Journal of Biomaterials and Nanobiotechnology
Vol.4 No.3(2013), Article ID:35329,9 pages DOI:10.4236/jbnb.2013.43037
Pharmacokinetic Study of Nanoparticulate Curcumin: Oral Formulation for Enhanced Bioavailability
![]()
Regional Institute of Education, National Council of Educational Research and Training (NCERT), Mysore, India.
Email: ravincert@gmail.com
Copyright © 2013 R. Ravichandran. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 22nd, 2013; revised February 23rd, 2013; accepted March 15th, 2013
Keywords: Curcumin; Nanoparticles; Pharmacokinetics; Bioavailability
ABSTRACT
Curcumin, a bioactive component of turmeric, which is a commonly used spice and nutritional supplement, is isolated from the rhizomes of Curcuma longa Linn. (Zingiberaceae). In recent years, the potential pharmacological actions of Curcumin in inflammatory disorders, cardiovascular disease, cancer, Alzheimer’s disease and neurological disorders have been shown. However, the clinical application of Curcumin is severely limited by its main drawbacks such as instability, low solubility, poor bioavailability and rapid metabolism. Multifarious nanotechnology-based drug delivery systems for Curcumin including liposomes, polymeric nanoparticles, solid lipid nanoparticles, micelles, nanogels, nanoemulsions, complexes and dendrimer/dimer, have been attempted to enhance the oral bioavailability, biological activity or tissue-targeting ability of Curcumin. We attempted the nanosuspensions based delivery of curcumin. Nanonisation renders curcumin completely dispersible in aqueous media. To enhance the curcumin absorption by oral administration, nanoparticulate solid oral formulation of curcumin was prepared by us and the resulting capsule was then examined for its efficiency on bioavailability in Male Wistar rats at a dose of 100 mg curcumin/kg body weight and the pharmacokinetic parameters were compared to those of normal curcumin powder and a commercial curcumin capsule CUR-500. The bio-distribution of curcumin in organs of rat was also studied. Nanoparticulation significantly raised the curcumin concentration in selective organs in the body. The results obtained provide promising results for nanoparticulate Curcumin to improve its biological activities. Enhanced bioavailability of curcumin in the form of nanoparticle is likely to bring this promising natural product to the forefront of therapeutic agents for treatment of human disease. The available information also strongly suggests that nano-formulation of ingredients such as curcumin may be used as a novel nutrient delivery system too.
1. Introduction
Turmeric (Curcuma longa Linn), is a crystalline compound which has been traditionally used in medicine and cuisine in India and other Asian countries. Curcumin, a hydrophobic polyphenol derived from the rhizome of the herb Curcuma longa has a wide spectrum of biological and pharmacological activities. Chemically, curcumin is a bis-α, β-unsaturated β-diketone (commonly called diferuloylmethane, Figure 1), which exhibits keto-enol tautomerism having a predominant keto form in acidic and neutral solutions and stable enol form in alkaline medium. Commercial curcumin contains approximately 77% diferuloylmethane, 17% demethoxycurcumin, and 6% bisdemethoxycurcumin. Traditionally, turmeric has been used for many ailments, particularly as an anti-inflammatory agent, and curcumin has been identified as the active
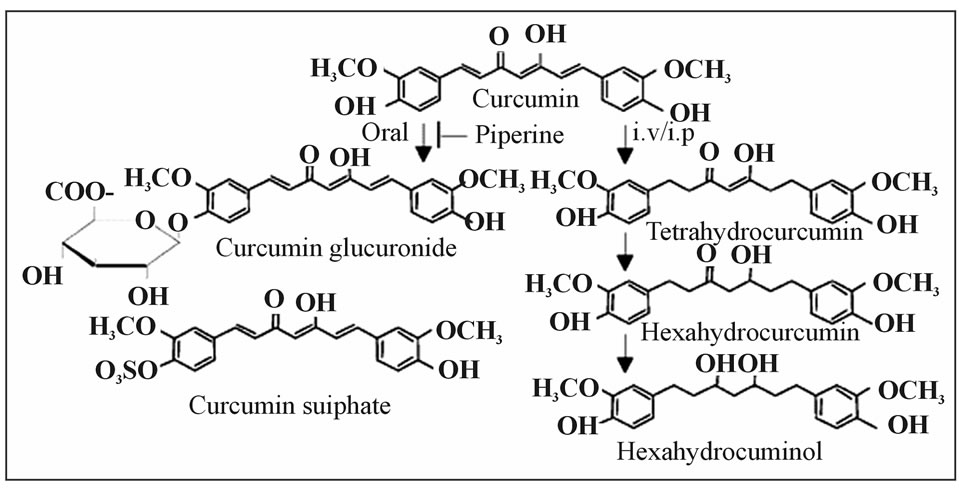
Figure 1. Metabolic reduction.
principle of turmeric [1]. Curcumin has been shown to exhibit antioxidant, anti-inflammatory [2-5] antimicrobial, and anticarcinogenic [6-10] activities. Additionally, the hepatoand nephro-protective [11-13] thrombosis suppressing [14] myocardial infarction protective [15-17] hypoglycemic [18-21] and antirheumatic [22] effects of curcumin are also well established. It is generally recognized that the therapeutic effectiveness of curcumin is limited due to its poor absorption from the gastrointestinal tract and poor bioavailability due to its rapid metabolism in the liver and intestinal wall. Oral doses result in only traces appearing in the blood, with most of the dose being excreted in the feces. The predominant metabolites in plasma following oral administration of curcumin were glucuronides and glucuronide/sulfates. The conjugative enzyme activities for glucuronidation and sulfation of curcumin were found in liver, kidney and intestinal mucosa. These results indicate that orally administered curcuminoids are absorbed from the alimentary tract and present in the general blood circulation after largely being metabolized to the form of glucuronide and glucuronide/sulfate conjugates. Various animal models [23,24] or human studies [25-28] proved that curcumin is extremely safe even at very high doses. For example, three different phase I clinical trials indicated that curcumin, when taken as high as 12 g per day, is well tolerated [26- 28]. Similarly, the efficacy of curcumin in various diseases including cancer has been well established [29]. Several clinical studies dealing with the efficacy of curcumin in humans can also be cited [1,30]. The pharmacological safety and efficacy of curcumin make it a potential compound for treatment and prevention of a wide variety of human diseases. In spite of its promising therapeutic index, the biological activity of curcumin is severely limited due to its poor bioavailability and hence has not yet been approved as a therapeutic agent. Effective methods to deliver such substances to increase their bioavailability have been a major challenge in current biomedical and food research. Earlier we have reported the preparation and characterisation of curcumin nanosuspension for enhanced solubility and dissolution velocity followed by the development of an oral curcumin nanocrystal capsule formulation [31]. The present study was designed to evaluate this capsule formulation for improved pharmacokinetic parameters and hence the bioavailability and food functionality following oral administration in rats.
2. Materials and Methods
2.1. Materials
Curcumin was a gift sample from Indsaff Inc., Bhubaneswar, India. Curcumin nanosuspensions were stabilized by Polyvinyl alcohol (PVA, molecular weight 90,000, Sigma-Aldrich, USA) and sodium dodecyl sulfate (Fluka Switzerland). Milli-Q Plus water, double-distilled water (Millipore, USA) was used as dispersion medium. The other chemicals were of analytical reagent grade (SRLMumbai, India).
2.2. Preparation of Curcumin Nanosuspensions
The curcumin nanosuspension on a lab scale is typically produced by pre-milling (with SDS 0.2%) followed by high pressure homogenization in pure water using a continuous Micron LAB 40 at room temperature, applying 20 homogenization cycles at 1500 bar. The formulation of curcumin nanosuspension was prepared using Curcumin 10%, Polyvinyl alcohol 2% and Water 88%.
2.3. Formulations of the Curcumin Capsule
Curcumin was admixed to the capsule excipients by a tumbler (Turbula, Willy A. Bachofen, Basel, Switzerland). The mixed powder was filled into hard gelatin capsule no. 2 using a simple filling capsule equipment for lab scale. The final product of the capsules were collected and immediately transferred into dry plastic containers and tightly sealed. Formulation: Curcumin nanocrystal 500 mg; Excipient (mg): Lactose 15; Avicel PH 102 190; Magnesium stearate 1.
2.4. Experimental Animals
Male Wistar rats weighing 250 g were used in this study in accordance with institutional guidelines and approval of local ethics authorities. The animals were fed with commercial pellet diet (Kamadenu Agencies, Bangalore, India) and water ad libitum. The animals were acclimatized to laboratory hygienic conditions for 10 days before starting the experiment. The animals were maintained in groups of six and were fasted for 8 h prior to the commencement of the study.
2.5. Animal Treatment
Male Wistar albino rats were kept under a twelve-hour light/dark cycle on standard lab chow. Animals were fasted overnight and received Curcumin nanocrystal-loaded capsules, marketed CUR-500 capsules and common curcumin powder at 100 mg/Kg body weight by oral gavage. At 30, 60, 90 and 120 min, animals were exsanguinated under terminal anaesthesia. Group size was 6 rats per time point. Whole blood was collected by cardiac puncture into heparinized tubes, centrifuged immediately at 7000 × g for 15 min, plasma was then decanted and stored at −80˚C until analysis. The organs (liver, heart, spleen, lung, kidney and brain) were removed and transferred into 50 ml tubes.
2.6. Sample Preparation
Curcumin and curcumin metabolites were extracted from plasma by solid phase extraction. Plasma (1 ml) was loaded onto a 1cc Oasis HLB cartridge, washed with 25:25:1 methanol:water:glacial acetic acid (1 ml), and eluted with 1 ml of methanol containing 2% glacial acetic acid. Eluant was evaporated to dryness at 45˚C under a stream of nitrogen, and the residue was re-suspended in 75 μl of 50% aqueous acetonitrile. Standard solutions of curcumin (5 - 1000 ng/ml) were prepared in 1 ml human plasma (obtained from local blood bank) and extracted as described above. Extraction efficiency was 59% with 2.5 and 4.5% intra and inter day variability, 99% accuracy and response was linear over the range 5 - 1000 ng/ml with an R2 value consistently of 0.999. The organs were weighed and homogenized in isotonic KCl. An aliquot (0.5 ml) of it was mixed with 2 ml of acetone: formic acid (9:1), and the mixture was immediately vortexed. The samples were then centrifuged at 6000 rpm for 10 min at 4˚C and the supernatant was collected and preserved at −20˚C before further sample assay. After centrifuging at 12,000 rpm for 15 min, 20 μl of supernatants were collected and analyzed by the HPLC system.
2.7. HPLC Analysis of Curcumin
A validated sensitive and selective high-performance liquid chromatography (HPLC) method using UV-vis detection was used for the determination and quantification of curcumin and its metabolites. The HPLC system consisted of a Shimadzu LC 6A HPLC instrument equipped with a solvent delivery pump, a Rheodyne injector valve and a variable wavelength UV detector. The column used was reversed phase C 18 analytical column (4.6 × 250 mm, particle size 5 µm), with mobile phase consisting of two components: A, 10 mM ammonium acetate pH 4.5; B, acetonitrile. Initial conditions were 95% A progressing to 55% A at 20 min and 5% A at 33 min. The flow rate was maintained at 1 mL/min at 45 ± 2˚C. The eluate was monitored at 420 nm. Retention time for curcumin, curcumin sulfate and curcumin glucuronide were 8, 7.4 and 7.1 min respectively. Free curcumin is completely insoluble in water therefore the concentration of curcumin was calculated using standard curve of curcumin in ethanol. The data was recorded and calculated using Winchrome software.
2.8. Pharmacokinetic Analysis
Pharmacokinetic calculations were performed on each individual set of data using the WinNonlin Standard Edition Version 2.1 by non-compartmental method. Pharmacokinetic results are represented as mean ± SEM. Statistical analysis was performed by t test (SPSS version 10.0) to compare different groups. The level of significance was set at p < 0.05.
3. Results and Discussion
Earlier we have developed a capsule containing oral solid formulation of nanoparticulate curcumin and investigated its dissolution behavior in different medium. The study was very successful and the data is being published. This Cur-NS-B had LD particle size distribution of 0.1 μm (< d 10%), 0.2 μm (< d 50%), 1.8 μm (< d 90%) and 2.8 μm (< d 99%). PCS size 306 nm, Zeta potential (mV) of −6.4 in water and −2.7 in original medium. Visual examination of crystals in nanosuspensions from images of the nanosuspensions from light microscopy and scanning electron microscopy showed fine stable homogeneous distribution. It showed very good physical and chemical stability over 3 and 6 months period respectively. A spray drying process was employed to obtain dried curcumin nanocrystals having good re-dispersability, saturation solubility and dissolution velocity. LD values were 0.13 μm (< d 10%), 0.4 μm (< d 50%), 3.1 μm (< d 90%) and 3.9 μm (< d 99%). PCS size was 321 nm and PI of 0.38. In general, the saturation solubility of the nanocrystals was distinctly 5 fold higher than for microparticles. Free curcumin is poorly soluble in aqueous media, with macroscopic undissolved flakes of the compound visible in the solution (Figure 2(a)); in contrast, nanoparticulate curcumin is a clear, dispersed formulation, with its hue derived from the natural colour of curcumin (Figure 2(b)). The results also showed the superiority of curcumin nanocrystals in dissolution behavior and was in agreement with the Noyes-Whitney equation. According to these results, curcumin nanocrystals are suitable for incorporation into solid dosage form, such as tablets, capsules, pellets etc. Accordingly a capsule containing these nanoparticulate curcumin was formulated as given in experimental section and tested for its bio-efficacy.
3.1. Pharmacokinetic Parameters of Curcumin and Its Metabolites in Rat Plasma
In the present investigation, Curcumin nanocrystal-loaded capsules, marketed CUR-500 capsules and common curcumin powder were chosen for the pharmacokinetics studies. Figure 3 shows the mean plasma curcumin concentration versus time profiles before and after oral administration of Curcumin nanocrystal-loaded capsules,
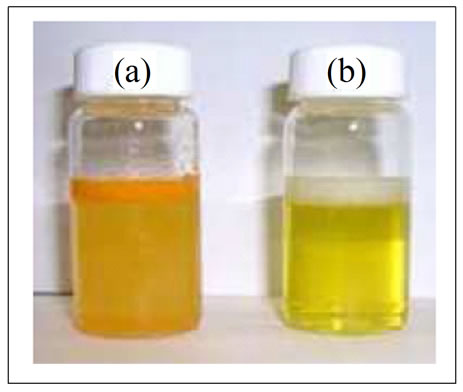
Figure 2. (a) Free curcumin is poorly soluble in aqueous media, and macroscopic flakes can be seen floating in the bottle. (b) In contrast, curcumin nanoparticles are fully dispersible in aqueous media.

Figure 3. Concentration of curcumin in rat plasma after a single oral administration of: Curcumin nanocrystal-loaded capsules, marketed CUR-500 capsules and common curcumin powder (100 mg curcumin/kg body weight). All nano data showed a significant difference at P < 0.01 (vs common curcumin powder group). Values are represented as means ± SEMs (n = 6).
marketed CUR-500 capsules and common curcumin powder, at a dose of 100 mg of curcumin/Kg body weight for each treatment group. The peak concentration (Cmax) and time of peak concentration (Tmax) were obtained directly from the individual plasma curcumin concentration versus time profiles. The area under the concentrationtime curve from 0 to 120 min (AUC0-120) was calculated using the trapezoidal method [32]. The AUC determines the bioavailability of the drug for a given dose of the formulation. These oral pharmacokinetic parameters are listed in Table 1. As shown in Figure 3, plasma curcumin concentrations were significantly higher in rats administrated Curcumin nanocrystal-loaded capsules than in those administrated marketed CUR-500 capsules or common curcumin powder, at all time points. The Cmax value of curcumin in the Curcumin nanocrystal-loaded
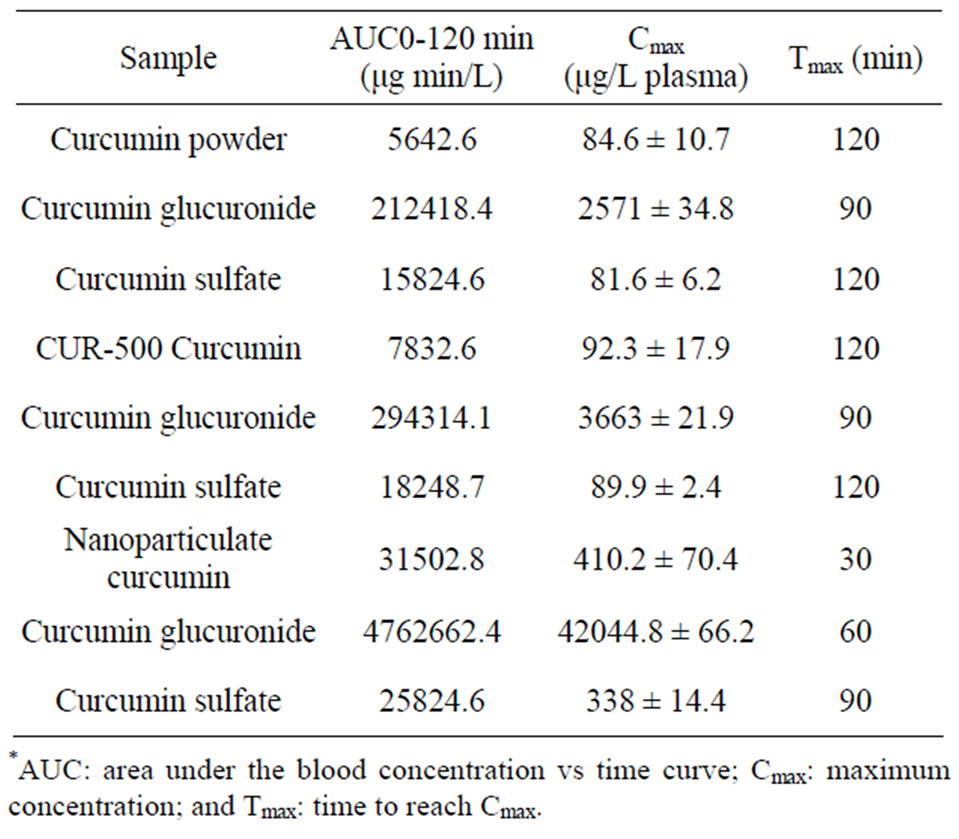
Table 1. Pharmacokinetic parameters derived from rat plasma.*
capsules group (410.2 ± 70.4 μg/L) was much higher than that obtained with marketed CUR-500 capsules (92.3 ± 17.9 μg/L) (Table 1). The AUC0-120 value of curcumin after oral administration of Curcumin nanocrystal-loaded capsules was 31502.8 μg min/L, which was 4 fold greater than that after marketed CUR-500 capsules administration. The values obtained for common curcumin powder was little less than that of CUR-500. Further we compared plasma levels of curcumin metabolites mainly curcumin glucuronide and curcumin sulfate in animals that had received either unformulated or nanoformulated curcumin (Table 1). A similar trend was observed here also. The metabolites were very many folds in much shorter time with nanoformulation as compared to nonformulated curcumin. Several lines of studies have demonstrated that administration of nanoparticles would enhance drug absorption and systemic bioavailability [33]. It could thus be possible that nanoparticulate curcumin also similarly exerts an activation effect on curcumin absorption in the gastrointestinal (GI) tract. Our experiment revealed that smaller the particle size greater the effect on enhanced curcumin absorption by oral administration (Figure 3). Accordingly, it seems that the nanonisation of curcumin leads to a substantial improvement in curcumin absorption. We considered three possible explanations of the above results: 1) Enhanced bioavailability of nanoformulation might be attributed to the direct uptake of nanoparticles through the GI tract, 2) increased permeability by surfactants, and 3) decreased degradation and clearance. First, the uptake of curcumin in a nano form could be accomplished through the GI tract, where particle size plays a dominant role in absorption rate [34]. The mechanisms involved in such uptake include the diffusion of particles through mucus and accessibility to an enterocyte surface, epithelial interaction and cellular trafficking, and exocytosis and systemic dissemination. A drug particle size of approximately 200 nm allows for efficient uptake in the intestine, particularly in the lymphoid sections of this tissue [35], and therefore bypass of the first-pass metabolism in the liver [36]. Second, GI absorption of drugs with low water solubility is enhanced when they are nanosuspension to increase surface area [37]. Thus, the surfactants involved in the formulations could affect the permeability and solubility of drugs across the membrane of the GI tract. Third, by incorporation into nano form, curcumin can be embedded into the phospholipid bilayer. This reduces its exposure to bacteria as well as enzymatic degradation during the absorption process. This also allows for prolonged contact with the intestinal wall due to the adhesive property that nano form exhibit toward the epithelial mucosal surface of the small intestine [38]. Accordingly, it seems that nanonisation of curcumin is highly advantageous for optimizing food functionality. Several studies have found that curcumin also has an antioxidant activity in vitro [39].
3.2. Pharmacokinetic Parameters of Curcumin in Rat Organs
The pharmacokinetic parameters of curcumin in rat organs are given in Table 2. In the curcumin powder and commercial product, the AUCs of curcumin in kidney (168.4; 194.6) and liver (76.2; 79.8) were larger than in other organs, indicating that more curcumin was in these two organs. The least was found in heart and brain (32 to 36). One possible reason for these results is that the systemic circulation of curcumin in the body is limited since a substantial amount of curcumin is distributed to the liver and kidney, where it is metabolized and eliminated [40]. However, when nanoparticulate curcumin was administrated, a significant amount of curcumin was found in spleen and lung, and the AUC of curcumin in these organs were 1624.2 and 458.8, respectively. The levels of nanoparticulate curcumin amassed in spleen tissue is closely related to phagocytic cell uptake in the reticuloendothelial system [41]. The lung accumulation contributes to the filtration of pulmonary capillary beds following nanoparticulate curcumin administration [42]. In the case of brain and heart the increase was only marginal compared to other organs but still being significant. Based on the finding that the main organs of distribution in the nanoparticulate curcumin treated group, are the spleen and lungs instead of the liver and kidney in conventional curcumin treated group, the advantage of nanoparticle in our study is credited with the fact that formulation has prohibited curcumin distributing to major organs metabolized drugs. The same observation could be made from the data obtained for Cmax and Tmax. However the Tmax data gives some more interesting features. While there is a general decrease in this value across the organs studied,
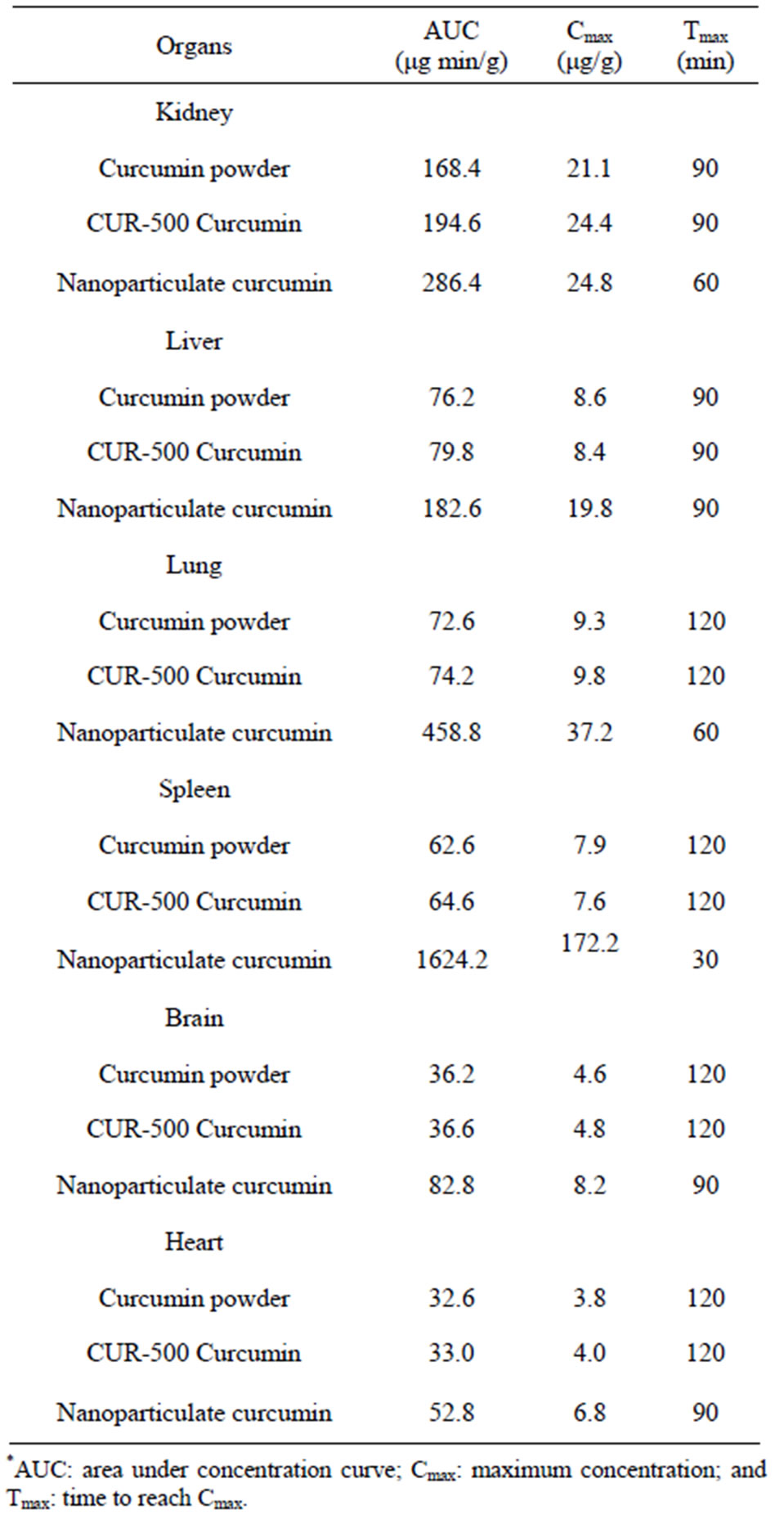
Table 2. Pharmacokinetic parameters derived from rat organs for curcumin content.
the decrease is remarkable in spleen and lungs. Thus nanoparticle proves to be much different in its reach, distribution and action.
According to these distribution results, curcumin can arrive in organs, where it can perform its pharmacodynamic activities, as demonstrated by previous studies. After nano-formulation, the concentration of curcumin in these organs was significantly increased. These pharmacokinetic data suggest that nanoparticulate curcumin might offer greater therapeutic effect than conventional curcumin from a pharmacodynamic perspective. Therefore, the distribution results of curcumin and nanoparticulate curcumin to the site of action are vital for dose determination, time to administration and toxicity in pre-clinical and clinical therapeutic research.
3.3. Problems of Curcumin Bioavailability
The reasons for reduced bioavailability of any agent within the body are low intrinsic activity, poor absorption, high rate of metabolism, inactivity of metabolic products and/or rapid elimination and clearance from the body. Studies to date have suggested a strong intrinsic activity and, hence, efficacy of curcumin as a therapeutic agent for various ailments. However, studies over the past three decades related to absorption, distribution, metabolism and excretion of curcumin have revealed poor absorption and rapid metabolism of curcumin that severely curtails its bioavailability [29]. The main problems of curcumin bioavailability are low serum levels, limited tissue distribution, apparent rapid metabolism and short half-life.
3.4. Promises
The absorption, bio distribution, metabolism, and elimination studies of curcumin have, unfortunately, shown only poor absorption, rapid metabolism, and elimination of curcumin as major reasons for poor bioavailability of this interesting polyphenolic compound. Some of the possible ways to overcome these problems are: Adjuvants, which can block metabolic pathways of curcumin, are one of the major means that are being used to improve its bioavailability; Liposomes, Micelles, and Phospholipid complexes are other promising novel formulations, which appear to provide longer circulation, better permeability, and resistance to metabolic processes. Recently a novel formulation to deliver curcumin embedding phospholipid vesicles or lipid-nanospheres (Cm) into tissue macrophages through intravenous injection has been developed [43]. More recently, curcumin or a curcumin analogue encapsulated in a colloidal drug a liposome is considered as excellent drug delivery systems since they can carry both hydrophilic and hydrophobic molecules [44]. Experimental evidences shows 47% to 56% enhanced intestinal absorption of curcumin when embedded with micelles then free curcumin in vitro [45] in rats. The molecular and chemical structure of curcumin plays a crucial role in its biological activity. Reports suggest change in antioxidant activity of curcumin due to isomerization. With a view to achieve improved biological activity of curcumin through structural modifications or curcumin derivatives and/or its analogues research was made by various research groups [46]. For example, a curcumin analogue EF-24 had shown increased antitumor activity in comparison to curcumin in vitro and in vivo, and increased bioavailability of EF-24 was also demonstrated by 60% and 35%, respectively in male and female mice [47]. Another strategy to improve the biological activity of curcumin is by chelation with various metals as compared to free curcumin. The presence of two phenolic groups and one active methelene group in a curcumin molecule makes it an excellent ligand for any chelation [48].
3.5. Nanoparticles
Recently, targeted and triggered drug delivery systems accompanied by nanoparticle technology have emerged as prominent solutions to the bioavailability of therapeutic agents. Nanoparticle-based delivery systems will probably be suitable for highly hydrophobic agents like curcumin circumventing the pitfalls of poor aqueous solubility. However, very few studies have been published citing curcumin nanoparticles. A recent study by Bisht et al. reported the synthesis, physicochemical characterization and cancer related application of a polymer-based nanoparticle of curcumin namely “nanoparticulate curcumin” with less than 100 nm size. Nanoparticulate curcumin, is made up of the micellar aggregates of cross-linked and random copolymers of Nisopropylacrylamide (NIPAAM), with N-vinyl-2-pyrrolidone (VP) and poly (ethyleneglycol) monoacrylate (PEG-A). Nanoparticulate curcumin, unlike free curcumin, is readily dispersed in aqueous media. Nanoparticulate curcumin was found to have similar in vitro activity as that of free curcumin in pancreatic cell lines. Like free curcumin, nanoparticulate curcumin also inhibits activation of the transcription factor NFκB, and reduces steady state levels of pro-inflammatory cytokines like interleukins and TNF-R. However, the authors neither determined the in vivo effect of nanoparticulate curcumin in mice nor its biodistribution to show any potential increase in efficacy of nanaocurcumin over free curcumin in vivo [49]. Solid lipid nanoparticles (SLNs) loaded with curcuminoids for topical application were developed and characterized by Tiyaboonchai et al. Curcuminoid loaded SLNs having 450 nm size were found to be stable for 6 months at room temperature and gave prolonged in vitro release of curcuminoids up to 12 h. Furthermore, the light and oxygen sensitivity of curcuminoids was strongly reduced by incorporating curcuminoids into this unique type of formulation. An in vivo study with healthy volunteers reveled the improved efficiency of a topical application cream containing curcuminoid loaded SLNs over that containing free curcumanoids [50]. Overall, nanoparticle based systems for curcumin delivery is still in its infancy and much progress is warranted in this area.
3.6. Nanoparticle Mediated Delivery Systems
Nanoparticles-based materials have attracted much attention in recent years because of their characteristic size and geometry dependent chemical and physical properties [27]. Nanoparticles are of great scientific interest as they are effectively a bridge between bulk materials and atomic or molecular structures. Literature survey suggests nanoparticle research is an area of intense scientific research, due to wide potential applications in human therapy. Nano particles are sized between 1 and 100 nm. Nanoparticles have a very high surface area to volume ratio. This makes the particles very reactive or catalytic [51]. Nanoparticles are easier to pass through cell membranes in organisms and get interacted rapidly with biological systems [51]. Recently, nanoparticle technology emerged as a potential area of targeted drug delivery systems and make biologically availability of therapeutic agent. Nanoparticle-mediated delivery systems will probably be the most suitable for highly hydrophobic agents like curcumin, circumventing its poor aqueous solubility [52,53]. However, very limited studies were made and the complete mechanism regarding nanoparticle mediated curcumin delivery system is still unknown [54].
4. Conclusion
The present work clearly demonstrated the superiority of nanoparticulate curcumin obtained through nanosuspension over normal/commercial curcumin in being more bioefficient, suggesting novel delivery strategies for curcumin in therapeutic applications. This pharmacokinetic study offers significant promises and is worthy of further exploration in attempts to enhance the bioavailability, medicinal value, and application of this interesting molecule from Mother Nature.
REFERENCES
- B. B. Aggarwal, A. Kumar and A. C. Bharti, “Anticancer Potential of Curcumin: Preclinical and Clinical Studies,” Anticancer Research, Vol. 23, No. 1A, 2003, pp. 363-398.
- O. P. Sharma, “Antioxidant Activity of Curcumin and Related Compounds,” Biochemical Pharmacology, Vol. 25, No. 15, 1976, pp. 1811-1812. doi:10.1016/0006-2952(76)90421-4
- A. J. Ruby, G. Kuttan, K. D. Babu, K. N. Rajasekharan and R. Kuttan, “Anti-Tumour and Antioxidant Activity of Natural Curcuminoids,” Cancer Letters, Vol. 94, No. 1, 1995, pp. 79-83. doi:10.1016/0304-3835(95)03827-J
- Y. Sugiyama, S. Kawakishi and T. Osawa, “Involvement of the Diketone Moiety in the Antioxidative Mechanism of Tetrahydrocurcumin,” Biochemical Pharmacology, Vol. 52, No. 4, 1996, pp. 519-525. doi:10.1016/0006-2952(96)00302-4
- R. C. Srimal and B. N. Dhawan, “Pharmacology of Diferuloyl Methane (Curcumin), a Non-Steroidal Anti-Inflammatory Agent,” Journal of Pharmacy and Pharmacology, Vol. 25, No. 6, 1973, pp. 447-452. doi:10.1111/j.2042-7158.1973.tb09131.x
- W. C. Jordan and C. R. Drew, “Curcumin––A Natural Herb with Anti-HIV Activity,” Journal of the National Medical Association, Vol. 88, No. 6, 1996, p. 333.
- G. B. Mahady, S. L. Pendland, G. Yun and Z. Z. Lu, “Turmeric (Curcuma longa) and Curcumin Inhibit the Growth of Helicobacterpylori, a Group 1 Carcinogen,” Anticancer Research, Vol. 22, No. 6C, 2002, pp. 4179-4181.
- M. K. Kim, G. J. Choi and H. S. Lee, “Fungicidal Property of Curcuma longa L. Rhizome-Derived Curcumin against Phytopathogenicfungi in a Greenhouse,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 6, 2003, pp. 1578-1581. doi:10.1021/jf0210369
- R. C. Reddy, P. G. Vatsala, V. G. Keshamouni, G. Padmanaban and P. N. Rangarajan, “Curcumin for Malaria Therapy,” Biochemical and Biophysical Research Communications, Vol. 326, No. 2, 2005, pp. 472-474. doi:10.1016/j.bbrc.2004.11.051
- R. Kuttan, P. Bhanumathy, K. Nirmala and M. C. George, “Potentialanticancer Activity of Turmeric (Curcuma longa),” Cancer Letters, Vol. 29, No. 2, 1985, pp. 197-202. doi:10.1016/0304-3835(85)90159-4
- Y. Kiso, Y. Suzuki, N. Watanabe, Y. Oshima and H. Hikino, “Antihepatotoxic Principles of Curcuma longa Rhizomes,” Planta Medica, Vol. 49, No. 3, 1983, pp. 185-187. doi:10.1055/s-2007-969845
- N. Venkatesan, “Curcumin Attenuation of Acute Adriamycin Myocardialtoxicity in Rats,” British Journal of Pharmacology, Vol. 124, No. 3, 1998, pp. 425-427. doi:10.1038/sj.bjp.0701877
- N. Venkatesan, D. Punithavathi and V. Arumugam, “Curcuminprevents Adriamycin Nephrotoxicity in Rats,” British Journal of Pharmacology, Vol. 129, No. 2, 2000, pp. 231- 234. doi:10.1038/sj.bjp.0703067
- R. Srivastava, M. Dikshit, R. C. Srimal and B. N. Dhawan, “Antithromboticeffect of Curcumin,” Thrombosis Research, Vol. 40, No. 3, 1985, pp. 413-417. doi:10.1016/0049-3848(85)90276-2
- M. Dikshit, L. Rastogi, R. Shukla and R. C. Srimal, “Prevention of Ischaemia-Induced Biochemical Changes by Curcumin & Quinidinein the Cat Heart,” Indian Journal of Medical Research, Vol. 101, 1995, pp. 31-35.
- C. Nirmala and R. Puvanakrishnan, “Protective Role of Curcuminagainst Isoproterenol Induced Myocardial Infarction in Rats,” Molecular and Cellular Biochemistry, Vol. 159, No. 2, 1996, pp. 85-93. doi:10.1007/BF00420910
- C. Nirmala and R. Puvanakrishnan, “Effect of Curcumin on Certainlysosomal Hydrolases in Isoproterenol-Induced Myocardial Infarctionin Rats,” Biochemical Pharmacology, Vol. 51, No. 1, 1996, pp. 47-51. doi:10.1016/0006-2952(95)02118-3
- M. Srinivasan, “Effect of Curcumin on Blood Sugar as Seen in Adiabetic Subject,” Indian Journal of Medical Science, Vol. 26, No. 4, 1972, pp. 269-270.
- P. S. Babu and K. Srinivasan, “Influence of Dietary Curcumin Andcholesterol on the Progression of Experimentally Induced Diabetesin Albino Rat,” Molecular and Cellular Biochemistry, Vol. 152, No. 1, 1995, pp. 13-21.
- P. S. Babu and K. Srinivasan, “Hypolipidemic Action of Curcumin, the Active Principle of Turmeric (Curcuma longa) in Streptozotocin Induced Diabetic Rats,” Molecular and Cellular Biochemistry, Vol. 166, No. 1-2, 1997, pp. 169-175. doi:10.1023/A:1006819605211
- N. Arun and N. Nalini, “Efficacy of Turmeric on Blood Sugar and Polyolpathway in Diabetic Albino Rats,” Plant Foods for Human Nutrition, Vol. 57, No. 1, 2002, pp. 41- 52. doi:10.1023/A:1013106527829
- S. D. Deodhar, R. Sethi and R. C. Srimal, “Preliminary Study Onantirheumatic Activity of Curcumin (Diferuloyl Methane),” Indian Journal of Medical Research, Vol. 71, 1980, pp. 632-634.
- T. N. Shankar, N. V. Shantha, H. P. Ramesh, I. A. Murthy and V. S. Murthy, “Toxicity Studies on Turmeric (Curcuma longa): Acutetoxicity Studies in Rats, Guineapigs & Monkeys,” Indian Journal of Experimental Biology, Vol. 18, No. 1, 1980, pp. 73-75.
- S. Qureshi, A. H. Shah and A. M. Ageel, “Toxicity Studies on Alpiniagalanga and Curcuma longa,” Planta Medica, Vol. 58, No. 2, 1992, pp. 124-127. doi:10.1055/s-2006-961412
- C. D. Lao, M. F. Demierre and V. K. Sondak, “Targeting Events Inmelanoma Carcinogenesis for the Prevention of Melanoma,” Expert Review of Anticancer Therapy, Vol. 6, No. 11, 2006, pp. 1559-1568. doi:10.1586/14737140.6.11.1559
- C. D. Lao, M. T. Ruffin, D. Normolle, D. D. Heath, S. I. Murray, J. M. Bailey, M. E. Boggs, J. Crowell, C. L. Rock and D. E. Brenner, “Dose Escalation of a Curcuminoid Formulation,” BMC Complementary and Alternative Medicine, Vol. 6, No. 1, 2006, p. 10. doi:10.1186/1472-6882-6-10
- A. L. Cheng, C. H. Hsu, J. K. Lin, M. M. Hsu, Y. F. Ho, T. S. Shen, J. Y. Ko, J. T. Lin, B. R. Lin, W. Ming-Shiang, H. S. Yu, S. H. Jee, G. S. Chen, T. M. Chen, C. A. Chen, M. K. Lai, Y. S. Pu, M. H. Pan, Y. J. Wang, C. C. Tsai and C. Y. Hsieh, “Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-Malignant Lesions,” Anticancer Research, Vol. 21, No. 4B, 2001, pp. 2895-900.
- G. Shoba, D. Joy, T. Joseph, M. Majeed, R. Rajendran and P. S. Srinivas, “Influence of Piperine on the Pharmacokinetics of Curcuminin Animals and Human Volunteers,” Planta Medica, Vol. 64, No. 4, 1998, pp. 353-356. doi:10.1055/s-2006-957450
- B. B. Aggarwal, C. Sundaram, N. Malani and H. Ichikawa, “Curcumin: The Indian Solid Gold,” Advances in Experimental Medicine and Biology, Vol. 595, 2007, pp. 1- 75. doi:10.1007/978-0-387-46401-5_1
- C. H. Hsu and A. L. Cheng, “Clinical Studies with Curcumin,” Advances in Experimental Medicine and Biology, Vol. 595, 2007, pp. 471-480. doi:10.1007/978-0-387-46401-5_21
- R. Ravichandran, “Preparation and Characterisation of Curcumin Nanosuspension for Enhanced Solubility and Dissolution Velocity,” International Journal of Nano and Biomaterials, Vol. 3, No. 2, 2010, pp. 153-186. doi:10.1504/IJNBM.2010.037803
- A. B. Mohsen, A. A. Abdulaziz, A. A. Mohamed and M. A. Mohamed, “In Vivo Evaluation of Arteether Liposomes,” International Journal of Pharmaceutics, Vol. 175, No. 1, 1998, pp. 1-7. doi:10.1016/S0378-5173(98)00182-3
- K. Maiti, K. Mukherjee, A. Gantait, B. P. Saha and P. K. Mukherjee, “Curcumin-Phospholipid Complex: Preparation, Therapeutic Evaluation and Pharmacokinetic Study in Rats,” International Journal of Pharmaceutics, Vol. 330, No. 1-2, 2007, pp. 155-163. doi:10.1016/j.ijpharm.2006.09.025
- N. Hussain, V. Jaitley and A. T. Florence, “Recent Advances in the Understanding of Uptake of Microparticulates across the Gastrointestinal Lymphatics,” Advanced Drug Delivery Reviews, Vol. 50, No. 1-2, 2001, pp. 107-142. doi:10.1016/S0169-409X(01)00152-1
- D. D. Stuart and T. M. Allen, “A New Liposomal Formulation for Antisenseoligodeoxynucleotides with Small Size, High Incorporation Efficiency and Good Stability,” Biochimica et Biophysica Acta, Vol. 146, 2000, pp. 3219- 3229.
- H. Yuan, J. Chen, Y. Z. Du, F. Q. Hu, S. Zeng and H. L. Zhao, “Studies on Oral Absorption of Stearic Acid SLN by a Novel Fluorometric Method,” Colloids and Surfaces B: Biointerfaces, Vol. 58, No. 2, 2007, pp. 157-164. doi:10.1016/j.colsurfb.2007.03.002
- R. N. Gursoy and S. Benita, “Self-Emulsifying Drug Delivery Systems (SEDDS) for Improved Oral Delivery of Lipophilic Drugs,” Biomedicine & Pharmacotherapy, Vol. 58, No. 3, 2004, pp. 173-182. doi:10.1016/j.biopha.2004.02.001
- S. J. Lim, M. K. Lee and C. K. Kim, “Altered Chemical and Biological Activities of All-Trans Retinoic Acid Incorporated in Solid Lipid Nanoparticle Powders,” Journal of Controlled Release, Vol. 100, No. 1, 2004, pp. 53-61. doi:10.1016/j.jconrel.2004.07.032
- T. Wisanu, L. Boonsom and L. Saisunee, “Flow Injection Analysis of Total Curcuminoids in Turmeric and Total Antioxidant Capacity Using 2,2Β0-Diphenyl-1-picrylhydrazyl Assay,” Food Chemistry, Vol. 112, 2009, pp. 494- 499.
- S. Schmidt, D. Gonzalez and H. Derendorf, “Significance of Protein Binding in Pharmacokinetics and Pharmacodynamics,” Journal of Pharmaceutical Sciences, Vol. 99, No. 3, 2010, pp. 1107-1122. doi:10.1002/jps.21916
- S. M. Moghimi, A. C. Hunter and J. C. Murray, “LongCirculating and Target-Specific Nanoparticles: Theory to Practice,” Pharmacological Reviews, Vol. 53, No. 2, 2001, pp. 283-318.
- E. Mastrobattista, G. A. Koning and G. Storm, “Immunoliposomes for the Targeted Delivery of Antitumor Drugs,” Advanced Drug Delivery Reviews, Vol. 40, No. 1-2, 1999, pp. 103-127. doi:10.1016/S0169-409X(99)00043-5
- K. Sou, S. Inenaga, S. Takeoka and E. Tsuchida, “Loading of Curcumin into Macrophages Using Lipid-Based Nanoparticles,” International Journal of Pharmaceutics, Vol. 352, No. 1-2, 2008, pp. 287-293. doi:10.1016/j.ijpharm.2007.10.033
- L. Li, B. Ahmed, K. Mehta and R. Kurzrock, “Liposomal Curcumin with and without Oxaliplatin: Effects on Cell Growth, Apoptosis, and Angiogenesis in Colorectal Cancer,” Molecular Cancer Therapeutics, Vol. 6, 2007, 1276- 1282. doi:10.1158/1535-7163.MCT-06-0556
- Z. Ma, A. Shayeganpour, D. R. Brocks, A. Lavasanifar and J. Samuel, “Highperformance Liquid Chromatography Analysis of Curcumin in Rat Plasma: Application to Pharmacokinetics of Polymeric Micellar Formulation of Curcumin,” Biomedical Chromatography, Vol. 21, No. 5, 2007, pp. 546-552. doi:10.1002/bmc.795
- C. A. Mosley, D. C. Liotta and J. P. Snyder, “Highly Active Anticancer Curcumin Analogues,” Advances in Experimental Medicine and Biology, Vol. 595, 2007, pp. 77- 103. doi:10.1007/978-0-387-46401-5_2
- A. Preetha, R. Banerjee and N. Huilgol, “Tensiometric Profiles and Their Modulation by Cholesterol: Implications in Cervical Cancer,” Cancer Investigation, Vol. 25, No. 3, 2007, pp. 172-181. doi:10.1080/07357900701209053
- H. Ohori, H. Yamakoshi, M. Tomizawa, M. Shibuya, Y. Kakudo, A. Takahashi, S. Takahashi, S. Kato, T. Suzuki, C. Ishioka, Y. Iwabuchi and H. Shibata, “Synthesis and Biological Analysis of New Curcumin Analogues Bearing an Enhanced Potential for the Medicinal Treatment of Cancer,” Molecular Cancer Therapeutics, Vol. 5, 2006, pp. 2563-2571. doi:10.1158/1535-7163.MCT-06-0174
- C. Karikar, A. Maitra, S. Bisht, G. Feldmann, S. Soni and R. Ravi, “Polymeric Nanoparticle-Encapsulated Curcumin (‘Nanoparticulate Curcumin’): A Novel Strategy for Human Cancer Therapy,” Journal of Nanobiotechnology, Vol. 5, 2007, p. 3. doi:10.1186/1477-3155-5-3
- W. Tiyaboonchai, W. Tungpradit and P. Plianbangchang, “Formulationand Characterization of Curcuminoids Loaded Solid Lipid Nanoparticles,” International Journal of Pharmaceutics, Vol. 337, No. 1-2, 2007, pp. 299-306. doi:10.1016/j.ijpharm.2006.12.043
- U. K. Parashar, P. S. Saxena and A. Srivastava, “Role of Na-nomaterials in Biotechnology,” Digest Journal of Nanomaterials and Biostructures, Vol. 3, No. 2, 2008, pp. 81-87.
- R. Ravichandran, “Nanoparticles in Drug Delivery: Potential Green Nanobiomedicine Applications,” International Journal of Green Nanotechnology: Biomedicine, Vol. 1, No. 2, 2009, pp. B108-B130.
- R. Ravichandran, “Nanotechnology-Based Drug Delivery Systems,” NanoBiotechnology, Vol. 5, No. 1, 2010, pp. 17-33. doi:10.1007/s12030-009-9028-2
- M. Sun, X. Su, B. Ding, X. L. He, X. J. Liu, A. H. Yu, H. X. Lou and G. X. Zhai, “Advances in NanotechnologyBased Delivery Systems for Curcumin,” Nanomedicine, Vol. 7, No. 7, 2012, pp. 1085-1100. doi:10.2217/nnm.12.80

