Atmospheric and Climate Sciences
Vol. 2 No. 1 (2012) , Article ID: 17132 , 6 pages DOI:10.4236/acs.2012.21012
Asymmetric Variation in Soil Carbon Emission in Sub-Tropics
1Institute of Natural Resources, Massey University, Palmerston North, New Zealand
2Department of Environmental Studies, University of Delhi, New Delhi, India
Email: R.Kant@massey.ac.nz
Received August 16, 2011; revised October 16, 2011; accepted November 3, 2011
Keywords: Soil Respiration; Carbon Emission; Bacterial Abundance; Fungal Population; Soil Depth; Climate Change
ABSTRACT
Carbon dioxide emission from soil, known as soil respiration, is one of the major sources of the atmospheric carbon. Understanding the relationship between emission rate and the factors associated with the emission process is important in global carbon emission management. The present study investigated soil respiration at three ecologically diverse locations in northern India. CO2 emission was measured in-situ by modified alkali absorption method at three different depths, top-soil (0 cm - 2 cm depth), mid-soil (20 cm depth) and deep-soil (40 cm depth) at each location. Rate of carbon emission from soil varied with location and time. The rate was higher at Riverine Zone (RZ) which had high soil moisture content and profuse ground vegetation compared to Hilly Zone (HZ) containing dry soil and scarce vegetation. The emission rate was also greater in grassland than the plantation area. Rate of carbon emission from soil was heterogeneous along different depths below the ground. Diel variation in emission rate was greater at HZ compared to RZ. Higher microbial population in soil was detected in RZ than HZ. However, the bacterial count out-numbered the fungal count in soils at most places. The study indicates a positive relationship between soil respiration rate and microbial abundance. The fungal population was strongly correlated with CO2 emission rate.
1. Introduction
Carbon dioxide concentration in the atmosphere has been increasing since the industrial revolution. However, the rate of increase has accelerated to about 0.5% per year during the last few decades [1]. The release of CO2 from soil due to autotrophic and heterotrophic respiration is also known as soil respiration, is the second largest source of CO2 efflux in the atmosphere [2]. The amount of CO2 release in the terrestrial ecosystem through soil respiration is approximately 11 times more than the contribution from fossil fuel burning [3].
Several soil physio-chemical and biological factors affect the rate of CO2 emission from soil [4]. These factors are interrelated, and show both synergistic and antagonistic effects on the emission rate. The physio-chemical properties of soil and the rate of carbon emission from the soil are intricately related and vary in soils [5,6]. Changes in day and night can also affect the soil temperature especially the top-soil that eventually affects biological activities in the soil [7].
There are various organisms in the soil that adds up to soil respiration rate but their contribution varies with soil factors including soil type, moisture content, pH, vegetation and is not well understood [8]. The major component of soil respiration is through decomposition of soil organic matter by the soil microorganisms [9]. Only a third of the total carbon metabolised by the microorganisms is utilised by them and the rest is lost in the form of CO2 in atmosphere. Among the soil microorganisms, bacteria and fungi play a major role in the soil respiration and their abundance varies with time and type of the soil [8].
Vegetation structure and density affect soil properties and mineralization process in the soil. Microbial abundance and distribution in the soil also get affected by the vegetation and species composition [10]. Vegetation screens the sunrays intensity and reduces the immediate drying off of soil. Thus, soil temperature and moisture content of soil are eventually regulated by the vegetation cover. Relative amount of air and water in the soil pore spaces affects the activities of these organisms in the soil and the ecosystem metabolism [11].
Understanding soil respiration in relation to different environmental conditions is important because a slight change in the condition could greatly affect the CO2 concentration in the atmosphere [2]. The study of soil respiration process in relation to microorganism abundance and atmospheric factors may help in understanding the process in global carbon emission. The present study was undertaken with two main objectives: 1) to quantify soil respiration rate in different ecosystems and 2) to quantify microbial distribution in soil and its relationship with CO2 emission. Any information on soil respiration could be of great importance in understanding the local terrestrial carbon budgets and to determine whether local terrestrial ecosystem acts as a sink or a source of carbon dioxide.
2. Materials and Methods
2.1. Experimental Sites
The study was conducted in the sub-tropics of northern region (Delhi) of India. In the territory of Delhi, two physio-graphic zones were selected on the basis of soil and vegetation pattern.
2.1.1. Hilly Zone (HZ)
The Hilly Zone is a ridge area, situated 28˚33'27"N and 77˚08'49"E, in the southern range of Delhi (Figure 1). This location is an extension of the oldest chain of mountains in India, The Aravallis. The soil in this location is comprised of sand stone rocks of the Delhi ridge, and the soil texture varies from sandy loam to clay loam. The pH of the soil is slightly acidic and has very low organic matter content [12]. The soil is very dry and the ground water level in the area is very deep. Due to uneven topography of the area, the soil erosion and formation of gullies are common. The region has sparse ground vegetation and the site is dominated by thorny Acacia woodland with Prosopis juliflora as a predominant species.
2.1.2. Riverine Zone (RZ)
The Riverine Zone is a part of riverbed soil, situated 28˚41'29"N and 77˚14'49"E, in the northern part of New Delhi. It is located about 2 km away from the river Yamuna (Figure 1). Texture of the soil in this area varies from silt to sandy loam. The soil is alkaline in nature because of salt pan formation during floods in the past years but the soil has high organic matter content [12]. The ground water level is quite high in this area. Two experimental sites were selected in this area, RZ-I and RZ-II. The RZ-I site is a plantation area, having different tree species and lacking ground vegetation, while RZ-II is covered by profuse ground vegetation, dominated by Desmostachya bipinnata.
2.2. Climate of Delhi
The semi-arid climate of Delhi is generally influenced by its remote inland location, Delhi ridge and the river Yamuna. It has extreme summer alternating with extreme winter and moderate rainfall. Therefore, the climate is not favourable for the growth of luxuriant vegetation. The average minimum and maximum ambient temperature
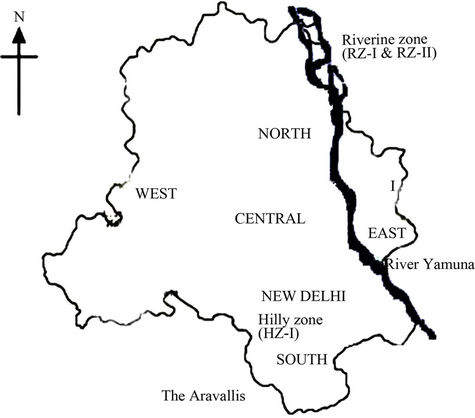
Figure 1. Locations of experimental sites, Hilly Zone (HZ) and Riverine Zone (RZ), are shown on Delhi map. HZ-I situated in the southern part of Delhi is a part of The Aravallis. Two locations of Riverine Zone (RZ-I and RZ-II), situated in the northern part of Delhi, were about 2 Km away from river Yamuna.
during the experiment months were 20.4˚C and 36.6˚C, respectively at 10 cm above ground level. The average rainfall during the period was l0.27 cm.
2.3. Soil Respiration Measurement
Soil respiration (SR) efflux was measured in situ by modified alkali absorption method [13]. Metallic cylinders (diameter 10 cm) of three different lengths 10 cm, 30 cm and 50 cm were used for measuring SR and collecting soil samples at <2 cm (top-soil), 20 cm (mid-soil), and 40 cm (deep-soil) deep, respectively. The amount of CO2 evolved from the ground was measured at every 24 h and the respiration values were converted per hour. CO2 efflux was measured for 12 h light and 12 h dark to compare day and night time variation in respiration rate.
2.4. Microbial Enumeration
Microbial biomass was estimated by the colony counting method. Microbes were first isolated by Serial Dilution Technique, at 10–5 dilution from 1 g soil, on PDA (Potato Dextrose Media, HiMedia) and NA (Nutrient Agar, Hi Media) for fungal and bacterial population, respectively. During isolation, Petri plates were incubated for 5 - 7 days at 28˚C for fungal population, while at 35˚C for bacterial isolation. Most Probable Number (MPN) procedure [14,15] and colony forming unit (CFU) were used for bacterial and fungal estimation, respectively.
2.5. Statistical Analyses
A goodness-of-fit test was used to test the distribution of data. The data on soil respiration and microbial distribution in the soils at different sites were analysed by analysis of variance (ANOVA) and the means were separated using a Tukey’s studentised range (HSD) test. Chi-square test was used to determine the diel variation in soil respiration rate. Relationship between soil respiration and microbial abundance were analysed by Linear Regression. All analyses were done at α = 0.05 level of significance.
3. Results
3.1. Soil Respiration at Different Sites
Soil respiration (SR) rate was higher at the Riverine Zone (RZ) compared to the Hilly Zone (HZ) (P < 0.05). Respiration rate was affected by the type of vegetation cover. The site with profuse ground vegetation RZ-II had increased respiration rate compared to the plantation site RZ-I which had less ground vegetation (P < 0.05) (Figure 2).
Respiration rate varied significantly with depths at both sites. The variation in soil respiration along depths at HZ-I was greater compared to RZ-I and RZ-II (Figure 2). Soil respiration was highest in deep-soil (256 mg C/m2/yr) compared to top-soil and mid-soil at HZ-I (ANOVA: F = 53.64; P < 0.0001). The trend in variations in soil respiration rate at RZ-I was similar to HZ-I
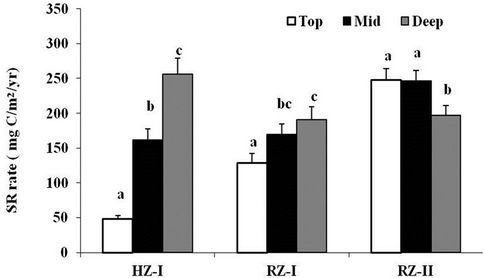
Figure 2. Variations in mean (±SE) soil respiration rate (SR rate) measured in-situ by modified alkali absorption method along three depths top (0 cm - 2 cm), mid (20 cm) and deep (40 cm) soils at three locations, HZ-I, RZ-I and RZ-II. Bars with the same letter are not significantly different (∝ = 0.05).
i.e. the highest respiration (191 mg C/m2/yr) was observed in the deep-soil compared to the lowest (128 mg C/m2/yr) in top-soil of RZ-I (ANOVA: F = 6.61; P < 0.05). However, the lowest soil respiration (197 mg C/m2/yr) was observed in deep-soil compared to top-soil and mid-soil at RZ-II (ANOVA: F = 38.93; P < 0.0001). Soil respiration rate was same in top-soil and mid-soil of RZ-II (P > 0.05).
3.2. Diel Variation in Soil Respiration Rate
Diel variation in soil respiration was observed at both Hilly and Riverine zones. At Hilly zone, a greater efflux of CO2 was observed during the night time than the day from the top-soil (P < 0.05). However, the amount of CO2 evolved from deep-soil from the same site was greater during day time than night (P < 0.05) (Table 1).
In Riverine zone, the amount of CO2 released during the day was not significantly different from the amount of CO2 released in the night from soils of RZ-II (P > 0.05). However, the amount of CO2 released was higher in night than day time when compared between top-soil and deep-soil of the RZ-I (P < 0.05) (Table 1).
3.3. Fungal and Bacterial Abundance
A variation in the combined population of bacteria and fungi was detected in the soils of Hilly and Riverine zones (P < 0.05). The deep-soil of Hilly zone was therichest in harbouring the microorganisms (362.5 × 105 per g of soil), compared to the soils from the other two depths of the same zone (ANOVA: F = 43.3; P < 0.05). However, microorganisms were most abundant (365 × 105 per g of soil), in mid-soil of Riverine zone (ANOVA: F = 144.9; P < 0.01). The deep-soil of Riverine zone had the least number of microorganisms present (Table 2).
When bacterial and fungal population were analysed separately, bacterial count outnumbered the fungal count in the soils from all the depths of both locations. The difference in the bacterial and fungal population was highest in the top-soil of Hilly zone and Riverine zone compared to the other two depths at those sites. However, the difference in fungal and bacterial population was least in the mid-soil of Hilly zone and deep-soil of Riverine zone.
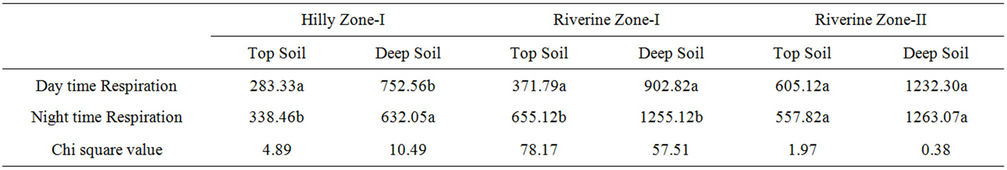
Table 1. Total amount of Carbon dioxide evolved (µg·C/cm2) during 12 h day and 12 h night at different sites. Same letter within a column indicates no significant difference (∝ = 0.05).
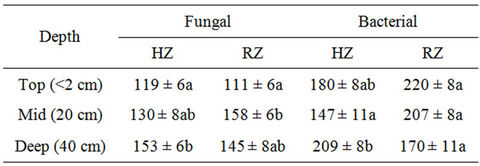
Table 2. Mean (±SE) fungal and bacterial count (×105) in soil along different depths at Hilly Zone (HZ) and Riverine Zone (RZ). Same letter within a column indicates no significant difference (∝ = 0.05).
There was a significant difference in the fungal population between the top-soil and deep-soil at Hilly zone, and between top-soil and mid-soil at Riverine zone (ANOVA: F = 9.66; P < 0.05) (Table 2). The bacterial population was highest in the deep-soil of Hilly zone, however, there was no significant difference in bacterial population in the soils among the three depths at Riverine zone (ANOVA: F = 8.81; P < 0.05) (Table 2).
3.4. Soil Respiration and Microbial Abundance
Soil respiration was positively correlated to the fungal and bacterial population at both sites (Analysis of Regression: F = 11.17, P < 0.01) (Figures 3 and 4). However, fungal abundance showed a stronger correlation with soil respiration rate (R2 = 0.76; P < 0.001) (Figure 3) compared to bacterial abundance (R2 = 0.12, P < 0.04) (Figure 4).
4. Discussion
Riverine zone, containing high soil moisture, had greater biological activity in the soil (higher soil respiration rate) compared to Hilly zone which had low soil moisture content. The availability of water affects the ecosystem metabolism and is an important factor influencing soil respiration processes [16]. High temperature in summer typically dried off the top-soil of Hilly zone which had sparse vegetation, and adversely affected the biological activity in the soil. The profuse vegetation also helped in improving the organic matter content in the soils of Riverine Zone which became favorable to biological activity and eventually increased the CO2 emission. Organic matter content in soil is affected by diversity and density of the vegetation cover [17].
The soil respiration rate in the soils of grassland area RZ-II was found greater than plantation area RZ-I. Soil in grassland generally harbours more water than forest and thus, increased the soil activities. In earlier studies, higher soil respiration was also observed in grassland than forest in humid subtropical climate in Mexico [18] and in Amazonian mature forest [19]. The variation in soil respiration rate along the soil depth was not uniform at Hilly and Riverine zones. Deep roots of Prosopis juliflora
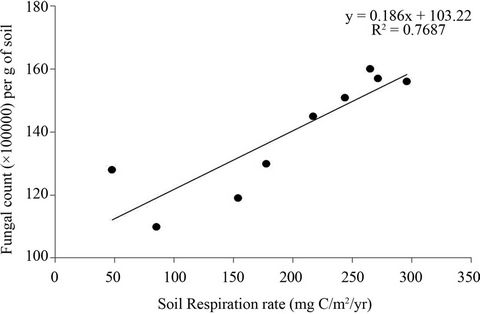
Figure 3. Relationship between fungal abundance (per g of soil) and soil respiration rate was determined by regression analysis.
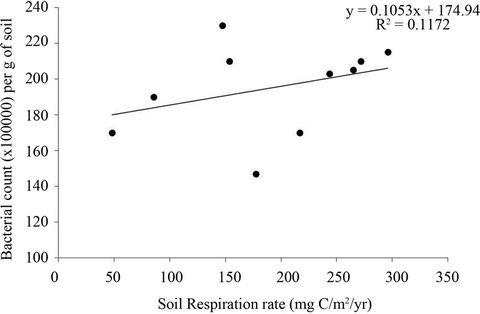
Figure 4. Relationship between bacterial abundance (per g of soil) and soil respiration rate was determined by regression analysis.
and other tree species were probably abundant in deepsoil of HZ-I and RZ-I, that might have helped in conserving the moisture in the soil. Thus greater soil respiration was recorded in deep soils. Similarly, the shallow roots of Desmostachya bipinnate at RZ-I probably favoured holding moisture in top-soil and mid-soil of RZ-II that led to increased respiration rate.
Changes in the day and night affect the temperature and moisture content of soil. The effect was much more in the top-soil as it was directly exposed to sun and heat. The effects gradually decreased with depth. However, the effects of day and night changes on the moisture content of soil might be less where the ground was covered with vegetation as no direct sunlight reaches the soil surface. Higher night time respiration from the top-soil in the forest area of Hilly zone and the plantation area of Riverine zone probably occurred because of decrease in soil temperature during the night [20] which favoured the growth and activities of the organisms in the soil. In general, high temperature instigates the respiration rate [21] but high temperature combined with low soil moisture reduces the soil respiration rate [22]. Respiration rate during the day and night did not change in the top-soil of grassland area RZ-II, because the profuse ground vegetation protected the soil from the temperature fluctuation in day and night.
Distribution of microorganism in soil varies due to several factors including soil temperature, moisture, pH, organic matter content and their interactions. The abundant vegetation at Riverine zone [20] improved the moisture and organic matter of the soil that influenced the microbial, combined bacterial and fungal, population [23,24]. Similarly, the microbial population also varied along the soil depth and most organisms were concentrated near the root systems in the soil. In Hilly zone, the deep root system of P. juliflora encouraged the microorganism abundance by conserving moisture and organic matter in the deep-soil when compared to top-soil and mid-soil. Similarly, the shallow root system of D. bipinnata allowed the abundant growth of microorganisms in mid-soil and top-soil. Roots of the grasses didn’t reach in deep-soil which could be the reason for the low population of these organisms at that depth. Soil moisture in mid-soil of RZ-II and deep-soil of HZ-I, facilitated microbial growth and soil respiration, respectively [25]. Sometime low soil moisture could reduce the CO2 efflux by limiting microbial contact with available substrate in the soil [26].
When fungal and bacterial population was compared at different depths, bacterial population was higher in the top-soil at both the sites. High temperature of the top-soil probably discouraged the fungal compared to bacterial growth [27]. Similarly, the high moisture content in the deep-soil of RZ-I due to very high ground water level discouraged the bacterial growth compared to fungal growth. Moreover, lack of root system in the deep-soil of RZ-II caused overall low microorganisms growth. Fungi and bacteria were most abundant in the deep-soil compared to soils at other depths at HZ. The high abundance probably occurred because of deep roots of P. juliflora. Similarly, shallow root system of D. bipinnata allowed the abundance growth of fungus in mid-soil of RZ. However, the bacterial population was highest in top-soil and least abundant in the deep-soil of RZ.
Soil respiration is a function of microbial abundance because microorganisms are the main group that produces CO2 from soil. However, the role of bacteria and fungus was not same in CO2 production form soil. Fungal population was highly correlated to the soil respiration rate. Fungi are more important in the forest soil and are more active at low temperatures than bacteria [28]. The high fungal population was observed in grassland area is supported by findings of Bardgett et al., [29] which might be the reason for high biological activity and respiration rate at that site.
The study suggests that vegetation coverage determines the physical and chemical properties of soil, which influence the microbial activity that controls the soil respiration rate. The microbial activity below the ground is not uniform and soil respiration process is a function of bacterial and fungal abundance in the soil [30]. However, fungal population is more responsible to CO2 emission than bacterial population. Deeper soil is more active than the surface soil in the dry area dominated by trees which have deep root system. However, in the grassland, the mid-soil is most active due to the shallow root system.
5. Acknowledgements
The research was financially supported by Centre for Environmental Management of Degraded Ecosystems (CEM-DE), University of Delhi. We thank staffs of Yamuna and Aravali Biodiversity Parks, New Delhi, India for their help during the study.
REFERENCES
- R. Lal, “Soil and the Green House Effects,” In: R. Lal, Ed., Soil Carbon Sequestration and the Green House Effect, SSSA Special Publication, Madison, 2001.
- W. H. Schlesinger and J. A. Andrews, “Soil Respiration and the Global Carbon Cycle,” Biogeochemistry, Vol. 48, No. 7, 2000, pp. 7-20. doi:10.1023/A:1006247623877
- C. K. Wang, J. Y. Yang and Q. Z. Zhang, “Soil Respiration in Six Temperate Forests in China,” Global Change Biology, Vol. 12, No. 11, 2006, pp. 2103-2114. doi:10.1111/j.1365-2486.2006.01234.x
- M. Rastogi, S. Singh and H. Pathak, “Emission of Carbon Dioxide from Soil,” Current Science, Vol. 82, No. 5, 2002, pp. 510-517.
- N. La Scala, J. Marques, G. T. Pereira and J. E. Cora, “Carbon Dioxide Emission Related to Chemical Properties of a Tropical Bare Soil,” Soil Biology & Biochemistry, Vol. 32, No. 10, 2000, pp. 1469-1473. doi:10.1016/S0038-0717(00)00053-5
- R. Kant and C. Ghosh, “Soil Respiration Study in Northern Ridge of Delhi Eco-Zone,” In: J. Singh, Ed., Environment and Development: Challenges and Opportunities, IK International, New Delhi, 2005.
- B. Wang, H. U. Neue and H. P. Samonte, “The Effect of Controlled Soil Temperature on Diel CH4 Emission Variation,” Chemosphere, Vol. 35, No. 9, 1997, pp. 2083- 2092. doi:10.1016/S0045-6535(97)00257-9
- J. Grace and M. Rayment, “Respiration in the Balance,” Nature, Vol. 404, 2000, pp. 819-820. doi:10.1038/35009170
- D. C. Coleman, D. A. Crossley Jr. and P. F. Hendrix, “Fundamentals of Soil Ecology,” 2nd Edition, Academic Press, New York, 2004.
- C. M. Fang and J. B. Moncrieff, “The Variation of Soil Microbial Respiration with Depth in Relation to Soil Carbon Composition,” Plant and Soil, Vol. 268, No. 1, 2005, pp. 243-253. doi:10.1007/s11104-004-0278-4
- J. J. Landsberg and S. T. Gower, “Application of Physiological Ecology to Forest Management,” Academic Press, San Diego, 1997.
- R. Kant, “Soil Respiration Study for Monitoring and Quantifying Soil Health in Different Habitats of Delhi Eco-Zone,” M.Phil. Thesis, School of Environmental Studies, University of Delhi, New Delhi, 2006.
- D. C. Coleman, “Soil Carbon Balance in a Sucessional Grassland,” Oikos, Vol. 24, 1973, pp. 195-199. doi:10.2307/3543875
- W. M. Porter, “Most Probable Number Method for Enumerating Infective Propagules of Vesicular Arbuscular Mycorrhizal Fungi in Soil,” Australian Journal of Soil Research, Vol. 17, 1979, pp. 515-519. doi:10.1071/SR9790515
- P. L. Woomer, “Most Probable Number Counts,” In: R. W. Weaver, Ed., Methods of Soil Analysis, Part 2. Microbiological and Biochemical Properties, SSSA, Madison, 1994.
- S. Vishnevetsky and Y. Steinberger, “Bacterial and Fungal Dynamics and Their Contribution to Microbial Biomass in Desert Soil,” Journal of Arid Environments, Vol. 37, No. 1, 1997, pp. 83-90. doi:10.1006/jare.1996.0250
- J. A. Pascual, C. Garcia, T. Hernandez, J. L. Moreno and M. Ros, “Soil Microbial Activity as a Biomarker of Degradation and Remediation Processes,” Soil Biology & Biochemistry, Vol. 32, No. 13, 2000, pp. 1877-1883. doi:10.1016/S0038-0717(00)00161-9
- A. Campos, “Response of Soil Surface CO2-C Flux to Land Use Changes in a Tropical Cloud Forest (Mexico),” Forest Ecology and Management, Vol. 234, No. 1-3, 2006, pp. 305-312. doi:10.1016/j.foreco.2006.07.012
- C. I. Salimon, E. A. Davidson, R. L. Victoria and A. W. F. Melo, “CO2 Flux from Soil in Pastures and Forests in Southwestern Amazonia,” Global Change Biology, Vol. 10, No. 5, 2004, pp. 833-843. doi:10.1111/j.1529-8817.2003.00776.x
- R. Kant, C. Ghosh, L. Singh and N. Tripathi, “Effect of Bacterial and Fungal Abundance in Soil on the Emission of Carbon Dioxide from Soil in Semi-Arid Climate in India,” Survival and Sustainability Part 1, 2011, pp. 151- 161. doi:10.1007/978-3-540-95991-5_16
- D. S. Schimel, “Terrestial Ecosystems and the Carbon-Cycle,” Global Change Biology, Vol. 1, 1995, pp. 77-91. doi:10.1111/j.1365-2486.1995.tb00008.x
- P. Ciais, M. Reichstein, N. Viovy, A. Granier, J. Ogee, V. Allard, M. Aubinet, N. Buchmann, C. Bernhofer, A. Carrara, F. Chevallier, N. De Noblet, A. D. Friend, P. Friedlingstein, T. Grunwald, B. Heinesch, P. Keronen, A. Knohl, G. Krinner, D. Loustau, G. Manca, G. Matteucci, F. Miglietta, J. M. Ourcival, D. Papale, K. Pilegaard, S. Rambal, G. Seufert, J. F. Soussana, M. J. Sanz, E. D. Schulze, T. Vesala and R. Valentini, “Europe-Wide Reduction in Primary Productivity Caused by the Heat and Drought in 2003,” Nature, Vol. 437, No. 7058, 2005, pp. 529-533. doi:10.1038/nature03972
- E. D. Sotta, P. Meir, Y. Malhi, A. D. Nobre, M. Hodnett and J. Grace, “Soil CO2 Efflux in a Tropical Forest in the Central Amazon,” Global Change Biology, Vol. 10, No. 5, 2004, pp. 601-617. doi:10.1111/j.1529-8817.2003.00761.x
- E. Brodie, S. Edwards and N. Clipson, “Bacterial Community Dynamics across a Floristic Gradient in a Temperate Upland Grassland Ecosystem,” Microbial Ecology, Vol. 44, No. 3, 2002, pp. 260-270. doi:10.1007/s00248-002-2012-1
- J. W. Raich and W. H. Schlesinger, “The Global Carbon-Dioxide Flux in Soil Respiration and Its Relationship to Vegetation and Climate,” Tellus, Vol. 44, No. 2, 1992, pp. 81-99. doi:10.1034/j.1600-0889.1992.t01-1-00001.x
- V. A. Orchard and F. J. Cook, “Relationship between Soil Respiration and Soil-Moisture,” Soil Biology & Biochemistry, Vol. 15, No. 4, 1983, pp. 447-453. doi:10.1016/0038-0717(83)90010-X
- J. Pietikainen, M. Pettersson and E. Baath, “Comparison of Temperature Effects on Soil Respiration and Bacterial and Fungal Growth Rates,” Microbiology Ecology, Vol. 52, No. 1, 2005, pp. 49-58. doi:10.1016/j.femsec.2004.10.002
- D. A. Lipson, C. W. Schadt and S. K. Schmidt, “Changes in Soil Microbial Community Structure and Function in an Alpine Dry Meadow Following Spring Snow Melt,” Microbial Ecology, Vol. 43, 2002, pp. 307-314. doi:10.1007/s00248-001-1057-x
- R. D. Bardgett, R. D. Lovell, P. J. Hobbs and S. C. Jarvis, “Seasonal Changes in Soil Microbial Communities along a Fertility Gradient of Temperate Grasslands,” Soil Biology & Biochemistry, Vol. 31, No. 7, 1999, pp. 1021-1030. doi:10.1016/S0038-0717(99)00016-4
- M. A. Aon, D. E. Sarena, J. L. Burgos and S. Cortassa, “Interaction between Gas Exchange Rates, Physical and Microbiological Properties in Soils Recently Subjected to Agriculture,” Soil & Tillage Research, Vol. 60, No. 3-4, 2001, pp. 163-171. doi:10.1016/S0167-1987(01)00191-X

