table 1. Interaction of anabasine with DDT.


Figure 1. Mass chromatograms of the transformation products of DDT.
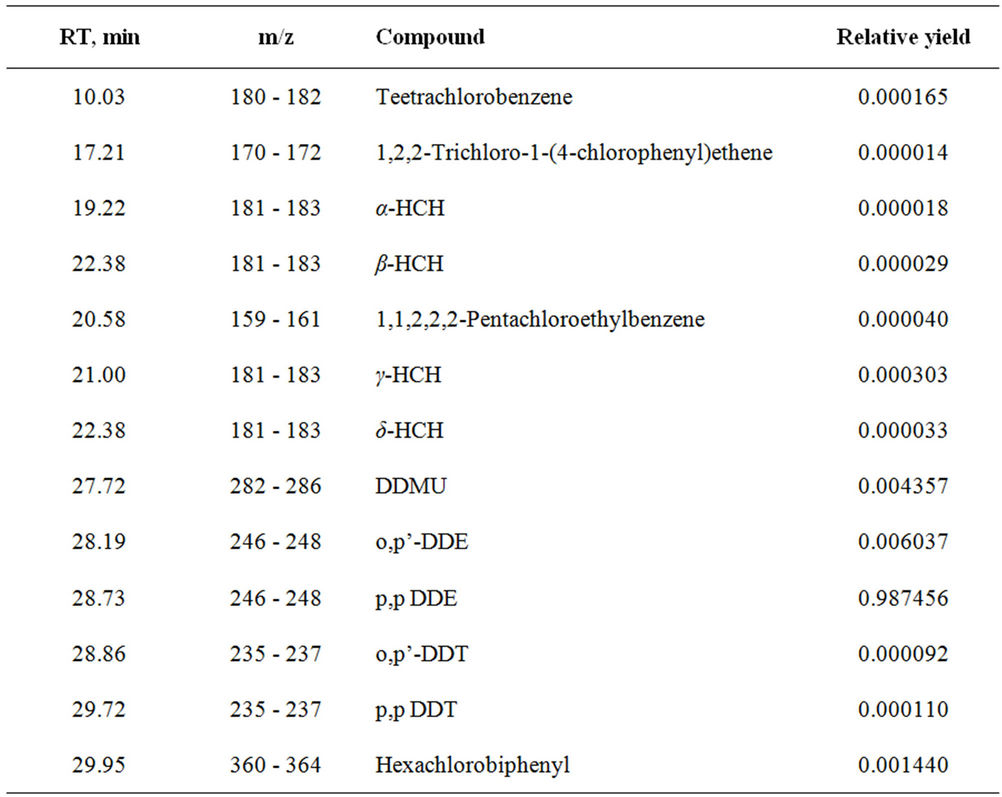
Table 2. Transformation products of DDT.
Researches on studying of interaction isolated from extractive sum of Anabasis aphylla with DDT in presence of humic acids in various ratio (1:2.2:0.5, 1:2.2:0.25) are lead. Degradation pesticide DDT in ratio of reagents 1:2.2:0.5 at room temperature during 7 days or at 50˚C - 60˚C during 24 hours occurs on 85% - 95% with forming DDE.
With the purpose of increase of a degree of degradation was investigated interaction of sum of alkaloids from ground up part of a plant Anabasis aphylla with DDT in presence of hydrogen peroxide (3%). Increasing of polychlorobiphenyls degradation in presence of H2O2 are well known [3,4]. It was determined, that during 5 days in presence H2O2 at room temperature occurs full transformations DDT to DDE.
Thus, the received data allow to conclude, that at interaction of alkaloid Anabasine with DDT occurs degradation to formation of less toxic DDE.
Results the lead researches on studying interaction sum of alkaloids, isolated from ground up part of a plant Anabasis Ahylla with DDT in various ratio (1:1, 2:1, 3:1) have shown, that thus there are detoxification DDT on 35% - 45%, 75% - 80% and 80% - 85% according to formation dichlorodiphenyldichloroethylene (DDE), and in presence of humic acids the degree of degradation is achieved up to 95% - 97% [5-7].
3. Hexachlorocyclohexane Degradation
Interaction of hexachlorocyclohexane with Anabasine in various conditions and a ratio of reagents—1:3; 1:6, without solvent or in ethanol solution. Results GC-MS analysis had shown, that in the case of reaction HCH with anabasine in a ratio 1:3 degradation goes with yield 76% (figure 2), and in a ratio 1:6 is 40%. Reaction carried out at room temperature and at 50˚C - 55˚C, at increased temperature reaction goes faster, but increased yield is not observed.
Received results show (table 3), that thus it is formed basically trichlorobenzenes (93.8%), are present insignificant quantity dichlorobenzene and tetrachlorobenzene.
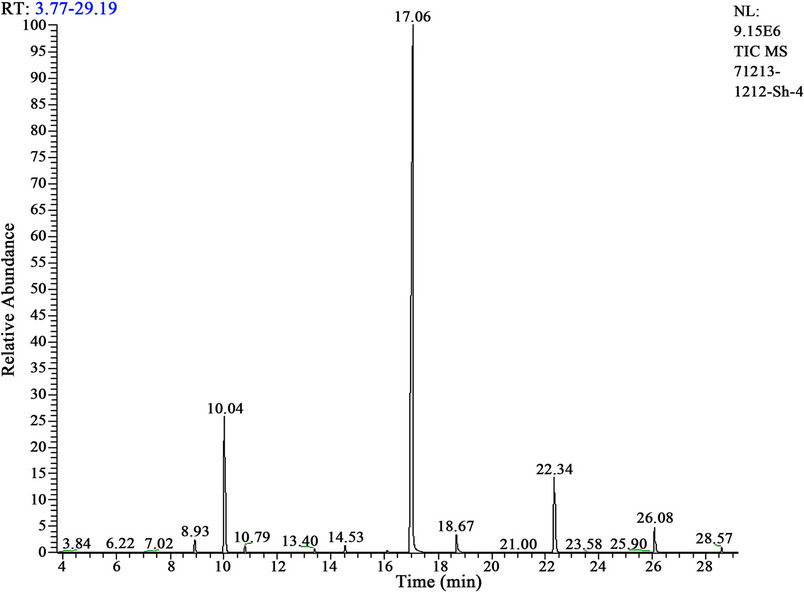
Figure 2. Mass-chromatogram of HCH degradation products.
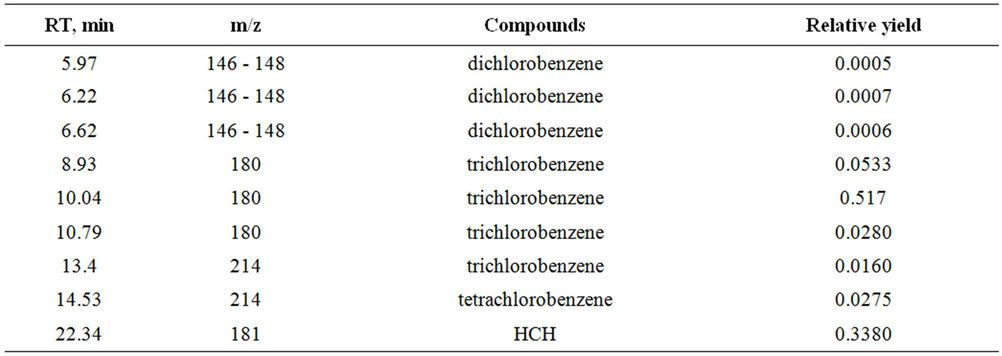
Table 3. Degradation products of HCH.
Reactions of a mix humic acids and extracts from a plant of Anabasis Aphylla with HCH in a ratio of reagents 1:0.1:1, 1:1:1, 1:2:1 in solution and without solvent are investigated. The optimal ratio was 1:2:1. Products of degradation have been studied by GC-MS (figure 3).
Results a quantitative estimation are resulted in table 4.

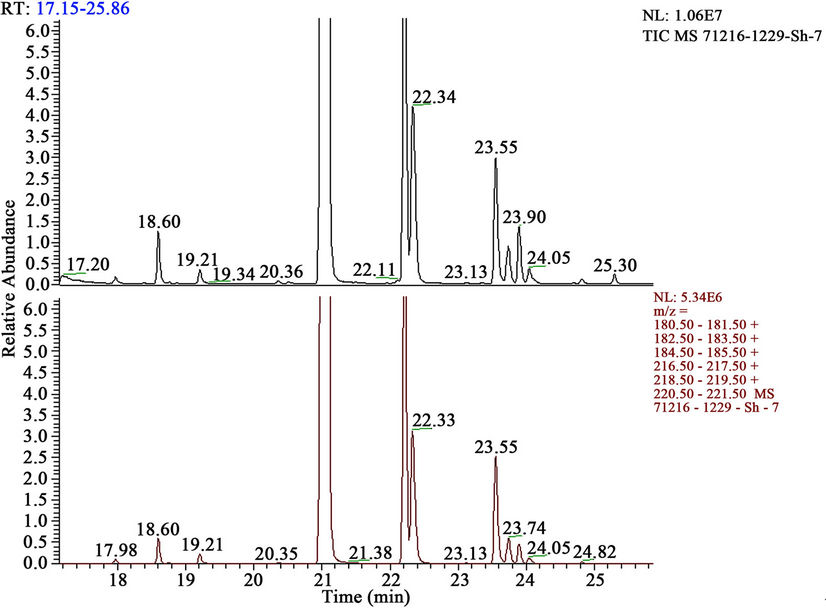
Figure 3. Mass-chromatograms of HCH + extracts of Anabasis Ahylla + humic acids.

Table 4. Transformation products of HCH with extracts of Anabasis Ahylla + humic acids.
From the data resulted in the table, it is visible, that thus 96%, 63% tetrachlorohexadiene (HCH) and insignificant quantity of tri-, tetrachlorobenzenes and pentachlorocyclohexadiene are formed.
It is investigated interaction sum of alkaloids from ground up part plant Anabasis aphylla, alkaloid of anabasine with HCH in presence 3% H2O2. It is established, that thus goes full detoxification of pesticide HCH (figures 4 and 5, tables 5 and 6).
Obtained results show, that thus basically it is formed tetrachlorocyclohexadiene (66.17%) and trichlorobenzene (28.99%) and a small quantity of tetrachlorobenzene and pentachlorocyclohexene.
In a case of interaction Anabasine with HCH in presence 3% H2O2 content of tetrachlorocyclohexadiene achieves up to 79.26%, and content of trichlorobenzene makes 16.57%.
Interaction tetrachlorobenzene with Anabasine, also with sum of alkaloids are not given positive results, i.e. thus there is no disintegration TeCB.
4. Experiments
4.1. Condition of GLC
GLC analysis (for reaction of tetrachlorobenzene) detector DIP, a column carry out on device Chromium-5: 2500 mm glass, diameter 3 mm, a nozzle: Inerton AW-HMDS 0.16 - 0.20; 5% SE-52, M.p. 260˚C, B.p. = 150˚C; VN2 = 30 ml/min, scale 16; test 1 mcl; Vpaper = 0.3 sm/min; sensitivity of a recorder –2 mV.
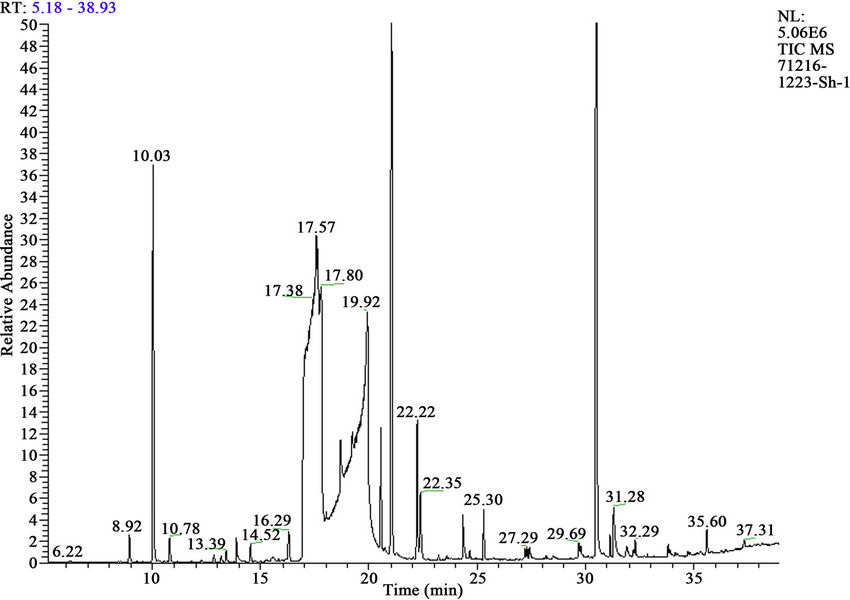
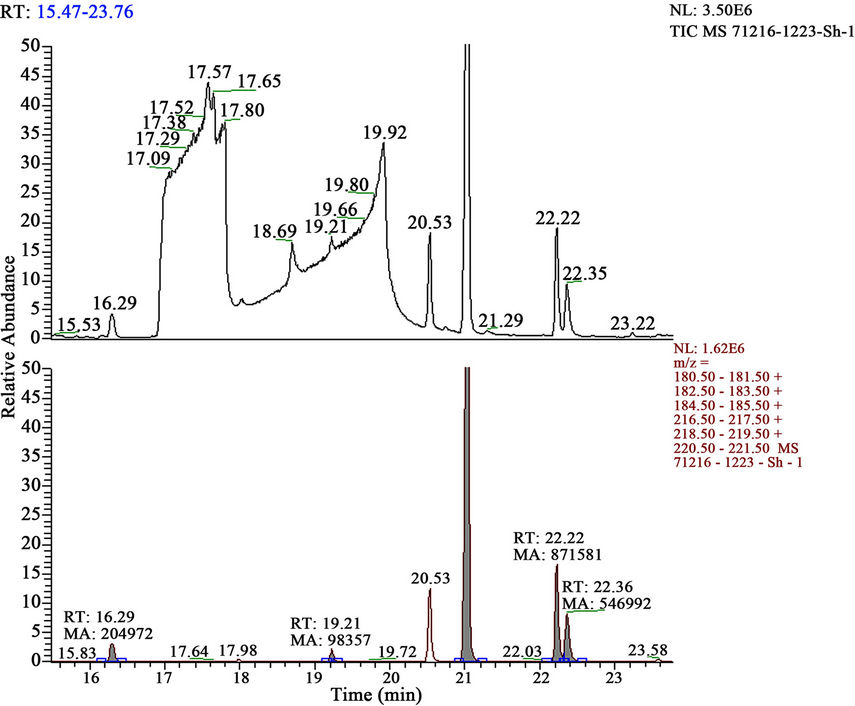
Figure 4. Mass chromatograms of reaction products of HCH with sum of alkaloids (Anabasis aphylla) + Н2О2.
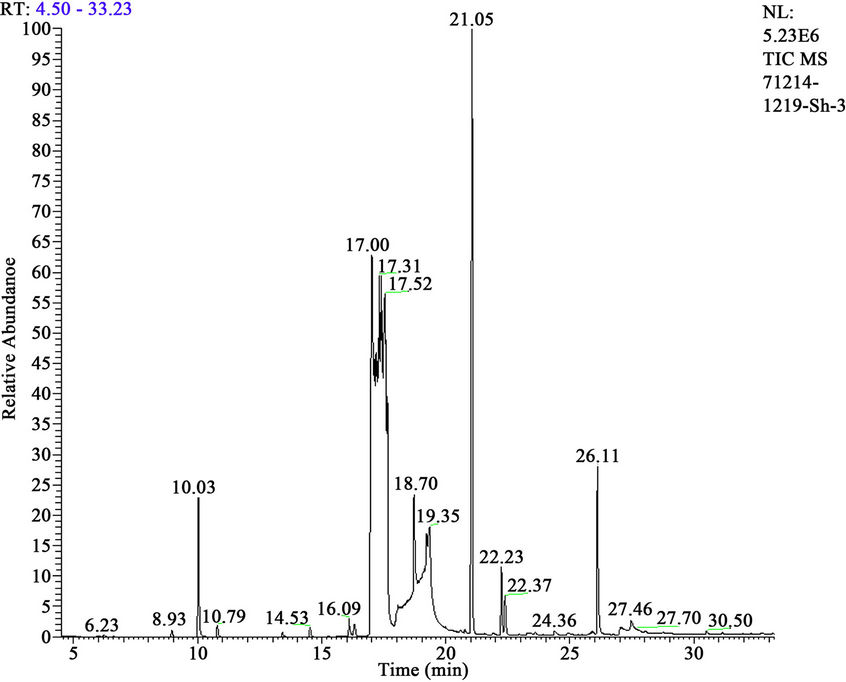
Figure 5. Mass chromatogram of products HCH degradation with Anabasine + Н2О2.
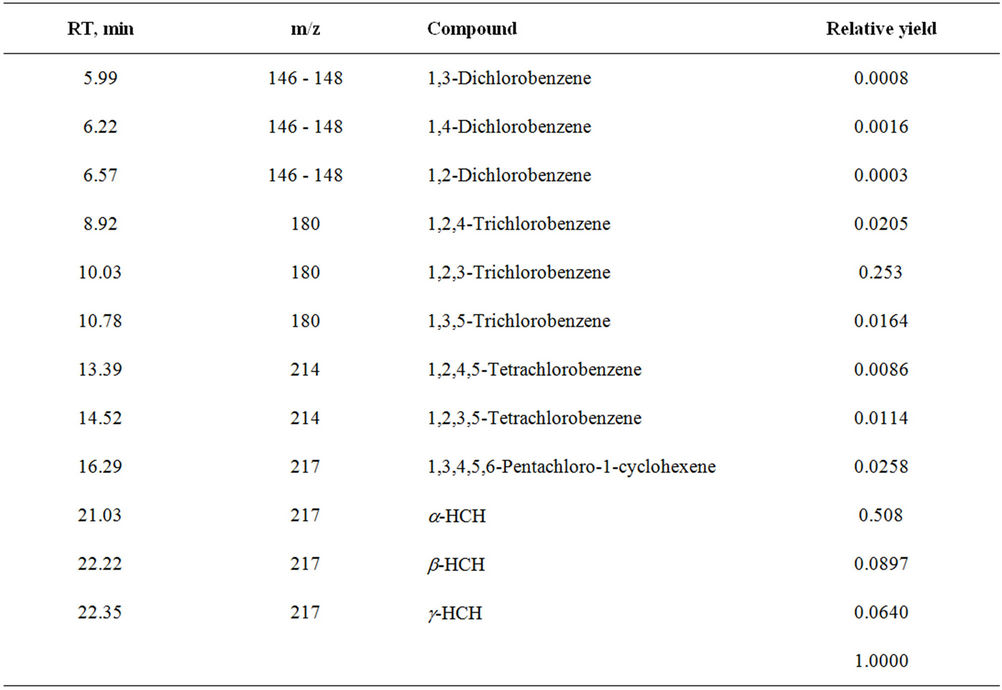
Table 5. Reaction products of HCH with sum of alkaloids (Anabasis aphylla) + Н2О2.

Table 6. Products of HCH degradation with Anabasine in presence H2O2.
4.1.1. GC-MS Analysis
The analysis was performed on gas chromatograph Trace GC with ion trap mass-spectrometric detector Polaris Q (Thermo Finnigan). Analysis conditions of: silica capillary column 7 m × 0.32 mm (DB-5ms, film thickness 0.25 micron), temperature programming from 60˚C (2 min) to 200˚C with a rate 6˚C/min then to 280˚C with a rate 8˚C/min, injector temperature 220˚C, interface- 220˚C, electron ionization at energy 70 eV, full scan 41 - 450 Da. Splitless injection (1:25).
4.1.2. GLC Analysis
GLC analysis carried out on chromatograph “Chromotec Crystal 5000”, detector EZD-2 temperature 250˚C, a column: 1500mm glass, diameter 3mm, a nozzle: SE-30, the evaporator temperature is 260˚C, VN2 = 30 ml/min; volume of test 2 ml;
4.2. Isolation of the Sum of Alkaloids from Anabasis Aphylla
500 g the dried up elevated part plant with a degree of crushing 1 - 2 mm fill in 2000ml 5% of a solution of ammonia, mix and leave at 12 hours. Then filtered, the rest extracted 2 more times on 1000 ml 5% solution of ammonia. All extracts unite and reextracted a mix petroleum and a sulfuric ethers a ratio 1:1. Solvent drive away on rotor evaporator. The yield 7.8 g (1.56% from airdried weigh).
4.3. Isolation of Anabasine by Column Chromatography with Aluminum Oxide
In column (height 80 sm and diameter 30 mm) fill in the “wet” way 300 g aluminum oxide of II degree of activity. There bring 5 g sum of alkaloids, isolated from of plant, dissolved in 10 ml petroleum ether (T. boil. 40˚ - 65˚). When the solution almost will completely be absorbed in the top layers of adsorbent, a column start to wash out by petroleum ether with the subsequent increase of polarity of eluent with addition of methanol. Collect on 50 ml. The contents of solutions supervise on TLC. From the moment of transition to the mixed solvent it is washed away Anabasine, extracts unite, solvent drive away also the rest overtake in vacuum. T. boil. 139˚/12 mm.
4.4. Isolation of HCH from Preparation “Hexachlorane”
10 g 12% p.s. pesticide “Hexachlorane” heated up with 100 ml heptane and filtered in a hot kind. A filtrate cooled and a dropped out deposit have filtered. Received hexachlorocyclohexane recrystalled from ethanol. Yield is 80%.
4.5. Reaction of Anabasine with DDT without Solvent
In a conic flask place 500 mg Anabasine and 380 mg DDT, well mix and heat up at 50˚C - 60˚C during 12 hours. The next day a reaction mixture check with help TLC. Results HPTLC show, that the new stain—DDE with the yield 85% was formed.
Reactions at temperature 20˚C, 40˚C carried out similarly.
4.6. Isolation by Column Chromatography
Reaction mixture dissolve in acetone and mix with a small amount silicagel. A column with a diameter 1,8sm, height 100 sm fill silica gel marks КСК 100 - 250. In quality of eluent use hexane. Collected on 100ml. In result it is isolated new compounds—DDE in quantity 210 mg (66%). M.p. 75˚C - 77˚C. Rf = 0.87 system hexane: acetone 6:1.
4.7. Reactions Plant Extracts with DDT
In a conic flask place on 100 mg extracts and on 50 mg DDT. Mix and at room temperature within 3 days, check on TLC, significant changes are not present, leave for 7 days, results TLC show, that quantity of DDT in a reaction mixture has decreased for 45% - 50% in a case extract III, in the case of 96% ethanol extract on 50% - 60%.
4.8. Interaction Humic Acid with DDT in Presence of Alkaloid in Solution
Sample humic acids in weight 120 mg have mixed with 11 mg alkaloid Anabasine, 12 mg DDT and have added 2.6 ml ethanol. Lead through 1 and 2 weeks TLC analyses did not give positive results, thus what or changes of initial compounds do not occur.
4.9. Interaction Humic Acid with HCH in Solution
100 mg sample of humic acids and 10 mg HCH have mixed in 2 ml acetone. The analysis on TLC gives a new stain on finish which differs from HCH by Rf value. It is resulted GLC analysis of a reaction mixture dissolved in toluene.
5. Conclusions
Reactions DDT with alkaloid Anabasine and extractive sum of alkaloids, isolated from ground up part plant Anabasis аphylla proceed more easy without solvent. It is established, that at interaction alkaloids of Anabasis aphylla with pesticide DDT occurs degradation to formation of less toxic DDE.
Results the carried out researches on studying interaction sum of alkaloids, isolated from ground up part plant Anabasis Ahylla, with DDT in various ratio (1:1, 2:1, 3:1) have shown, that thus there are detoxification pesticide DDT on 35% - 45%, 75% - 80% and 80% - 85% according to formation dichlorodiphenyldichloroethylene (DDE), and in presence humic acids the degree degradation achieves to 95% - 97%.
6. Acknowledgements
The work was made by financial support of the INTAS M3-7271.
REFERENCES
- http://www.nashislova.ru/she/page/fentiuram.3737
- Kunimasa Morita, Masahiro Ogata and Takashi Hasegawa, “Chlorophyll Derived from Chlorella Inhibits Dioxin Absorption from the Gastrointestinal Tract and Accelerates Dioxin Excretion in Rats,” Environmental Health Perspectives, Vol. 1091, No. 3, 2001, pp. 289-294. doi:10.1289/01109289
- J. J. Pignatello and G. Chapa, “Degradation of PCBs by Ferric Ion, Hydrogen Peroxide and UV Light,” Environmental Toxicology and Chemistry, Vol. 13, No. 3, 1994, pp. 423-427. doi:10.1002/5620130309
- G. Köller, M. Möder and K. Czihala, “Peroxidative Degradation of Selected PCB: A Mechanistic Study,” Chemosphere, Vol. 41, No. 12, 2000, pp. 1827-1834. doi:10.1016/S0045-6535(00)00132-6
- A. P. Orekhov, “Chemistry of Alkaloids,” in Russian, 2nd Edition, AS USSR, Moscow, 1955.
- Kh. M. Shakhidoyatov, M. M. Khakimov, N. I. Mukarramov, N. K. Khidyrova, Kh. U. Khodjaniyazov and B. A. Urakov, “The Ways of Degradation of Proof Chlororganic Pollutants by Plant Extracts,” 7th International Symposium on the Chemistry of Natural Compounds, Tashkent, 16-18 October 2007, p. 147.
- Kh. M. Shakhidoyatov, M. M. Khakimov, N. I. Mukarramov and B. A. Urakov, “The Use of Alkaloid Containing Plant Extracts for Detoxification of DDT,” Materials of XVIII Mendeleev Congress, Moscow, 23-29 September 2007, p. 131.

